Abstract
Dental composites undergo material property changes during exposure to the oral environment and may release compounds of potential toxicity, such as bisphenol A. Degradation of dental composites was studied in a simplified overlayer model in which bisphenol A diglycidyl methacrylate (BisGMA) was covalently bound to a porous silicon oxide surface. It was hypothesized that the chemical structure of this overlayer would allow release of bisphenol A, BisGMA, and the decomposition products thereof, upon exposure to water for an extended period. Liquid chromatography mass spectrometry found leaching of intact BisGMA and several degradation products that contained the bisphenol A moiety from the overlayer into distilled water after 2 wks of aging. The absence of bisphenol A release from the overlayer reduces concerns regarding its potential health risk in dental composites. Nevertheless, health concerns might arise with respect to BisGMA and the leached degradation products, since they all contain the bisphenol A moiety. Abbreviations: BisGMA, bisphenol A diglycidyl methacrylate; HPLC, high-performance liquid chromatography; LCMS, liquid chromatography mass spectrometry; MA, methacrylic acid; MPS, 3-(trimethoxysilyl) propyl methacrylate; m/z, mass-to-charge ratio; and TIC, total ion chromatogram.
Keywords: hydrolysis, degradation, dental composite, liquid chromatography mass spectrometry
INTRODUCTION
Dental composites of polymer resins and glass filler particles have an esthetically pleasing appearance and adhere well to dental enamel and dentin (Ferracane, 1995; Jones, 2001; Ortengren et al., 2001). The resin is usually composed of methacrylate polymers such as bisphenol A diglycidyl methacrylate (BisGMA) and tetraethyleneglycol dimethacrylate. Coupling agents are used to bind the filler particles and the polymer resin covalently, to impart better mechanical properties to the composite (Ferracane, 1995; Jones, 2001; Halvorson et al., 2003). 3-(trimethoxysilyl) propyl methacrylate (MPS) is a common coupling agent used to bind the filler particle to the methacrylate end of BisGMA (Liu et al., 2001; Halvorson et al., 2003).
Both physical and chemical processes affect the degradation of dental composites in the oral cavity (Ferracane, 1995, 2006; Goperfich, 1996). Chemical degradation of dental composites can be caused by hydrolysis and/or enzyme catalysis from saliva and enzymes in the oral environment, weakening the composite material sufficiently to reduce restoration longevity and mechanical properties (Ferracane, 1995, 2006; Geurtsen, 1998; Santerre et al., 2001). Degradation changes the composite microstructure by forming pores or openings from which degradation products, residual monomers, and oligomers can be released (Goperfich, 1996; Geurtsen, 1998).
Composite degradation releases organic compounds into tissues, where they can accumulate in the body and may be of medical concern. Various studies and an expert panel have argued that bisphenol A poses a significant health risk to humans (Crain et al., 2007; Dolinoy et al., 2007; Vandenberg et al., 2007; vom Saal et al., 2007). Bisphenol A is not used directly in dental materials (Ferracane, 2006), but BisGMA and other components of dental composites are chemical derivatives of bisphenol A and may degrade into related species with potentially suspect health effects.
Degradation of the dental composites in the oral environment can proceed by hydrolysis of the siloxane bond (Drummond et al., 1998; Xiao et al., 1998; Lateef et al., 2002; Zhou et al., 2006). Hydrolysis of ester bonds in the complex is another degradation pathway. The majority of resin elution studies have been done in water to mimic oral conditions (Sideridou and Achilias, 2005). Water diffuses into the composite and accumulates at the interface between the resin and filler material, reacting with the silane coupler and filler material to release degradation products into solution (Drummond et al., 1998; Geurtsen, 1998). Water diffusion can also release unreacted resin monomers into the solution (Oysaed et al., 1988; Ferracane et al., 1995; Goperfich, 1996; Drummond et al., 1998).
