Abstract
The oncogene erbB2 is overexpressed in 20% to 30% human breast cancers and is most commonly overexpressed in estrogen receptor (ER)-negative breast cancers. Transgenic mice expressing erbB2 develop ER-negative mammary tumors, mimicking human breast carcinogenesis. Previously, we have shown that activator protein 1 (AP-1) regulates proliferation of ER-negative breast cancer cells. We hypothesized that blockade of AP-1 in mouse mammary epithelial cells will suppress ER-negative tumorigenesis induced by erbB2. Trigenic erbB2 mice were generated by crossing a bigenic pUHD-Tam67/MMTV-rtTA mouse to a MMTV-erbB2 mouse. The resulting trigenic mice develop tumors and express a doxycycline-inducible c-Jun dominant negative mutant (Tam67) in the mammary glands. In vivo AP-1 blockade by Tam67 expression delayed mammary tumor formation in MMTV-erbB2 mice by more than 11 weeks. By 52 weeks of age, 100% (18 of 18) of the untreated animals had developed mammary tumors, whereas 56% (9 of 16) of the doxycycline-treated trigenic mice developed tumors. In addition, the tumors that arose in the AP-1-blocked erbB2 mice failed to express Tam67. Twenty-five percent of the doxycycline-treated MMTV-erbB2 mice survived more than 72 weeks of age without developing mammary tumors. Examination of normal-appearing mammary glands from these mice showed that AP-1 blockade by Tam67 also significantly prevents the development of premalignant lesions in these glands. The expression of erbB2 either in normal mammary tissue or in mammary tumors was not altered. Our results show that blocking the AP-1 signaling in mammary cells suppresses erbB2-induced transformation, and show that the AP-1 transcription factor is a critical transducer of erbB2. These results provide a scientific rationale to develop targeted drugs that inhibit AP-1 to prevent the development of ER-negative breast cancer.
Despite the cancer-preventive activity of selective estrogen receptor (ER) modulators and the aromatase inhibitors, it is clear that none of these hormonal therapies prevent all breast cancers. In breast cancer prevention trials testing hormonal therapies, many ER-positive breast cancers were prevented; however, none of the ER-negative breast cancers was prevented (1). Thus, more effective agents for the prevention of ER-negative breast cancer are urgently needed. The results of many prevention trials show the feasibility of preventing breast cancer using medical therapy. However, they also show that more effective agents are needed.
In addition to estrogen, many other growth factors have been shown to be critical growth regulators for breast cells. ER-negative breast cancer often requires peptide growth factors to support their growth. The growth factors and their receptors offer potential targets for the treatment and prevention of breast cancer. One such growth factor receptor, erbB2, has already been effectively targeted to treat those breast cancers that overexpress this protein. We have previously targeted the epidermal growth factor receptor using small molecular inhibitors. We have sought to inhibit signal transduction at a more distal point within the cell where multiple growth factor signals converge.
We have selected the activator protein 1 (AP-1) transcription factor for targeting because it is involved in estrogen signaling (through transcription factor cross talk) and because it has been shown to be activated in poor-prognosis breast cancers (2). The AP-1 transcription factor is a key component of many signal transduction pathways and consists of dimers of Jun (c-Jun, Jun B, and Jun D), Fos (c-Fos, Fos B, Fra-1, and Fra-2), or other closely related factors such as activating transcription factor proteins (3-6). Differential expression and activation of Jun and Fos members allow these factors to control proliferation, apoptosis, oncogene-induced transformation, and invasiveness (7-12). Recently, AP-1 factors have been shown to be important regulators of breast cancer cell growth. Jun and Fos members are variably expressed in human breast tumors, with up to 20% to 40% showing high levels of activated c-Jun (13, 14). Consistent with this observation is the finding that AP-1 is activated by growth factors and growth factor receptors that are involved in breast tumorigenesis, including erbB2, insulin-like growth factors, and estrogen (15-17). In addition, we have previously shown that c-Jun overexpression in MCF-7 breast cancer cells produces highly invasive and hormone-resistant tumors (14). Increased levels of c-Jun and phospho-c-Jun in human breast tumors are associated with low ER expression (18, 19), tamoxifen resistance (13, 14), increased invasion (14), and poor prognosis (20). All of these studies show that AP-1 activation is commonly seen in highly aggressive breast cancers that have a poor prognosis.
The observation that AP-1 is involved in the regulation of the growth of breast cancer cells led us to attempt to block this transcription factor to prevent the development of breast cancer. Previously, we developed a c-Jun dominant-negative mutant, Tam67, to inhibit this transcription factor. This c-Jun mutant lacks the transactivation domain of c-Jun but retains the DNA binding and dimerization domains. We and others have used Tam67 to investigate the role of AP-1 in a variety of cell types (8, 21-25). Our studies have shown that blockade of AP-1 signaling in breast cancer cells suppresses the growth of premalignant and malignant breast cells (26), inhibits breast cancer xenograft tumor growth (27), and blocks proliferation induced by many different growth factors including epidermal growth factor, insulin-like growth factor, fibroblast growth factor, and heregulin (27). All of these studies show that it is possible to block cell growth by inhibiting AP-1 activity.
