Abstract
Many genes in the Candida albicans ergosterol biosynthetic pathway are controlled by the transcriptional activator Upc2p, which is upregulated in the presence of azole drugs and has been suggested to regulate its own transcription by an autoregulatory mechanism. The UPC2 promoter was cloned upstream of a luciferase reporter gene (RLUC). UPC2-RLUC activity is induced in response to ergosterol biosynthesis inhibitors and in response to anaerobicity. In both conditions, induction correlates with the magnitude of sterol depletion. Azole inducibility in the parental strain was approximately 100-fold, and in a UPC2 homozygous deletion strain was 17-fold, suggesting that in addition to autoregulation, UPC2 transcription is controlled by a novel, Upc2p-independent mechanism(s). Curiously, basal UPC2-RLUC activity is 5-fold higher in the deletion strain, which may be an indirect consequence of the lower sterol level in this strain, or a direct consequence of repression by an autoregulatory mechanism. These results suggest that transcriptional regulation of UPC2 expression is important in the response to antifungal drugs, and that this regulation occurs through Upc2p-dependent as well as novel Upc2p-independent mechanisms.
Keywords: Candida albicans, UPC2, azoles, resistance, ergosterol biosynthesis, autoregulation
INTRODUCTION
The pathogenic yeast Candida albicans causes oral, vaginal and systemic disease in immunocompromised hosts, and vaginal infection in immune competent hosts. Significant mortality is seen with systemic disease, which is most commonly seen in neutropenic patients, such as those receiving transplant chemotherapy. Candida infections are one of the most common opportunistic infections associated with AIDS, and usually manifests as oral disease in these patients (Pfaller & Diekema, 2004).
The most frequently used antifungals for treatment of oral candidiasis are the azoles which inhibit ergosterol biosynthesis. Resistance to the azoles has emerged due to the fungistatic nature of these drugs and their frequent use for prophylaxis (Pfaller & Diekema, 2004). The azoles, such as fluconazole (FLC) and clotrimazole (CLO), act by targeting the ergosterol biosynthesis enzyme lanosterol 14-α-demethylase which is encoded by the gene ERG11 (White et al., 1998). Other ergosterol biosynthesis inhibitors act either up or downstream of Erg11p. These include terbinafine (TER) that inhibits the ERG1 gene product, fenpropimorph (FEN) that inhibits Erg2p, and lovastatin (LOV) that inhibits Hmg1p. Inhibition of sterol synthesis at any of these points results in upregulation of many genes within the pathway (Arthington-Skaggs et al., 1996; Dimster-Denk & Rine, 1996; Henry et al., 2000; Song et al., 2004). Expression of many of these genes has recently been shown to be controlled by the master sterol transcriptional regulator Upc2p (MacPherson et al., 2005; Silver et al., 2004).
C. albicans Upc2p (CaUpc2p) is a Zn2Cys6 cluster transcription factor and is homologous at the sequence and functional levels to the Saccharomyces cerevisiae paralogs UPC2 and ECM22 (ScUPC2 and ScECM22) (MacPherson et al., 2005; Silver et al., 2004). CaUpc2p is required for upregulation of ERG11 (Oliver et al., 2007) and other sterol biosynthesis genes in response to sterol depletion (MacPherson et al., 2005; Silver et al., 2004), and it activates transcription of target genes by binding to a conserved core sequence known as the sterol response element (SRE) (MacPherson et al., 2005). The CaUPC2 homozygous deletion is hypersensitive to ergosterol biosynthesis inhibitors as well as to certain drugs that target the cell wall, demonstrating that this transcription factor is central to the response to many antifungal drugs (MacPherson et al., 2005; Silver et al., 2004).
Interestingly, the CaUPC2 promoter itself contains a putative SRE (MacPherson et al., 2005), suggesting transcriptional self-regulation. It is generally accepted that transcriptional self-activation accounts for the majority of control of ScUPC2 expression, but this hypothesis has previously only been supported by indirect experimental evidence. Transcriptional profiling of a mutant containing a hyperactive allele of ScUPC2 (UPC2-1) revealed an increase in ScUPC2 mRNA when compared to wild-type, suggesting that ScUPC2 was self-activated in the UPC2-1 strain (Wilcox et al., 2002). Another study using a ScUPC2-lacZ fusion showed that deletion of the SRE causes a significant, although not complete reduction in the anaerobic inducibility of the reporter, some of which appears to be due to an increase in basal activity of the promoter lacking the SRE (Abramova et al., 2001). Both of these studies were conducted using S. cerevisiae strains containing the ScUPC2 paralog ScECM22, and inducibility of ScUPC2 may be affected by the presence of ScECM22. Studies showing that ScUPC2 expression is induced by azole drugs have not shown whether inhibition of the ergosterol biosynthetic pathway with other antifungal drugs also results in a ScUPC2 transcriptional response. The work in this study characterizes the transcriptional activation profile of CaUPC2 in response to sterol depletion mediated by sterol synthesis inhibitors and anaerobicity, and investigated the hypothesis that CaUPC2 expression is self-regulated.
MATERIALS AND METHODS
Abbreviations
Abbreviations are used throughout the text for drugs used in this study as follows: clotrimazole (CLO), fenpropimorph (FEN), fluconazole (FLC), lovastatin (LOV), nikkomycin Z (NKZ), nourseothricin (NAT) and terbinafine (TER).