Previous work from our group examined the leaching of inorganic ions from commercial composites after aging in ethanol, water, and artificial saliva solutions (Zhou et al., 2005). Other work has examined the leaching of organic components from commercial dental composites when aged under various conditions (Goperfich, 1996; Geurtsen, 1998; Lee et al., 1998; Spahl et al., 1998; Noda et al., 1999; Ortengren et al., 2001; Sideridou and Achilias, 2005; Stefova et al., 2005; Ferracane, 2006). However, commercial dental composites contain initiators, stabilizers, inhibitors, and various additives that may be proprietary and/or vary with manufacturer. This complex composition severely complicates the study of chemical degradation in commercial dental composites.
Our group therefore developed a simplified model of the resin-filler particle-bonding interface to allow for hypothesis-testing of degradation mechanisms and simplification of degradation analysis (Zhou et al., 2006). The overlayer model system consists of three elements: a polymer resin, a glass filler, and a silane coupler. The chemical linkages that comprise this model include a siloxane bond that forms between filler and silane, as well as a covalent bond that forms between the silane’s organofunctional group and reactive groups of the resin matrix (Halvorson et al., 2003). The resin used in the model was BisGMA, a nanoporous silicon chip was used to mimic the silica filler particles, and MPS was the silane coupler utilized between the two. The current work studied the degradation of this model overlayer, after being aged in water, by liquid chromatography mass spectrometry (LCMS). A two-week aging period was chosen, since this was found to be the interval of maximum release of BisGMA from commercial dental composites (Stefova et al., 2005).
MATERIALS & METHODS
Instrumentation
The high-performance liquid chromatography (HPLC) system utilized consists of a vacuum membrane degasser, a gradient elution pump, and an autosampler (SpectraSYSTEMS SCM 1000, P4000, and AS 3000, all from Finnigan Mat, San Jose, CA, USA). The HPLC is connected to an ion trap mass spectrometer (LCQ Classic, Finnigan Mat) equipped with an electrospray ionization source. A visible-light-curing system (Triad 2000, Dentsply International, York, PA, USA) was used for curing and polymerization.
Materials
Standard chemicals and silicon wafers have been described previously (Zhou et al., 2006). A reverse-phase chromatography column was used for HPLC analysis (3.5 μm beads, 3.0 × 150 mm, Symmetry C18 model 186000695, Waters Corporation, Milford, MA, USA). Spectroscopic-grade methanol was used for the separation, along with triple-de-ionized water.
Preparation and Aging of Composite Overlayer Model System
Nanoporous oxidized silicon wafers were prepared as described previously (Zhou et al., 2006), by electrolytic oxidation, then reacted with MPS/toluene solution to form a methacryloyl overlayer. A BisGMA solution was subsequently added and photopolymerized to form a BisGMA-methacryloyl overlayer, which was washed to remove unbound BisGMA.
The BisGMA-methacryloyl overlayers were aged in deionized water for 2 wks. The degradation products that remained in the water used for aging were extracted into ethyl acetate, which was then evaporated. The dry extract was then mixed with methanol and analyzed by LCMS.
LCMS Analysis
Methacrylic acid, vinyl pyrrolidinone, triethanolamine, bisphenol A, Eosin Y, and BisGMA (Zhou et al., 2006) were used as standards to determine known retention times on the column in LCMS. Several of these compounds were studied because they were used to prepare the overlayer, while others were potential degradation products.
An injected sample volume of 10 μL was used for both standards and samples. Forty-minute separations were used, with a 60% to 100% methanol:water gradient at a flow rate of 0.3 mL/min and a constant 0.1% formic acid concentration. Mass spectra for all standards and aged samples were recorded over the mass range of m/z 80 to 950, and total ion chromatograms (TIC) were reported. The entire sample-aging and analysis process was performed at room temperature. We analyzed multiple samples to identify degradation products of the BisGMA-methacryloyl overlayer, as described further below, and only products that consistently appeared are reported in the Table and mentioned in the Results and Discussion (below).
Table.