We are currently investigating the role of AP-1 in the intact mammary glands. To this end, we have generated a transgenic mouse that expresses this AP-1 inhibitor in mammary tissue. Using this unique mouse model, we previously showed that AP-1 regulates postnatal mammary gland development (28). Because the AP-1 transcription factor is required for ductal branching and terminal end bud outgrowth and is activated by many oncogenes, we have investigated whether AP-1 blockade can prevent oncogene-induced transformation and premalignant lesions. Our results show that in vivo blockade of the AP-1 transcription factor in adult mice effectively suppresses ER-negative mammary tumor formation induced by oncogene erbB2 and significantly blocks the development of premalignant lesions. These results suggest that AP-1 factor may be an effective target for the prevention of breast cancer.
Materials and Methods
Generation and genotyping of transgenic mice
The experimental FVB strain mouse for breeding and generation of transgenic mice was purchased from the Center for Comparative Medicine at Baylor College of Medicine. The FVB strain MMTV-rtTA mouse was generated and described previously (29). All studies involving the mice were regulated under the protocol approved by Baylor Institutional Animal Care and Use Committee. The generation of bigenic pUHD-Tam67/MMTV-rtTA mice was previously described (28). Briefly, the Tam67 transgene, regulated by a tetracycline-inducible promoter (prepared from the pUHD-Tam67 vector; ref. 26), was microinjected into FVB/F1 one-cell embryos both at the Baylor College of Medicine Transgenic Mouse Facility and at the National Cancer Institute-Frederick. The pUHD-Tam67 transgenic mice were backcrossed with wild-type FVB mice to produce heterozygous Tam67-positive (Tam67+/-) and Tam67-negative mice (Tam67-/-). The heterozygous Tam67-positive mice were backcrossed with siblings to generate homozygous Tam67 transgenic mice (Tam67+/+). These homozygous Tam67 mice were crossed with the MMTV-rtTA mouse to produce a heterozygous pUHD-Tam67+/-/MMTV-rtTA+/- bigenic mouse. To generate trigenic mice, the heterozygous pUHD-Tam67+/-/MMTV-rtTA+/- bigenic mouse was crossed with a homozygous MMTV-erbB2 mouse [The Jackson Laboratory, strain FVB/N-TgN(MMTVneu) 202Mul]. Offsprings carrying pUHD-Tam67, MMTV-rtTA, and MMTV-erbB2 transgenes were identified by PCR analysis of tail DNA. Primers used in genotyping are Tam67 (forward, 5′-ATGGACTACAAGGACGACGA-3′; reverse, 5′-GCGATTCTCTCCAGCTTC-3′) and MMTV-erbB2 (forward, 5′-CGGAACCCACATCAGGCC-5′; reverse, 5′-TTTCCTGCAGCAGCCTACGC-3′). MMTV-rtTA PCR reactions were carried out as previously described (29). PCR products were separated on a 1.5% agarose gel, and mice found to carry these transgenes were selected for experiments.
Doxycycline treatment
Doxycycline was given as previously described (28). Briefly, experimental and doxycycline(+) control mice were fed doxycycline-water that contains doxycycline in drinking water at a 2 mg/mL concentration (Sigma-Aldrich). The drinking water was replaced every 3 d.
Measurement of mammary tumors
Mammary tumors were measured as previously described (30, 31). Briefly, tumor measurements were made twice a week with electronic calipers (Mitutoyo), and tumor volume was determined by multiplying the square of the width by the length and dividing by two. A mammary tumor was defined as a palpable mass of ≥100 mm3 in volume. Individual tumor size and tumor location for each animal were recorded twice weekly. Weights of all mice were recorded weekly. At the time of sacrifice, each tumor was resected and separate portions were (a) processed for histologic analysis or (b) frozen for future use in biomarker studies, or (c) selected mammary tumors were explanted into tissue culture to prepare in vitro tumor cell lines.
Histology and biomarker analysis
Histology was done as previously described (28). The mammary glands were collected from FVB mice carrying combinations of the following trangenes: Tam67+/-, MMTV-rtTA+/-, and MMTV-erbB2+/-. The samples were fixed in 4% paraformaldehyde in PBS solution overnight and then embedded in paraffin. Tissue sections were then mounted onto slides and processed for H&E staining or immunohistochemical staining as previously described (27). Briefly, 4-μm tissue sections were cut and mounted onto slides. The slides were deparaffinized and endogenous peroxidase was blocked in 3% hydrogen peroxide buffer. The anti-Flag (1:100; M2, Sigma-Aldrich) antibody was used as the primary antibody followed by a biotinylated rabbit anti-mouse antibody (1:100). Peroxidase activity was visualized with 3,3′-diaminobenzidine or NovaRed chromagen intensified with 0.2% osmium tetroxide. Other primary antibodies used include erbB2 (1:100; Lab Vision) and anti-ERα (1:800; Santa Cruz Biotechnology). The slides were counterstained with hematoxylin for 1 min followed by mounting with a coverslip. Positively staining cells were counted in 40 fields and data were expressed as a percentage of total epithelial cells counted. ErbB2 staining was recorded as 0, 1, or 2 points to indicate none, moderate, or strong staining. Scoring of the erbB2 staining was done by a blinded independent pathologist. Averages of staining scores were plotted and statistically tested with Student’s t tests.