Strains and growth conditions
C. albicans strain BWP17 (ura3::λ434/ura3::λimm434his1::hisG/his1::hisG arg4::hisG/arg4::hisG) and its derivative D-6 (upc2::URA3/upc2::ARG4) were transformed with UPC2-RLUC expression constructs containing the nourseothricin resistance marker SAT1 (generously provided by Dr. Joachim Morchauser) to create strains CaUPC2-750WT (strain TW16201) and CaUPC2-750D (strain TW16202). Strains were maintained on YEPD (10 g Difco yeast extract, 20 g Bacto peptone, and 20 g dextrose per liter) containing 200 μg/ml nourseothricin (NAT). Innocula prepared for luciferase assays and ergosterol quantitation were grown in CSM (0.75 g CSM (Bio101 Inc, Vista, CA) 5.0 g ammonium sulfate, 1.7 g yeast nitrogen base without amino acids or ammonium sulfate, and 20 g dextrose per liter) with 200 μg/ml NAT to provide selection. Growth during assays was carried out in CSM lacking nourseothricin to avoid pleiotrophic effects of the selective agent.
Creation of UPC2 constructs containing the Renilla reniformis luciferase reporter
The plasmid pCRW3 containing the Renilla luciferase reporter plasmid was generously provided by D.R. Soll (Srikantha et al., 1996). To construct the reporter plasmid containing the nourseothricin resistance marker, the plasmid pA83 (Reuss et al., 2004) was used to amplify the SAT1 marker with the oligonucleotides SAT1Kpn and SAT1EcoRV (Table 1). The resulting PCR fragment was cloned into the vector pCR-Topo (Invitrogen, Carlsbad, CA), after which the SAT1 marker was excised and ligated into EcoRV and KpnI digested pCRW3 to create pCRW3-SAT1. This was done such that the SAT1 marker would be transcribed in the opposite direction of the reporter gene, to avoid potential RLUC activity that could result from incomplete termination of SAT1 transcription. To create the CaUPC2-RLUC fusion, 750bp of CaUPC2 sequence upstream of the initiating ATG were amplified from the plasmid pGEM-HIS-UPC2 (Silver et al., 2004) using oligonucleotides UPC2Kpn and UPC2Sma. The resulting fragment was cloned into KpnI-XmaI digested pCRW3-SAT1 to create pUPC2-RLUC. This plasmid was then linearized using NsiI and integrated at the ADE2 locus of C. albicans strains according to an integration strategy previously used in this laboratory (Song et al., 2004).
TABLE 1.
Oligonucleotides used for reporter plasmid construction and transformant screening
| Oligonucleotide name | Sequence (5’- 3’) |
|---|---|
| SAT1Kpn | TACAACGGTACCCAGCGTCAAAACTAGAGAATAATAAG |
| SAT1EcoRV | TACAACGATATCGATTTCTAGAAGGACCAC |
| UPC2Kpn | CTCTCGGTACCATGGATGTTGGTATATCAGG |
| UPC2Sma | CTCTCCCCGGGAAATGGCTTTTTTGTTGAAAAA |
| RLUC | CACCACTGCGGACCAGTTATCATCCGTTTCC |
| ADE2 | CAGTTAAATAGTCTTCATATC |
C. albicans transformation
C. albicans strains were transformed using the lithium acetate-heat shock method described previously (Sanglard et al., 1996) with modifications. Briefly, 500 uL of YEPD overnight cultures were diluted into 50 ml of fresh YEPD and grown for 5 h at 30°C with shaking at 180 RPM. Cells were prepared by pelleting, washing once with sterile water, and resuspending in 0.1M Lithium acetate in TE pH 7.5 (Li-TE). 5-10 ug of NsiI linearized plasmid DNA and 10 ug carrier DNA (sheared herring sperm DNA, Invitrogen) was used for each transformation. Cells were incubated overnight with DNA in 40% PEG 3350 in Li-TE, heat shocked for 30 m at 42°C and washed once in 1M sorbitol. This was followed by 4 h of growth at 30°C with shaking at 180 RPM in YEPD in the absence of nourseothricin selection after which cells were plated on YEPD plates containing 200 μg/ml nourseothricin as described previously (Reuss et al., 2004).
PCR and Southern blot screening of transformants
Genomic DNA from pUPC2-RLUC transformed BWP17 and D6 was prepared from cells grown overnight in YEPD-NAT using glass bead lysis as described previously (Hoffman & Winston, 1987). Transformants were initially screened for positive integration of NsiI digested pUPC2-RLUC at the ADE2 locus using the oligonucleotides ADE2 and RLUC (Table 1). PCR positive transformants were then confirmed by Southern blotting to contain the pUPC2-RLUC construct in single copy. Briefly, approximately 10 ug of genomic DNA was digested with KpnI overnight and run on a 0.7% agarose gel and blotted overnight to a nitrocellulose membrane. The blot was probed with 32P end-labeled RLUC oligonucleotide probe (Table 1). Transformants containing pUPC2-RLUC in single copy at the ADE2 locus were used for luciferase assay.
Drugs and conditions for UPC2-RLUC activity
Drugs used for induction of the UPC2 reporter construct include the azoles FLC (Pfizer, New York, NY, stock concentration of 3 mg/ml in water) at final concentrations of 0.1-100 μg/ml, and CLO (Sigma-Aldrich, stock concentration 10 mg/ml in DMSO) at a final concentration of 10 μg/ml. TER (Novartis, Vienna, Austria, stock concentration of 10 mg/ml in DMSO) was used at a final concentration of 100 μg/ml, FEN (Sigma-Aldrich, stock concentration of 10 mg/ml in DMSO) at a final concentration of 100μg/ml, nikkomycin Z (NKZ) (Sigma-Aldrich, stock concentration 5 mg/ml in water) at final concentration of 10-100 μg/ml, and LOV (Calbiochem, San Diego, CA, stock concentration 10 mg/ml in ethanol) at a final concentration of 20 μg/ml. In assays where non-water vehicle was used, the no-drug controls were also treated with vehicle. For anaerobic conditions, AnaeroPack anaerobic catalysts (Mitsubishi Gas Chemical, New York, NY) were used in GasPack anaerobic jars (BD, Franklin Lakes, NJ). All drug or anaerobicity induction experiments were carried out in 5 ml volumes in 50 ml conical tubes at 30°C with shaking at 180rpm for 6, 24, or 48 h.