Retention Times and Apparent Parent Ions for Species Leached from BisGMA-Methacryloyl Overlayer Samples Aged for 2 Wks in Water, as Measured by LCMS
| Retention Time (min) | m/z | Name | % Observed (30 trials) |
|---|---|---|---|
| 16.0 ± 0.5 | 530 | BisGMA, [MNH4]+ | 93 |
| 11.3 ± 0.5 | 462 | BisGMA-MA, [MNH4]+ | 67 |
| 25.3 ± 0.6 | 376 | BisGMA-2MA, [MH]+ | 57 |
| 24.2 ± 0.3 | 363, 747 | Singly and Doubly Dehydrated BisGMA-2MA, [MNa]+, [M2CH3OH]+ | 37 |
| 19.1 ± 0.5 | 620 | Unknown Derivative 1 | 60 |
| 20.6 ± 0.5 | 634 | Unknown Derivative 2 | 77 |
| 21.8 ± 0.5 | 644 | Unknown Derivative 3 | 40 |
| 33.5 ± 0.4 | 663 | Overlayer polymer or BisGMA dimer | 60 |
Mass peaks suspected of being parent ions were analyzed by tandem mass spectrometry, but none of them displayed a sufficient signal-to-noise ratio to allow for further structural elucidation, since no fragment ions were observed. The only exception was that the ammoniated parent ion of BisGMA [MNH4]+ at m/z 530 yielded abundant fragment ions.
We took extensive precautions to remove impurities and sample cross-contamination from the analysis, including cleaning glassware with potassium chromate/sulfuric acid solution and rinsing the HPLC column after each analysis. Various controls were performed, including the analysis of methanol exposed to all glassware and water from aging nanoporous silicon oxide wafers, which lacked the BisGMA-methacryloyl overlayer.
Data Analysis Software
We used commercial software (ACD MS Fragmenter, Advanced Chemistry Development Inc., Toronto, ON, Canada) to simulate fragmentation of parent ion chemical structures by using the MH+ pseudoparent ion as the starting ion for the fragmentation pathway. We used other commercial software (ACD MS Manager, Advanced Chemistry Development Inc.) to analyze and process HPLC data, including chromatographs and mass spectra to compare multiple datasets.
RESULTS
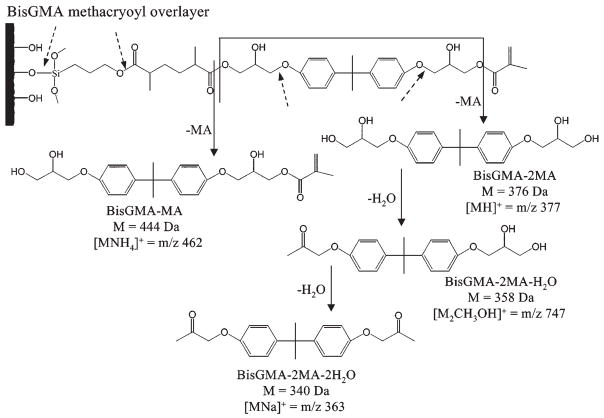
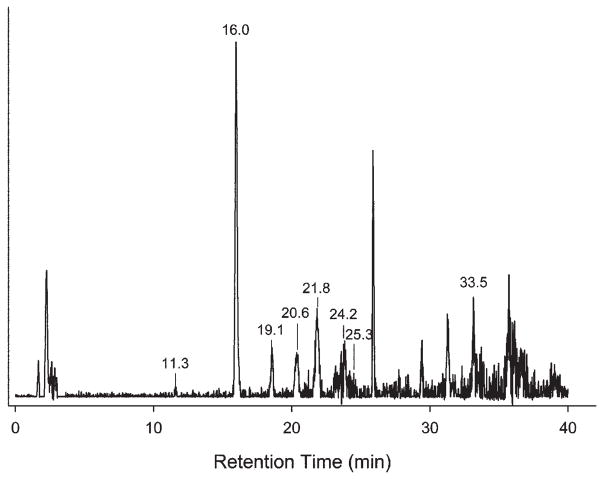
We used LCMS to determine the chemical degradation products of the BisGMA-methacryloyl overlayer after 2 weeks’ aging in water (Fig. 1). The solid arrows identify bonds that hydrolyzed to form degradation products as well as their secondary dehydration products. The broken arrows indicate bonds that could potentially - but did not - undergo hydrolysis. A typical TIC of LCMS of the overlayer aged for 2 wks in water is shown in Fig. 2, with the results summarized in the Table. Intact BisGMA eluted at around 16 min, as confirmed with analysis of the BisGMA standard, and was observed in all but a few samples analyzed. The BisGMA parent (M) complexed with an ammonium ion to form [MNH4]+, which appeared in the mass spectra at m/z 530. This m/z 530 peak was observed for both the aged samples and the standard (data not shown).