Establish of mammary tumor cell lines and cell growth assays
Selected mammary tumors from control and experimental mice were resected and explanted into tissue culture dishes to prepare in vitro tumor cell lines or frozen for future use for biomarker studies. These cells were grown in DMEM containing 10% fetal bovine serum, 1% glutamine, 1% penicillin/streptomycin, and 1% fungizone.
Western blot analysis
Tissue lysates were prepared by incubating the ground mammary glands in lysis buffer, and total cell lysates were prepared from the cell lines derived from the mammary tumors that arose in the experimental mice. Protein concentrations in these lysates were measured by bicinchoninic acid analysis. Equal amounts of total cellular protein extract (40 μg) were electrophoresed on a 10% acrylamide gel and transferred onto a nitrocellulose membrane (Amersham). The primary antibodies used were anti-Flag M2 (1:2,000 dilution; Sigma-Aldrich) and anti-β-actin (1:8,000; Sigma-Aldrich). An antimouse or antirabbit antibody (1:4,000; Amersham) was used as the secondary antibody. The Western blotted bands were visualized using the enhanced chemiluminescence procedure (Amersham).
Statistical analysis
Statistical significance was obtained using Kaplan-Meier curves and assessed by the generalized Wilcoxon test for tumor-free survival data. Student’s t test was used to compare mammary gland markers and transgene in animals treated with or without doxycycline, using Prism GraphPad Software. Tumor multiplicity was determined by counting total number of tumors occurring in each animal up to the time of sacrifice. Multiplicity was summarized by means and SEs and compared by ANOVA. Fisher’s exact tests were done for the comparison of the number of mice showing premalignant lesions.
Results
Generation of trigenic mice
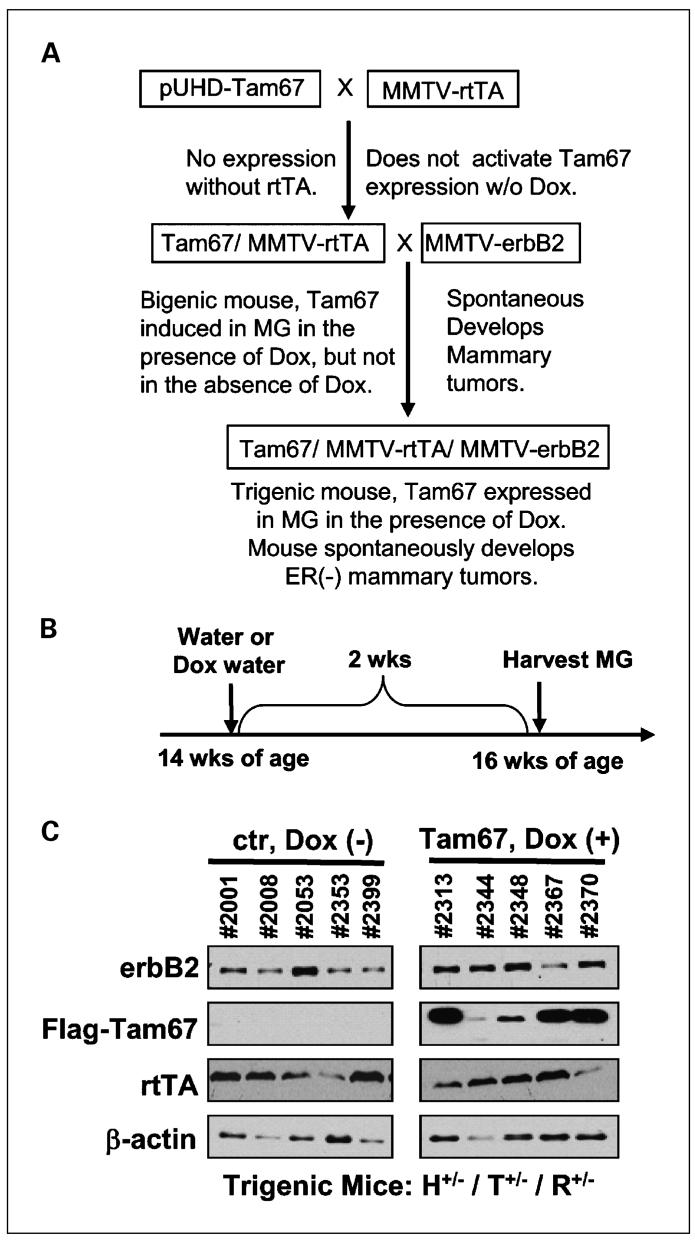
We have previously used an inducible Tam67 vector that was stably transfected into MCF-7 cells to study the role of AP-1 in controlling the growth of breast cancer cells (26, 32, 33). Using this system, we constructed a Tet-On Flag-Tam67 transgene, which was microinjected into pronuclei of fertilized mouse oocytes as previously described (28). Founder mice were screened by Southern blot analysis (data not shown) and PCR analysis of tail DNA samples. Two independent founder lines were established for breeding and experimental purposes that were subsequently bred to homozygosity (pUHD-Flag-Tam67+/+). We then crossed these mice with a MMTV-rtTA transgenic mouse (29) to generate bigenic pUHD-Tam67+/-/MMTV-rtTA+/- mice (28). The MMTV-rtTA transgenic mouse does not show any mammary-specific phenotype, but breeds, nourishes, and behaves normally as compared with its FVB strain counterparts (29). We then crossed the heterozygous pUHD-Tam67+/-/MMTV-rtTA+/- bigenic mouse with homozygous MMTV-erbB2 mice to produce trigenic pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice (Fig. 1A). All female offspring generated in the trigenic breeding were categorized according to their genotypes. At the start of the experiment, we measured the expression of erbB2 in mammary glands of the trigenic pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice. Two weeks of doxycycline treatment induced Tam67 expression but did not affect the expression of erbB2 and rtTA in mammary glands (Fig. 1B and C). No expression of Tam67 was detected in control mice that did not receive doxycycline. A total of four genotypes were maintained: (a) pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/-; (b) pUHD-Tam67+/-/MMTV-rtTA-/-/MMTV-erbB2+/-; (c) pUHD-Tam67-/-/MMTV-rtTA+/-/MMTV-erbB2+/-; and (d) pUHD-Tam67-/-/MMTV-rtTA-/-/MMTV-erbB2+/-. At the age of 3 months (13 weeks), when the mammary glands were fully developed, approximately half of the mice in each category were continuously fed the expression inducer doxycycline (which was dissolved in drinking water) until the end of experiment (when a mammary tumor reaches 1,000 mm3 in volume).
Fig. 1.
Generation of trigenic pUHD-Tam67/MMTV-rtTA/MMTV-erbB2 mice and expression of the transgenes in mammary glands. A, bigenic pUHD-Tam67/MMTV-rtTA mice were generated by crossing heterozygous or homozygous pUHD-Tam67 mice with MMTV-rtTA mice. Offspring were screened by PCR of tail DNA. Identified bigenic pUHD-Tam67/MMTV-rtTA mice were crossed with MMTV-erbB2 mice. Offspring were screened for the presence of Tam67, rtTA, and erbB2 transgenes. B, five mice each were set for control or doxycycline treatment groups. The adult trigenic pUHD-Tam67/MMTV-rtTA/MMTV-erbB2 mice were fed either regular water or doxycycline-water for 2 wk, and then mammary glands were collected in tissue lysates for Western blot analysis. C, ErbB2, Flag-Tam67, rtTA, and β-actin were detected in mammary glands of control and doxycycline-treated mice by Western blotting with specific antibodies.
In vivo AP-1 blockade decreases mammary tumor incidence
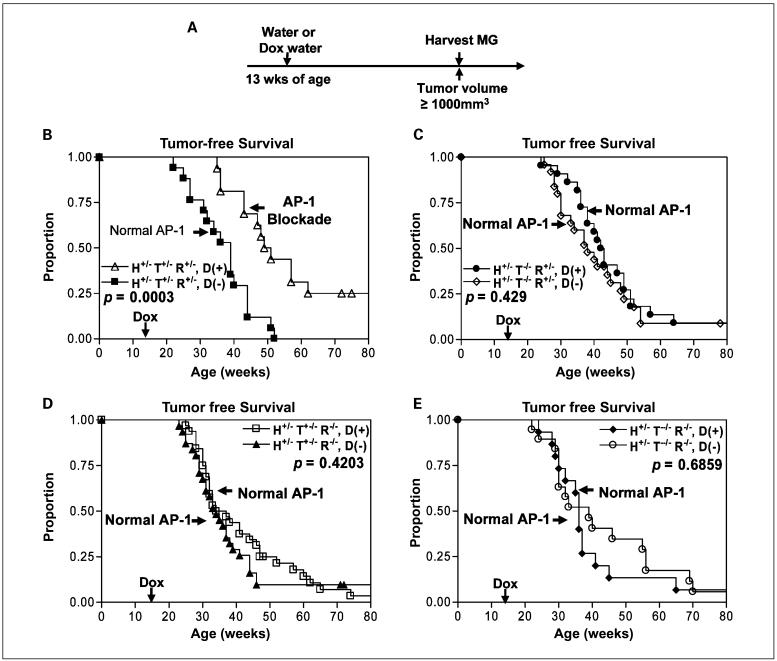
The adult transgenic mice were fed doxycycline-containing water at the age of 3 months until the mammary tumors reached a volume ≥1,000 mm3 (Fig. 2A) or until 80 weeks of age if they did not develop tumors. We then monitored the mammary tumor development and collected data for the incidence of mammary tumors in all eight groups of transgenic erbB2 mice fed with or without doxycycline (Table 1). At the time when 100% of the untreated trigenic pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice (18 of 18) had developed mammary tumors, 56% (9 of 16) of the AP-1-blocked mice developed mammary tumors. Thus, we conclude that in vivo AP-1 blockade by Tam67 in the mammary glands reduces mammary tumor incidence in erbB2 mice. However, the growth rate of the mammary tumors that did arise in all eight groups was not significantly different (data not shown).