Luciferase assay of UPC2-RLUC activity
Luciferase assays were performed as described previously (Srikantha et al., 1996) with modifications described in (Song et al., 2004). Because of inter-assay variability, data presented are representative of three independent experiments. Intra-assay variability was assessed by growing three independent colonies of both UPC2-RLUC strains in the presence and absence of 100 μg/ml FLC. This experiment confirmed that within a given assay, variability is low (under 10%).
Ergosterol quantitation experiments
Total ergosterol levels were measured as described previously (Arthington-Skaggs et al., 1999). Cultures were inoculated such that the optical density at 600 nm was 0.2 in a total volume of 25 ml CSM. Cells were grown in the presence and absence of drug, or grown anaerobically for 48 h before harvesting. Equivalent OD units of each culture were used to allow for direct comparison of ergosterol levels between strains and conditions. Wavelength scans of samples were performed from 210 nm to 340 nm.
RESULTS
Creation of C. albicans strains expressing CaUPC2-RLUC fusions
Previous work has shown that exposure to azoles and other ergosterol biosynthesis inhibitors induces the expression of ergosterol biosynthetic genes as well as their transcriptional activator CaUpc2p (Silver et al., 2004). In order to study the effect of these drugs over prolonged exposure, however, it was necessary to create reporter fusions to avoid difficulties in extracting high quality RNA at late time points. Additionally, to address the role of CaUpc2p self-activation in CaUPC2 transcriptional induction, it was necessary to express the CaUPC2-RLUC fusion in our Δupc2/Δupc2 strain D6. This allowed for monitoring changes between wild type and the deletion strain to test the effect of endogenous CaUpc2p on CaUPC2 transcriptional inducibility. The Renilla reniformis luciferase reporter from plasmid pCRW3 (Srikantha et al., 1996) was used to monitor changes in CaUPC2 transcriptional activation. To express CaUPC2-RLUC in both strains tested in this study, the nourseothricin resistance marker SAT1 was cloned into pCRW3, and 750 bp of the CaUPC2 promoter was fused to the RLUC gene in this plasmid (see Materials and Methods for details).
CaUPC2 expression is highly regulated at the transcriptional level in response to azole drugs
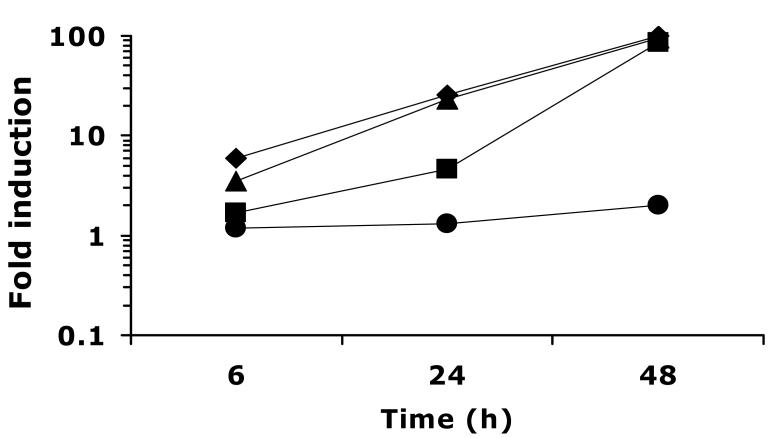
To assess the role of CaUPC2 transcriptional activation in response to ergosterol depletion in a WT strain, CaUPC2-750WT was grown in the presence and absence of various antifungal drugs that target the ergosterol biosynthesis pathway. The effect of a range of FLC concentrations (0.1-100 μg/ml) on CaUPC2-RLUC expression was tested at 6, 24 and 48 h. The drug concentrations were chosen to test the effect of concentrations of drug below, at and above the MIC of FLC for the wild-type strain. This analysis revealed that CaUPC2 transcriptionally responds to FLC exposure at concentrations near or above the MIC (1.0 μg/ml, Fig 1). Exposure to a sub-inhibitory concentration of FLC (0.1 μg/ml) does not induce UPC2 transcription to a significant degree (Fig. 1). The presence of 1.0, 10 or 100 μg/ml of FLC, however, causes activation of CaUPC2-RLUC activity, and this induction increases with time, to a maximum of about 100-fold inducibility at 48 h (Fig 1). Interestingly, prolonged incubation with more than 1.0 μg/ml FLC causes a greater induction at earlier time points, but by 48 h, 1.0 μg/ml of FLC is sufficient to induce a maximal transcriptional response. The effect of another azole, CLO, was similar to that of FLC at the concentration tested (10 μg/ml, Fig 2). The concentration of CLO used was based on previous work (Song et al., 2004) and is reflective of the MIC of CLO for the C. albicans strains used in this study.
Fig. 1.
Time course of UPC2-RLUC induction in WT by a range of FLC concentrations. CaUPC2-750WT was grown in the presence and absence of FLC at 0.1 (circles), 1.0 (squares), 10 (triangles), or 100 (diamonds) μg/ml. Luciferase activity was assayed at 6, 24 and 48 h. Data are presented as fold induction, or the luciferase activity in the presence of drug relative to the luciferase activity in the absence of drug. The results are representative of three independent experiments.