Figure 1.
Structure and proposed reaction of BisGMA-methacryloyl overlayer in the presence of water. Hydrolysis of resin ester bonds caused degradation products of BisGMA to appear, indicated by the solid arrows. Dehydration of the BisGMA – 2MA also occurred, leading to the loss of one equivalent of water with each reaction. Potential hydrolysis reactions that did not lead to observed products are indicated by broken arrows.
Figure 2.
Total ion chromatograms (TIC) of extract from methacryloyl BisGMA monolayer on nanoporous silicon aged for 2 wks in de-ionized water. Unlabeled peaks were not reproducible.
Other eluted peaks in the TIC, corresponding to degradation and the derivative products of BisGMA, were formed after aging. The peaks at 11.3 and 25.3 min corresponded to BisGMA minus one or both of the methacrylate groups hydrolyzed at the ester group bond (denoted in the Table as BisGMA – MA and BisGMA – 2MA, respectively). BisGMA - MA has a mass of 444 Da, which complexed with an ammonium ion to form the ion observed at m/z 462, [MNH4]+. BisGMA - 2MA has a mass of 376 Da, which complexed with a proton to form the ion observed at m/z 377, denoted as [MH]+. The BisGMA – 2MA degradation product has also been referred to as Bishydroxypropoxyphenylpropane (BisHPPP) and was previously observed to elute from dental composites (Santerre et al., 2001). Other workers also found BisGMA to lose methacrylate groups via hydrolysis of their ester bonds (Shajii and Santerre, 1999; Smith et al., 2001; Kostoryz et al., 2003; Finer and Santerre, 2004).
A degradation peak eluted at 24.2 min corresponded to a dehydration reaction of BisGMA - 2MA that loses a hydroxyl group and forms a carbonyl bond. The spectra displayed 2 peaks at m/z 363 and 747 (data not shown). The first peak corresponded to the compound of mass 340 Da, which is BisGMA - 2MA dehydrated twice and complexed with sodium to form the [MNa]+ ion at m/z 363 (Fig. 1). The second peak corresponded to the dimer of BisGMA - 2MA dehydrated once to form the compound at mass 358 Da, which complexed with an additional methanol from the solvent to form the [M2CH3OH]+ ion at m/z 747.
An additional degradation peak eluted at 33 min and displayed a parent ion at m/z 731, with a major fragment at m/z 663. This compound has not been positively identified, but its mass and consideration of possible side-reactions indicate that it likely corresponds to a hydrolysis product of the BisGMA dimer, a derivative of the overlayer containing multiple BisGMA components, or the product of hydrolysis of the siloxane bond coupling the overlayer to the substrate.
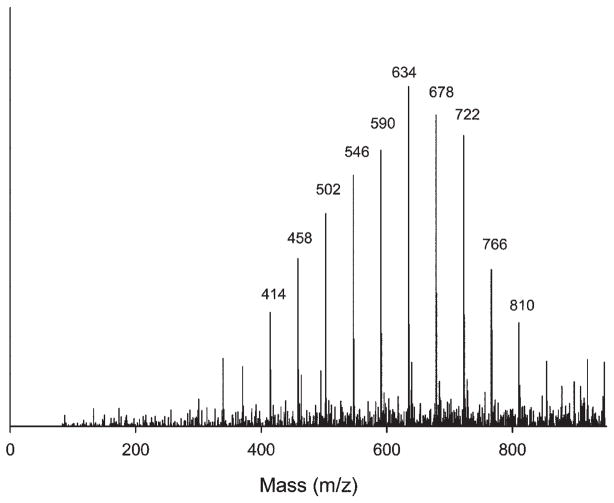
Three polymeric derivatives appeared in the TIC at retention times of 18 to 21 min (Table). The mass spectrum of the 20.6-min peak (labeled “Unknown Derivative 2” in the Table) displayed a typical distribution of oligomers centered at m/z 634 with a peak spacing of Δm/z 44 (Fig. 3). Unknown Derivatives 1 and 3 appeared at 19.1 and 21.8 min retention time in the TIC. They both also displayed polymer-like mass spectra, with similar Δm/z 44 peak spacing, but centered at m/z 620 and 644, respectively (data not shown, but similar to Fig. 3). Polymeric derivatives have appeared in previous studies of commercial dental composites (Smith et al., 2001). Other peaks occasionally appeared in the TIC beyond 25 min, but with insufficient reproducibility to warrant further discussion. However, none of the standard compounds, including methacrylic acid and bisphenol A, appeared in the analysis of the aged solutions.