Fig. 2.
In vivo AP-1 blockade by Tam67 suppresses erbB2-induced mammary tumorigenesis. A, treatment scheme. The adult trigenic pUHD-Tam67/MMTV-rtTA/MMTV-erbB2 mice together with other unitransgenic and bitransgenic control mice were fed either regular water or doxycycline (Dox)-water at the age of 3 mo (13 wk) for the entire duration of experiment. Mice that developed mammary tumors ≥1,000 mm3 were sacrificed for pathologic examination. B, Kaplan-Meier plot of the proportion of animals free of mammary tumors versus weeks of age is shown for the trigenic pUHD-Tam67/MMTV-rtTA/MMTV-erbB2 mice. Control group (n = 16) were fed regular drinking water, whereas AP-1-blocked group (n = 18) were fed doxycycline-water to turn on Tam67 expression in the mammary glands. Tumor measurements were made twice per week. Statistical analysis was done with the generalized Wilcoxon test. C, bigenic mice with erbB2 and rtTA transgenes but without the Tam67 gene were divided into two groups. All animals have normal AP-1 activity. Doxycycline(-) group [D(-); n = 22] were fed regular drinking water, whereas doxycycline(+) group [D(+); n = 25] were fed doxycycline-water as control for doxycycline effect. Kaplan-Meier plot and statistical analysis are treated the same as described in B. D, bigenic mice with erbB2 and Tam67 transgenes but without the activator rtTA gene were divided into two groups. All animals have normal AP-1 activity. Doxycycline(-) group (n = 32) were fed regular drinking water, whereas doxycycline(+) group (n = 31) were fed doxycycline-water as control for doxycycline effect. Kaplan-Meier plot and statistical analysis are treated the same as described in B. E, unigenic mice with erbB2 transgene but without the activator rtTA gene and Tam67 transgene were divided into two groups. All animals have normal AP-1 activity. Doxycycline(-) group (n = 15) were fed regular drinking water, whereas doxycycline(+) group (n = 19) were fed doxycycline-water as control for doxycycline effect. Kaplan-Meier plot and statistical analysis are treated the same as described in B.
Table 1.
AP-1 blockade reduces erbB2-induced mammary tumor incidence
| Purpose | Genotype | No. mice | No. mice that developed mammary tumor | Tumor incidence (%) |
|---|---|---|---|---|
| Expt. | H+/- T+/- R+/-, Dox(+) | 16 | 9* | 56 |
| Control | H+/- T+/- R+/-, Dox(-) | 18 | 18 | 100 |
| Control | H+/- T-/- R+/-, Dox(+) | 22 | 22 | 100 |
| Control | H+/- T-/- R+/-, Dox(-) | 25 | 23 | 92 |
| Control | H+/- T+/- R-/-, Dox(+) | 32 | 31 | 97 |
| Control | H+/- T+/- R-/-, Dox(-) | 31 | 28 | 90 |
| Control | H+/- T-/- R-/-, Dox(+) | 15 | 14 | 93 |
| Control | H+/- T-/- R-/-, Dox(-) | 19 | 18 | 95 |
NOTE: H+/-, Her2 heterozygous; T+/-, Tam67 heterozygous; R+/-, rtTA heterozygous; Dox, doxycycline. Cutoff time was at 80 wk of age.
When 100% (18 of 18) of group 2 control mice developed mammary tumors, 56% (9 of 16) of group 1 experimental mice developed mammary tumors.
AP-1 blockade delays and partially prevents mammary tumor development in trigenic pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice
In vivo AP-1 blockade by Tam67 delayed mammary tumor formation in MMTV-erbB2 mice by 11 weeks as shown by Kaplan-Meier curves and assessed by the generalized Wilcoxon test (P = 0.0003; Fig. 2B). We also found that AP-1 blockade by inducible Tam67 in mammary gland prevented mammary tumor formation in 25% of the mice (4 of 16) at the age of 72 weeks. Mammary tumor development in all control groups showed no significant difference within each category (Fig. 2C-E). From these data, we conclude that in vivo AP-1 blockade is able to delay and partially prevent erbB2-induced mammary tumors.