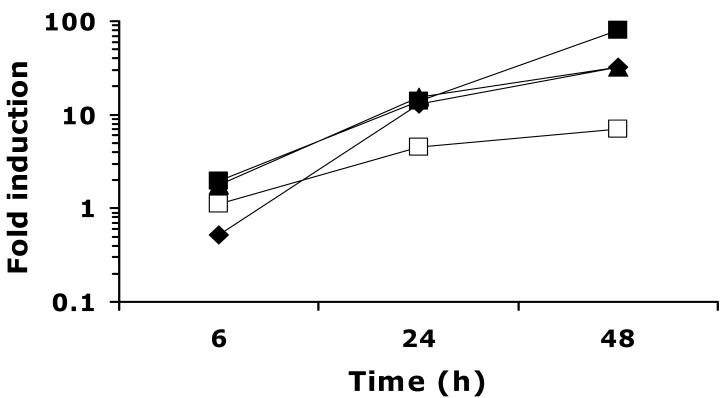
Fig. 2.
Time course of UPC2-RLUC induction by additional ergosterol biosynthesis inhibitors. CaUPC2-750WT was grown in the presence and absence of CLO at 10 μg/ml (solid diamonds), TER at 100 μg/ml (solid triangles), FEN at 100 μg/ml (solid squares) or LOV at 20 μg/ml (open squares). Luciferase activity was assayed at 6, 24 and 48 h. Data are presented as fold induction, or the luciferase activity in the presence of drug relative to the luciferase activity in the absence of drug. The results are representative of three independent experiments.
CaUPC2 expression is transcriptionally induced in response to multiple ergosterol biosynthesis inhibitors
CaUpc2p has been shown to be a global regulator of sterol biosynthesis genes in response to levels of ergosterol or other late stage intermediates of the sterol pathway (MacPherson et al., 2005; Silver et al., 2004). Because of this, it was of interest to test whether CaUPC2 is transcriptionally induced in the presence of inhibitors of ergosterol biosynthesis enzymes other than the azoles which target ERG11. To test this, CaUPC2-750WT was grown in the presence or absence of FEN, TER, and LOV. These drugs were chosen because they target enzymes of both the early (LOV targets Hmg1p,) and late (TER targets Erg1p, which is upstream of Erg11p; FEN targets Erg2p, downstream of Erg11p) parts of the ergosterol biosynthetic pathway. As shown in Figure 2, TER and FEN (solid triangles and solid squares, respectively) treatment induces transcription of CaUPC2 to levels similar to those seen with azole exposure (Fig. 1), and this induction increases with prolonged growth in the presence of drug. Incubation with 20 μg/ml LOV also induced a CaUPC2 transcriptional response, although the overall fold induction was lower than that seen with other ergosterol biosynthesis inhibitors (approximately 5-fold, Fig 2, open squares).
CaUPC2 expression is induced in response to ergosterol biosynthesis inhibitors in the absence of endogenous CaUpc2p
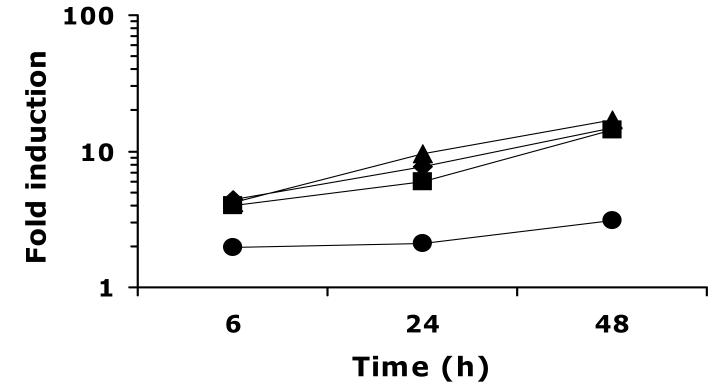
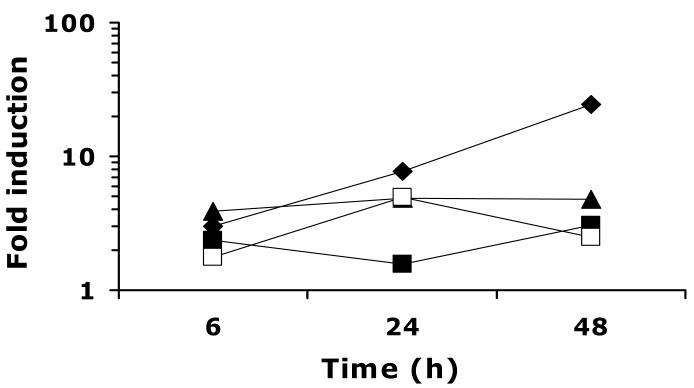
To test the hypothesis that CaUPC2 expression is transcriptionally self-regulated, CaUPC2-750D6, the Δupc2/Δupc2 strain expressing CaUPC2-RLUC, was grown in the presence and absence of ergosterol biosynthesis inhibitors. This allowed for monitoring of the CaUPC2 reporter activity in the absence of endogenous CaUpc2p. Interestingly, growth of CaUPC2-750D6 in the presence and absence of 0.1-100 μg/ml of FLC resulted in UPC2 transcriptional induction, albeit to a lower level than that seen in CaUPC2-750WT (Fig. 3). The concentration of FLC required to induce a maximal transcriptional response beginning at early time points in this strain was 1.0 μg/ml. Incubation of CaUPC2-750D6 with CLO, FEN, TER (Fig. 4 solid diamonds, solid squares and solid triangles, respectively) or LOV (Fig. 4, open squares) also resulted in induction of the CaUPC2-RLUC fusion, but with overall inducibility lower than that seen in the wild-type strain.