Figure 3.
MS of Unknown Derivative 2 of 20.5-min peak from TIC, with a Δm/z 44 between adjacent peaks.
The reproducibility of the eluted peaks was evaluated over a total of 30 trials (only reproducible peaks are given in the Table). For example, intact BisGMA was observed in 93% of the samples. The degradation peaks of the resin, BisGMA with the loss of one or both methacrylate groups due to hydrolysis of the ester bonds of BisGMA, were seen in 67% and 57% of the samples, respectively. The dehydrated BisGMA - 2MA products were seen in 37% of the samples.
DISCUSSION
The polymerization process in dental composites is never complete, and unreacted monomers can remain in the composite after the curing process (Ferracane, 2006). Here, covalently bound BisGMA was cleaved at its ester bond via hydrolysis and released into the aging solution. Some of the BisGMA observed here might have derived from monomers strongly adsorbed by, rather than covalently bound to, the nanoporous surface, but adsorbed BisGMA was minimized by a thorough washing of the overlayer prior to aging. Adsorbed species mimic the leaching of trapped material such as BisGMA that occurs in real composites, although the two processes occur on very different timescales.
Water diffuses into a composite during aging and causes chemical degradation products, which can be released into the oral cavity (Geurtsen, 1998). Chemical changes such as oxidation or hydrolytic attack of functional groups or polymer chain scission can result from water absorbing into the composite (Ferracane, 2006). Aging of the BisGMA-methacryloyl overlayer in water caused hydrolysis of the ester bonds to form the BisGMA – MA and BisGMA – 2MA products, resulting from loss of one or two methacrylic acid groups, respectively. These results indicate that protection of the ester groups in dental composites against hydrolysis may reduce the release of compounds containing the bisphenol A moiety into the oral cavity.
Various expected degradation products were not observed. Aging in aqueous solution of siloxane coupled overlayers can lead to hydrolysis of the siloxane bond (Xiao et al., 1998; Lateef et al., 2002), but products clearly associated with cleavage of this bond were not identified. Esters, ethers, urethanes, and hydroxyl groups in a resin are also susceptible to hydrolytic degradation. Thus, the overlayer could potentially degrade at the ether groups adjacent to the aromatic rings or the ester group of the silane coupler, but such products were not observed.
The absence of bisphenol A release from the model overlayer examined here is consistent with studies of dental composites (Santerre et al., 2001; Ferracane, 2006) and reduces concerns regarding its potential health risk in this application (Crain et al., 2007; Dolinoy et al., 2007; Vandenberg et al., 2007; vom Saal et al., 2007). Nevertheless, health concerns might arise with respect to BisGMA and the other degradation products, since they all contain the bisphenol A moiety.
Acknowledgments
This work was funded by the National Institute of Dental and Craniofacial Research (DE-07979).