Expression of erbB2 and ERα in mammary glands and mammary tumors
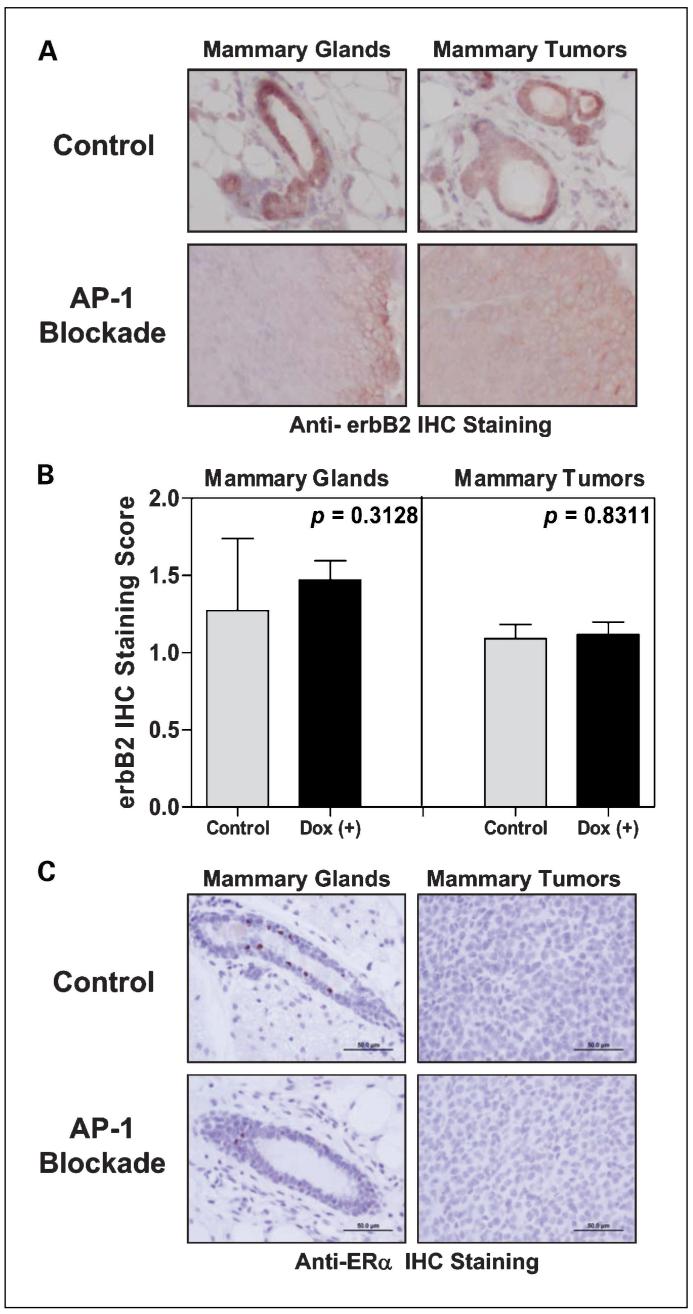
We have previously shown that Tam67 can be induced in ∼40% to 60% of mammary epithelial cells in our Tet-On inducible system (28). Because mammary tumors still arose in the AP-1-blocked mammary glands, we next examined the expression of the AP-1 inhibitor Tam67 in pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice. To ensure that Tam67 expression did not affect the expression of the transforming oncogene, we first examined the expression of erbB2 in mammary tissue and in tumors at the end of experiments. Staining with an anti-erbB2 antibody, we found no differences of erbB2 expression in normal mammary tissue and in mammary tumors that arose in either control or AP-1-blocked mice (P = 0.3128 for erbB2 expression in normal mammary tissue, P = 0.8311 for mammary tumors; Fig. 3A and B). We also determined the expression of ERα in normal mammary epithelial cells and in mammary tumors from control and doxycycline-treated mice. As shown in Fig. 3C, ERα is expressed in normal mammary ductal cells but not in mammary tumors. Thus, the trigenic mice develop ER-negative mammary carcinoma just as do the parental MMTV-erbB2 mice.
Fig. 3.
ER-negative mammary tumor development and erbB2 expression. A, expression of erbB2 in the normal mammary tissue and in the mammary tumors of trigenic erbB2 mice. Normal mammary tissue and mammary tumors samples were collected and embedded in paraffin. The 4-μm slides were prepared for staining with anti-erbB2 antibody. ErbB2 staining was viewed under a standard microscope and pictures were taken at ×10 magnification. Representative pictures are shown. Bar, 100 μm. B, manual scoring of the intensity of erbB2 staining was done by a pathologist on double-blinded samples. Scoring scale: 0, no or negligible erbB2 staining; 1, moderate intensity of erbB2 staining; and 2, strong erbB2 staining. Average intensity scores for the normal mammary tissue and mammary tumors were plotted separately for comparison purpose. C, ERα immunohistochemical (IHC) staining was done and viewed under a standard microscope and pictures were taken at ×20 magnification. Representative pictures are shown. Bar, 50 μm.