Fig. 3.
Time course of UPC2-RLUC induction in Δupc2/Δupc2 by a range of FLC concentrations. CaUPC2-750D6 was grown in the presence and absence of FLC at 0.1 (circles), 1 (diamonds), 10 (squares), or 100 (triangles) μg/ml. Luciferase activity was assayed at 6, 24 and 48 h. Data are presented as fold induction, or the luciferase activity in the presence of drug relative to the luciferase activity in the absence of drug. The results are representative of three independent experiments.
Fig.4.
Time course of UPC2-RLUC induction in Δupc2/Δupc2 by additional ergosterol biosynthesis inhibitors. CaUPC2-750D6 was grown in the presence and absence of CLO at 10 μg/ml (solid diamonds), TER at 100 μg/ml (solid triangles), FEN at 100 μg/ml (solid squares) or LOV at 20 μg/ml (open squares). Luciferase activity was assayed at 6, 24 and 48 h. Data are presented as fold induction, or the luciferase activity in the presence of drug relative to the luciferase activity in the absence of drug. The results are representative of three independent experiments.
CaUPC2-RLUC basal activity is higher in the absence of endogenous CaUpc2p
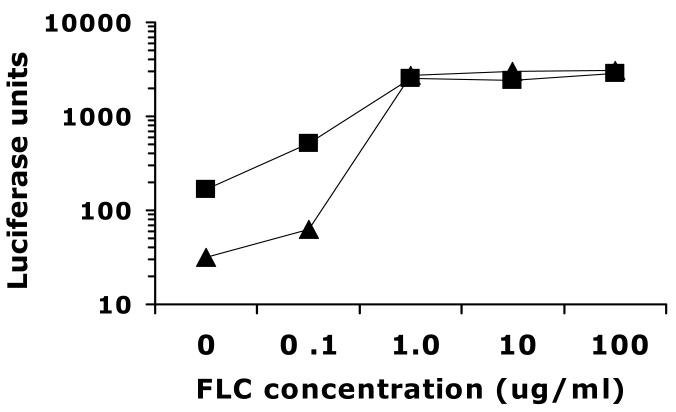
The observation that CaUPC2 fold induction is lower in the CaUPC2 homozygous deletion suggested either that CaUPC2 is autoregulated, or that there are pleiotrophic effects due to the genetic deletion. To address this, the basal CaUPC2-RLUC activities of both strains were compared, as well as the absolute level of activity in the presence of azoles, so that the two strains could be directly compared to each other. Interestingly, the decrease in fold induction seen in CaUPC2-750D6 is due primarily to an increased basal level of expression in the absence of drug (Fig. 5). The CaUPC2 deletion strain exhibits approximately 5-fold higher basal activity when compared to the wild-type strain. Additionally, the CaUPC2-RLUC activity is higher in the deletion strain at the lowest concentration of FLC tested (0.1 μg/ml). Most importantly, the maximal level of promoter activity in the presence of FLC is the same in both strains (Fig. 5), indicating that the CaUPC2 promoter is activated transcriptionally in response to drug to the same degree in both strains. The relationship of the CaUPC2-RLUC activities between both strains was highly reproducible despite variation in absolute levels of luciferase activity between experiments. Additionally, this phenomenon was consistent in assays using other antifungal drugs (data not shown).
Fig. 5.
Levels of UPC2-RLUC activity in WT and Δupc2/Δupc2 over a range of FLC concentrations. CaUPC2-750WT (triangles) CaUPC2-750D6 (squares) were grown for 48 hours in various fluconazole concentrations. Luciferase activity is expressed as the specific activity of luciferase corrected for total protein content. The results are representative of three independent experiments.
CaUPC2 expression is induced in response to anaerobicity
It has previously been shown that a CaUPC2 homozygous deletion is impaired for anaerobic growth (MacPherson et al., 2005). Additionally, it has been shown that CaERG11, which is under the control of CaUpc2p, is anaerobically induced (Song et al., 2004), and it is known that CaErg11p requires oxygen to be functional. These data suggest that CaUPC2 is transcriptionally activated in the absence of oxygen. To test this, CaUPC2-750WT and CaUPC2-750D6 were grown aerobically and anaerobically in the presence and absence of 100 μg/ml FLC. Although the strain D6 is impaired for anaerobic growth compared to BWP17, it is able to undergo several generations of growth, presumably due to ergosterol stores. After 48 h of anaerobic growth, CaUPC2-RLUC activity was increased approximately 23—fold and 2.3—fold for the WT and deletions strains, respectively (Fig.6). The lack of an additional effect when 100 μg/ml FLC is added during anaerobic growth indicates that the effects of FLC and anaerobicity are not additive (Fig 6).
Fig. 6.
Anaerobic inducibility of UPC2-RLUC activity in WT and Δupc2/Δupc2. Data were collected after 48 hrs of growth. UPC2-RLUC induction in 100 μg/ml FLC is shown in black bars, induction during anaerobic growth is shown in grey bars, and induction in the presence of 100 μg/ml FLC during anaerobicity in hatched bars. Data are presented as fold induction, or the luciferase activity in the presence of drug relative to the luciferase activity in the absence of drug. The results are representative of three independent experiments.