References
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, et al. An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol. 2007;24:225–239. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond JL, Botsis J, Zhao D, Samyn J. Fracture properties of aged and post-processed dental composites. Eur J Oral Sci. 1998;106:661–666. doi: 10.1046/j.0909-8836.1998.eos106208.x. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Hopkin JK, Condon JR. Properties of heat-treated composites after aging in water. Dent Mater. 1995;11:354–358. doi: 10.1016/0109-5641(95)80034-4. [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res. 2004;69:233–246. doi: 10.1002/jbm.a.30000. [DOI] [PubMed] [Google Scholar]
- Geurtsen W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687–695. doi: 10.1046/j.0909-8836.1998.eos10602ii04.x. [DOI] [PubMed] [Google Scholar]
- Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- Halvorson R, Erickson R, Davidson C. The effect of filler and silane content on the conversion of resin-based composite. Dent Mater. 2003;19:327–333. doi: 10.1016/s0109-5641(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Jones FH. Teeth and bones: applications of surface science to dental materials and related biomaterials. Surf Sci Rep. 2001;42:75–205. [Google Scholar]
- Kostoryz EL, Eick JD, Glaros AG, Judy BM, Welshons WV, Burmaster S, et al. Biocompatibility of hydroxylated metabolites of BisGMA and BFDGE. J Dent Res. 2003;82:367–371. doi: 10.1177/154405910308200508. [DOI] [PubMed] [Google Scholar]
- Lateef SS, Boateng S, Hartman TJ, Crot CA, Russell B, Hanley L. GRGDSP peptide bound silicone membranes withstand mechanical flexing in vitro and display enhanced fibroblast adhesion. Biomaterials. 2002;23:3159–3168. doi: 10.1016/s0142-9612(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Lee SY, Huang HM, Lin CY, Shih YH. Leached components from dental composites in oral simulating fluids and the resultant composite strengths. J Oral Rehabil. 1998;25:575–588. doi: 10.1046/j.1365-2842.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Ding J, Chambers DE, Debnath S, Wunder SL, Baran GR. Filler-coupling agent-matrix interactions in silica/polymethyl -methacrylate composites. J Biomed Mater Res. 2001;57:384–393. doi: 10.1002/1097-4636(20011205)57:3<384::aid-jbm1181>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Noda M, Komatsu H, Sano H. HPLC analysis of dental composites components. J Biomed Mater Res. 1999;47:374–378. doi: 10.1002/(sici)1097-4636(19991205)47:3<374::aid-jbm12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil. 2001;28:1106–1115. doi: 10.1046/j.1365-2842.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- Oysaed H, Ruyter IE, Sjovik Kleven IJ. Release of formaldehyde from dental composites. J Dent Res. 1988;67:1289–1294. doi: 10.1177/00220345880670100901. [DOI] [PubMed] [Google Scholar]
- Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–151. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- Shajii L, Santerre JP. Effect of filler content on the profile of released biodegradation products in micro-filled bis-GMA/TEGDMA dental composite resins. Biomaterials. 1999;20:1897–1908. doi: 10.1016/s0142-9612(99)00087-3. [DOI] [PubMed] [Google Scholar]
- Sideridou I, Achilias D. Elution study of unreacted BisGMA, TEGDMA, UDMA, and BisEMA from light cured dental resins and resin composites using HPLC. J Biomed Mater Res (Appl Biomater) 2005;74:617–626. doi: 10.1002/jbm.b.30252. [DOI] [PubMed] [Google Scholar]
- Smith RE, Eick JD, Yourtee DM. Liquid chromatography and purification of dental monomers. J Liq Chrom Rel Technol. 2001;24:531–542. [Google Scholar]
- Spahl W, Budzikiewicz H, Geurtsen W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J Dent. 1998;26:137–145. doi: 10.1016/s0300-5712(96)00086-3. [DOI] [PubMed] [Google Scholar]
- Stefova M, Ivanova V, Muratovska I. Identification and quantification of BisGMA and TegDMA released from dental materials by HPLC. J Liq Chrom Rel Technol. 2005;28:289–295. [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill Bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S-J, Textor M, Spencer ND, Sigrist H. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998;14:5507–5516. doi: 10.1023/a:1018501804943. [DOI] [PubMed] [Google Scholar]
- Zhou M, Drummond JL, Hanley L. Barium and strontium leaching from aged glass particle/resin matrix dental composites. Dent Mater. 2005;21:145–155. doi: 10.1016/j.dental.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wu C, Edirisinghe PD, Drummond JL, Hanley L. Organic overlayer model of a dental composite analyzed by laser desorption postionization mass spectrometry and photoemission. J Biomed Mater Res A. 2006;77:1–10. doi: 10.1002/jbm.a.30591. [DOI] [PubMed] [Google Scholar]