Induction of Tam67 in mammary glands and mammary tumors
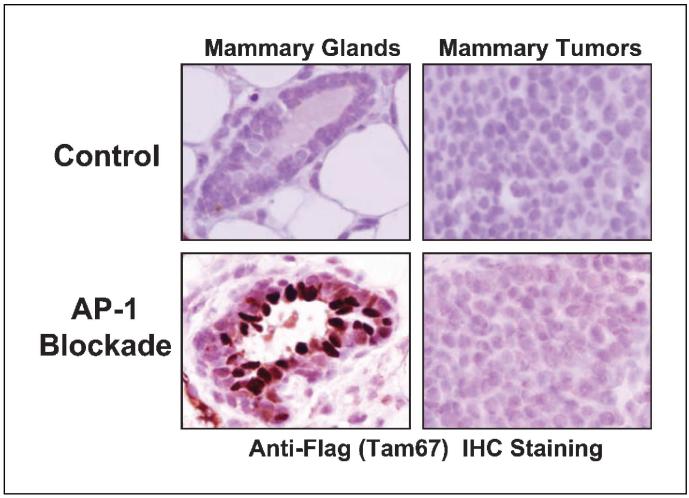
We next used an anti-Flag antibody to detect Tam67 protein in control and AP-1-blocked mice that developed mammary tumors. As expected, Tam67 was not seen in normal mammary tissue or the tumors that arose in control mice (Fig. 4). In AP-1-blocked mice, doxycycline induced Tam67 in the normal mammary tissue as expected. However, Tam67 was not expressed or was expressed only at a very low level in the mammary tumors that arose in the doxycycline-treated, AP-1-blocked mice (Fig. 4). In these mice, the adjacent normal mammary tissue expressed Tam67 whereas the tumor did not. We also examined Tam67 protein expression in normal mammary tissue and tumors by Western blotting (data not shown). In five of six mammary tumors arising in the doxycycline-treated mice, we detected no Tam67 expression. We observed a very low level of Tam67 protein in 1/6 of the mammary tumors (in <1% of tumor cells, sporadically located in peripheral portion of the tumor). In each of these mice, we observed a high level of Tam67 in the normal mammary tissue of the AP-1-blocked mice (data not shown). To exclude the possibility that the loss of Tam67 induction is due to loss of the transgenes, we confirmed by PCR that the Tam67 transgene, as well as the rtTA and erbB2 transgenes, was present in mammary tumors from the AP-1-blocked mice (data not shown). These results show that Tam67 was induced in normal mammary tissue, but minimally in the mammary tumors that arose in doxycycline-treated, AP-1-blocked mice. Thus, in all cells in which tumors arose in the doxycycline-treated trigenic mice, Tam67 expression was lost or greatly reduced, suggesting that breast tumors cannot arise in the presence of AP-1 blockade.
Fig. 4.
The mammary tumors that arose in doxycycline-treated trigenic mice do not express the AP-1 inhibitor Tam67. Expression of Flag-tagged Tam67 was detected by staining with anti-Flag antibody. Staining was viewed under ×40 magnification. Representative pictures are shown.
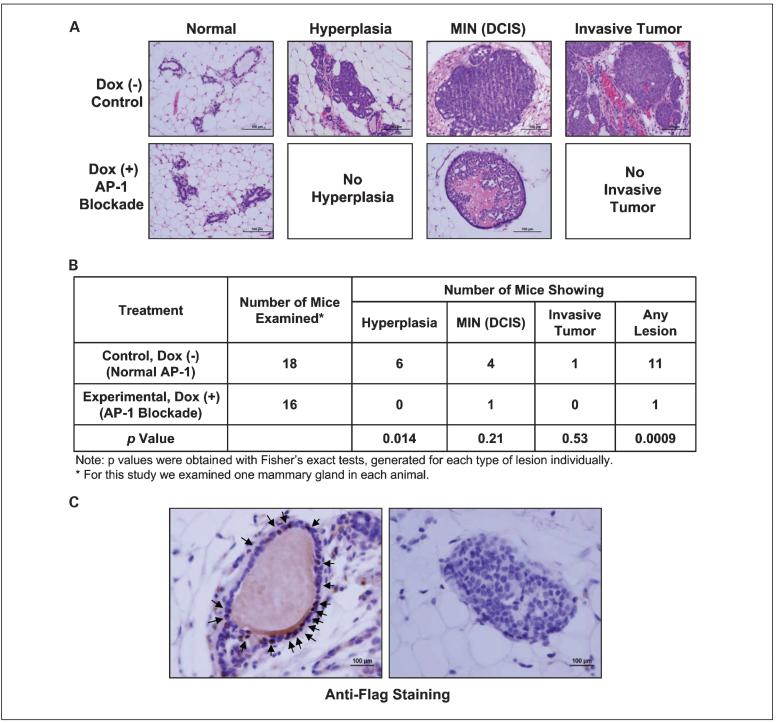
AP-1 blockade prevents the development of premalignant lesions
Mammary tumorigenesis in MMTV-erbB2 transgenic mice proceeds through discrete steps. These mice develop lesions showing hyperplasia, mammary intraepithelial neoplasia [equivalent to human ductal carcinoma in situ (DCIS)], and microscopic invasive tumors (34), which mimic human breast carcinogenesis. To assess whether in vivo AP-1 blockade by Tam67 expression prevents development of these premalignant lesions, we examined the normal-appearing mammary glands that were collected from control (no doxycycline treatment) and experimental (doxycycline treatment) trigenic erbB2 mice and compared the number of mammary glands showing premalignant lesions on H&E-stained mammary gland sections. We found that Tam67 expression significantly suppressed the development of hyperplasias (P = 0.0138; Fig. 5A and B) and also reduced DCIS and microscopic invasive breast cancers, although the reduction of these lesions did not reach statistical significance. However, there was a statistically significant decrease in the total number of premalignant and microscopic invasive lesions (see Fig. 5B). These results suggest that AP-1 blockade by Tam67 expression not only inhibits mammary tumor formation but also prevents the development of premalignant lesions in the mammary glands.