CaUPC2 transcriptional induction correlates with sterol depletion
Because CaUPC2 transcription increases over time with drug exposure, it was of interest to test whether this response may be linked to total cellular ergosterol levels. To measure ergosterol content in response to various inhibitors, cells were grown for 48 h (the time-point when the transcriptional response is maximal) and total sterols were extracted and measured spectrophotometrically as described previously (Arthington-Skaggs et al., 1999). Sterol levels were shown to correlate well with the degree of CaUPC2 induction over a range of FLC concentrations, (Table 2) as increasing amounts of FLC resulted in a decrease in total sterol in CaUPC2-750WT, with the maximal degree of ergosterol depletion being seen with 100 μg/ml FLC (5.85% of total ergosterol in cells grown in the absence of FLC). Similarly, with other ergosterol biosynthesis inhibitors as well as anaerobicity, a decrease in ergosterol production was seen (Table 2).
TABLE 2.
Ergosterol content of cells grown in sterol depleting conditions
| CaUPC2-750WT | CaUPC2-750D6 | |||
|---|---|---|---|---|
| ERG contenta) | Induction levelb) | ERG contenta) | Induction levelb) | |
| No Drug | 100.00 | N/A | 74.66 | N/A |
| FLC (ug/ml): | ||||
| 0.1 | 80.36 | 2.02 | 65.21 | 3.09 |
| 1 | 52.06 | 86.58 | 27.49 | 14.97 |
| 10 | 4.74 | 95.72 | 12.19 | 14.44 |
| 100 | 5.85 | 99.66 | 3.07 | 17.02 |
| LOV 20 ug/ml | 60.32 | 7.08 | 43.51 | 2.48 |
| TER 100 ug/ml | 55.20 | 31.97 | 85.77 | 4.78 |
| FEN 100 ug/ml | 23.04 | 79.57 | 39.07 | 3.06 |
| Anaerobicity | 21.72 | 23.49 | 44.81 | 2.31 |
Expressed as percent of WT in absence of drug.
UPC2-RLUC induction level at 48 h expressed as fold induction.
Similar decreases in total ergosterol levels were seen with the CaUPC2-750D6 strain, in that exposure to ergosterol biosynthesis inhibitors results in reduced ergosterol production (Table 2). The CaUPC2-750D6 strain exhibits ergosterol depletion over a range of FLC concentration, which parallels the CaUPC2 transcriptional induction shown in Figure 3. It is important to note that while the Δupc2Δ/upc2 strain has lower total ergosterol than does the wild-type strain following FLC treatment, the intrinsic level of ergosterol in the deletion strain is lower even in the absence of drug treatment. The deletion strain shows a decrease in total ergosterol following exposure to FEN, LOV or anaerobicity (39.07%, 43.5%, and 44.81% of ergosterol level in BWP17 in absence of drug, respectively). Interestingly, the ergosterol level in the Δupc2Δ/upc2 strain did not decrease in response to TER. As previously reported (Silver et al., 2004), the deletion strain exhibits a lower level of ergosterol than the wild-type (74.66%, Table 2) in the absence of drug, which correlates with the difference between wild-type and deletion strain basal CaUPC2 reporter activity in the absence of drug as shown in Figure 5.
Chemical inhibition of cell wall biosynthesis does not affect CaUPC2 induction
Based on altered susceptibility of C. albicans Δupc2/Δupc2 to the chitin synthase inhibitor NKZ (Silver et al., 2004), as well as the role of ScUPC2 in regulation of cell wall gene (Abramova et al., 2001), it was hypothesized that CaUPC2 may transcriptionally respond to NKZ treatment. Growth of CaUPC2-750WT or CaUPC2-750D in the presence of 10 or 100 μg/ml of NKZ did not alter CaUPC2 expression (data not shown). In parallel, NKZ exposure did not alter total ergosterol levels in either strain (data not shown).
DISCUSSION
The goal of this study was to characterize the role of transcriptional inducibility of CaUPC2 in response to antifungal drugs that target ergosterol biosynthesis. An important component of ScUPC2 transcriptional regulation has previously been hypothesized to be positive auto-regulation (Abramova et al., 2001; Wilcox et al., 2002). Therefore, this study also addressed whether CaUPC2 regulates its own transcription by using a CaUPC2 homozygous deletion strain and a CaUPC2 luciferase reporter construct.
Initial characterization of the CaUPC2 transcriptional response to ergosterol depletion showed that in the wild-type strain CaUPC2-750WT, CaUPC2 is highly regulated at the transcriptional level. With prolonged exposure to many ergosterol biosynthesis inhibitors, CaUPC2-RLUC activity increased up to 100-fold. This suggests that in addition to other potential mechanisms of regulation of the ergosterol biosynthetic pathway, transcriptional control of the major sterol regulator CaUPC2 may play an important role in the response to antifungal drugs. Previous work has suggested a post-translational control mechanism regulating ScUpc2p in S. cerevisiae (Davies et al., 2005; Vik & Rine, 2001). Data suggested a model in which ScUpc2p is present in the nucleus and is bound by a repressor during sterol-rich conditions. Upon sterol depletion, however, the repression is released and ScUpc2p binds to and activates sterol biosynthesis genes (Davies et al., 2005). An alternative model is based in analogy to the mammalian sterol regulator SREBP and was proposed in a recent review (White & Silver, 2005). In this model, the N-terminal CaUpc2p DNA binding domain is anchored to a membrane via four predicted transmembrane spans found in the C-terminal portion of the protein. Upon sterol depletion, it is proposed that a cleavage event liberates the DBD which can then translocate to the nucleus and activate target genes. This model is consistent with S. cerevisiae localization experiments that show that the C-terminally tagged Upc2p is not nuclear localized (C. Marie and T. White unpublished observations). The current evidence cannot determine which model is correct, but either model suggests an important post-translational regulatory mechanism. This work demonstrates that in addition to these proposed mechanisms, transcriptional activation of CaUPC2 likely plays an important role in regulating downstream genes via a large increase in abundance of CaUPC2 mRNA and, putatively, its subsequent protein product.
It was also shown that transcriptional induction became maximal after 48 h of growth in the presence of drug. This observation is consistent with the hypothesis that it is depletion of sterols that triggers CaUPC2 upregulation, as sterol depletion will only become severe after prolonged exposure to inhibitors.
The observation that inhibition of multiple steps in the ergosterol biosynthetic pathway results in an increase in CaUPC2-RLUC activity is consistent with evidence that CaUpc2p acts as a global regulator of sterol biosynthesis genes. These data are consistent with previous results from this laboratory showing that CaERG11-RLUC activity is responsive to inhibition of multiple steps in the ergosterol biosynthetic pathway (Song et al., 2004). It is important to note that all of the genes for enzymes that were inhibited in this study (HMG1, ERG1, ERG11, ERG2) contain putative CaUpc2p binding sites within their promoters (MacPherson et al., 2005; Silver et al., 2004). In addition, microarray analysis suggests that transcriptional activation of each of these drug targets in response to FLC is CaUpc2p dependent (unpublished results). When these data are taken together with the level of sterol depletion caused by these inhibitors, it seems likely that the signal that induces CaUPC2 expression and subsequent upregulation of ERG genes may be the lack of ergosterol or a late sterol pathway intermediate. Indeed, this work has shown that inhibition of multiple steps in the biosynthetic pathway results in a decrease in the end product ergosterol, and therefore it seems likely that this decrease serves as a signal to induce expression of the transcriptional activator of the pathway, CaUpc2p. This hypothesis is supported by the observation that ergosterol depletion (Table 2) correlates with induction of CaUCP2-RLUC activity (Figures 1-4).
The response of CaUPC2 to anaerobicity is also consistent with previous data. In S. cerevisiae, anaerobic growth is not possible in the absence of exogenous ergosterol, and this appears to be largely due to the dependence of Erg11p on molecular oxygen as a cofactor, as well as the heme requirement of this enzyme (Setiadi et al., 2006; White et al., 1998). C. albicans, however, can grow anaerobically in the absence of exogenous ergosterol, and this growth is accompanied by an increase in ERG11 expression (Setiadi et al., 2006; Song et al., 2004). The anaerobic induction of ERG11 is likely due to the oxygen dependence of the sterol pathway, which utilizes 12 molecules of O2 for every 1 molecule of ergosterol synthesized (Hughes et al., 2005). This study shows that the amount of ergosterol biosynthesis under anaerobic conditions is clearly decreased when compared to aerobically grown cells, although not to the same degree as seen with drug inhibition. This intermediate degree of anaerobic sterol depletion is paralleled with an increase in CaUPC2-RLUC activity that is somewhat lower than what is seen with direct chemical inhibition of ergosterol biosynthesis. Previous studies have also shown that a CaUPC2 deletion strain is deficient in anaerobic growth (MacPherson et al., 2005), suggesting that transcriptional activation of the ergosterol biosynthetic pathway by CaUpc2p is essential in anaerobicity, which is consistent with the data presented in this study. It is important to note that the anaerobic induction experiment in this study was performed using aerobically grown innocula, so that luciferase activity reflects the adaptation to anaerobicity, not true anaerobic growth.
The CaUPC2 deletion was previously shown to exhibit hypersensitivity to cell wall perturbing agents such as NKZ (Silver et al., 2004), but it is unclear whether this sensitivity is a direct result of CaUpc2p activation of cell wall associated genes, or a pleitrophic effect resulting from altered membrane sterol composition. ScUpc2p transcriptionally activates some cell wall associated proteins in response to anaerobicity, such as those in the DAN/TIR family (Abramova et al., 2001). This evidence suggested that perhaps the effect of ScUPC2 deletion on cell wall sensitivity is due to a direct effect of ScUpc2p on cell wall gene expression. If the effect was the result of transcriptional activation by CaUpc2p, it was expected that CaUPC2 would transcriptionally respond to treatment with NKZ. When this was tested, however, there was no change in CaUPC2-RLUC activity in the presence of NKZ. Additionally, NKZ did not alter total ergosterol levels. These data suggest that CaUPC2 transcriptional activation is specific to alterations in the sterol biosynthetic pathway. The NKZ susceptibility of the CaUPC2 deletion mutant may due to pleitrophic effects of the lower sterol level of the mutant rather than direct control of expression of cell wall associated genes by CaUpc2p.
The comparison of CaUPC2-RLUC activity between the wild-type and CaUPC2 deletion mutant suggests either direct autoregulation by CaUpc2p or an indirect consequence of the lower basal level of total sterol in the deletion strain. The difference in fold-induction between the two strains clearly demonstrates that the CaUPC2 mutant has an altered regulation of CaUPC2 promoter activity (Figures 1-4). The lower fold inducibility of CaUPC2-RLUC in Δupc2/Δupc2 suggests an important component of transcriptional self-regulation, which is consistent with the limited evidence reported for S.cerevisiae (Abramova et al., 2001; Davies et al., 2005) as well as the presence of a putative CaUpc2p binding site within the CaUPC2 promoter. The increase in basal activity of the Δupc2/Δupc2 strain when compared to wild-type, however, suggests more than one possibility. It is possible that CaUpc2p acts as a transcriptional repressor at its own promoter, but there has been no previous evidence to support this. Alternatively, the intrinsically lower level of ergosterol in the Δupc2/Δupc2 mutant (Silver et al., 2004) may account for the increased CaUPC2-RLUC activity in the absence of drug. This indirect effect may mask direct consequences of CaUPC2 deletion on CaUPC2-RLUC activity, and further study including nested deletions and direct binding to the putative SRE is needed to definitively address autoregulation. These studies are currently underway in this laboratory. While the intrinsically lower level of sterol in the deletion strain may explain the higher level of UPC2-RLUC activity in the absence of drug, recent work has demonstrated the CaUpc2p indeed does bind to the CaUPC2 promoter (Znaidi et al., 2008). This evidence, along with the data presented in the present study, suggests that at least some component of UPC2 inducibility is transcriptionally self-regulated. The CaUPC2-RLUC inducibility that remains in the Δupc2/Δupc2 strain strongly suggests that in addition to CaUpc2p, a novel sterol responsive transcription factor also controls CaUPC2 expression. This is consistent with previous work in which deletion of the ScUpc2p binding site within the ScUPC2 promoter reduced, but did not eliminate ScUPC2 inducibility (Abramova et al., 2001). Identification of additional transcription factors controlling CaUPC2 expression is currently being addressed in this laboratory and will contribute to our understanding of how C. albicans cells respond to antifungal drugs.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Joachim Morchhauser (University of Wurzburg, Wurzburg, Germany) for providing plasmid pA83 containing the SAT1 marker, David Soll (University of Iowa, Iowa City) for providing the Renilla reniformis luciferase containing plasmid pCRW3, and Aaron Mitchell (Columbia University, New York, NY) for providing strain BWP17. We thank members of the White laboratory for their valuable comments and support.
This research was funded by NIH NIDCR grants R01 DE11367 and R01 DE14161.
REFERENCES
- Abramova NE, Cohen BD, Sertil O, Kapoor R, Davies KJ, Lowry CV. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics. 2001;157:1169–1177. doi: 10.1093/genetics/157.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthington-Skaggs BA, Crowell DN, Yang H, Sturley SL, Bard M. Positive and negative regulation of a sterol biosynthetic gene (ERG3) in the post-squalene portion of the yeast ergosterol pathway. FEBS Lett. 1996;392:161–165. doi: 10.1016/0014-5793(96)00807-1. [DOI] [PubMed] [Google Scholar]
- Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1999;37:3332–3337. doi: 10.1128/jcm.37.10.3332-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BS, Wang HS, Rine J. Dual activators of the sterol biosynthetic pathway of Saccharomyces cerevisiae: similar activation/regulatory domains but different response mechanisms. Mol Cell Biol. 2005;25:7375–7385. doi: 10.1128/MCB.25.16.7375-7385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimster-Denk D, Rine J. Transcriptional regulation of a sterol-biosynthetic enzyme by sterol levels in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3981–3989. doi: 10.1128/mcb.16.8.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KW, Nickels JT, Edlind TD. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2000;44:2693–2700. doi: 10.1128/aac.44.10.2693-2700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hughes AL, Todd BL, Espenshade PJ. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell. 2005;120:831–842. doi: 10.1016/j.cell.2005.01.012. [DOI] [PubMed] [Google Scholar]
- MacPherson S, Akache B, Weber S, De Deken X, Raymond M, Turcotte B. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob Agents Chemother. 2005;49:1745–1752. doi: 10.1128/AAC.49.5.1745-1752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver BG, Song JL, Choiniere JH, White TC. cis-acting Elements Within the Candida albicans ERG11 Promoter Mediate the Azole Response through the Transcription Factor Upc2p. Eukaryot Cell. 2007 doi: 10.1128/EC.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss O, Vik A, Kolter R, Morschhauser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi ER, Doedt T, Cottier F, Noffz C, Ernst JF. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J Mol Biol. 2006;361:399–411. doi: 10.1016/j.jmb.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Silver PM, Oliver BG, White TC. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot Cell. 2004;3:1391–1397. doi: 10.1128/EC.3.6.1391-1397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JL, Harry JB, Eastman RT, Oliver BG, White TC. The Candida albicans lanosterol 14-alpha-demethylase (ERG11) gene promoter is maximally induced after prolonged growth with antifungal drugs. Antimicrob Agents Chemother. 2004;48:1136–1144. doi: 10.1128/AAC.48.4.1136-1144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha T, Klapach A, Lorenz WW, Tsai LK, Laughlin LA, Gorman JA, Soll DR. The sea pansy Renilla reniformis luciferase serves as a sensitive bioluminescent reporter for differential gene expression in Candida albicans. J Bacteriol. 1996;178:121–129. doi: 10.1128/jb.178.1.121-129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik A, Rine J. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:6395–6405. doi: 10.1128/MCB.21.19.6395-6405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TC, Silver PM. Regulation of sterol metabolism in Candida albicans by the UPC2 gene. Biochem Soc Trans. 2005;33:1215–1218. doi: 10.1042/BST20051215. [DOI] [PubMed] [Google Scholar]
- Wilcox LJ, Balderes DA, Wharton B, Tinkelenberg AH, Rao G, Sturley SL. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J Biol Chem. 2002;277:32466–32472. doi: 10.1074/jbc.M204707200. [DOI] [PubMed] [Google Scholar]
- Znaidi S, Weber S, Al-Abdin OZ. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot Cell. 2008;7:836–847. doi: 10.1128/EC.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.