Fig. 5.
AP-1 blockade by Tam67 expression prevents premalignant lesions. A, normal-appearing mammary glands were collected and paraffin embedded from the trigenic mice that developed mammary tumors. Mammary tissue sections were stained with H&E. Selective fields containing hyperplasia, mammary intraepithelial neoplasia (MIN; DCIS), and invasive mammary tumors. B, comparison of the number of mice with premalignant lesions in doxycycline-treated or untreated trigenic mice as described in A. One normal-appearing number 3 mammary gland was removed from each mouse and the number and type of premalignant lesions in each mammary gland were assessed microscopically after H&E staining. C, representative fields of the mammary gland that showed DCIS from the mouse of the doxycycline-treated, AP-1-blocked experimental group. The mammary tissue was stained for TAM67 expression by anti-Flag immunohistochemical staining. Left, a normal duct that stained positive for TAM67 (arrows); right, the DCIS lesion that arose in this doxycycline-treated mouse. No TAM67 expression is seen in the DCIS cells.
We next examined whether the lesions that arose in these animals expressed Tam67. Although no hyperplasia lesions were seen in the doxycycline-treated mice, one DCIS lesion was seen in the doxycycline-treated mice. We carried out immunohistochemical staining of this mammary gland for Tam67 (using an anti-Flag antibody). Tam67 expression was detected in the normal mammary ducts in this gland, but its expression was lost in the DCIS lesion (Fig. 5C). Although this analysis was of only one gland, these results show that loss of Tam67 can occur in premalignant lesions.
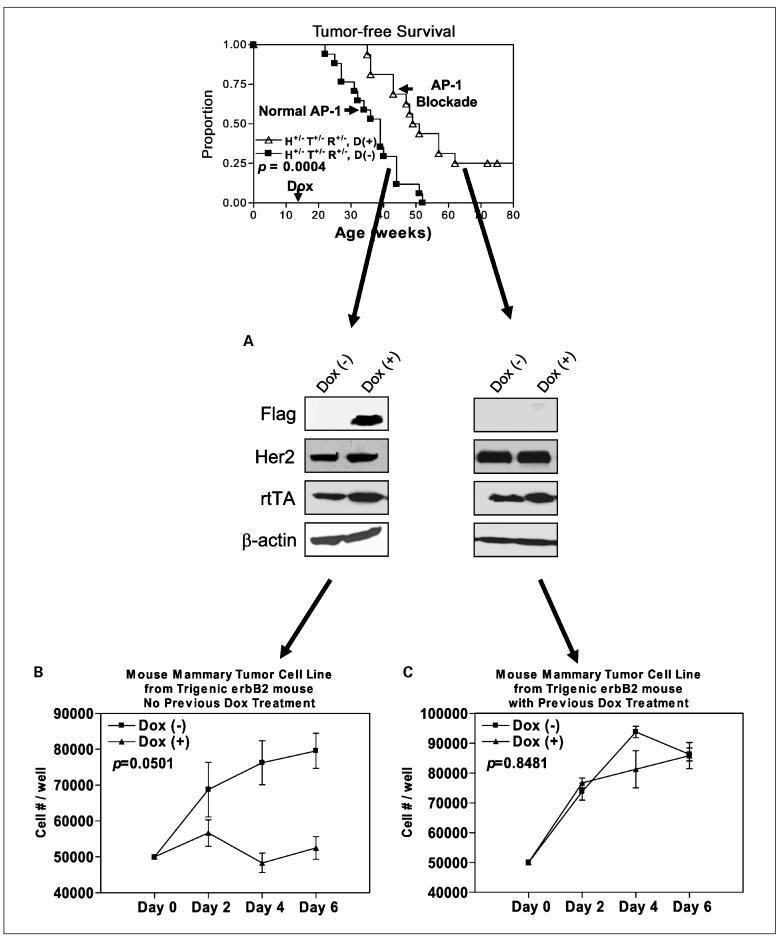
Primary tumor cell lines derived from mammary tumors in trigenic MMTV-erbB2 mice show comparable characteristics
To further investigate the tumors that arose in doxycycline-treated mice, we next examined the inducibility of Tam67 in the cell lines derived from mammary tumors in pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice that were or were not treated with doxycycline. As shown in Fig. 6A, Tam67 could be induced in the cell lines derived from mammary tumors that never received doxycycline (cell lines from pUHD-Tam67+/-/MMTV-rtTA+/-/MMTV-erbB2+/- mice, no doxycycline treatment group). However, Tam67 was not induced in the tumor cell lines derived from mice that had been exposed to doxycycline treatment. We then conducted cell growth assays with these cells and found that the cell lines that arose in the absence of doxycycline showed reduced growth when doxycycline was added to induce Tam67 (Fig. 6B), whereas the cells derived from tumors that arose in the presence of doxycycline were not inhibited when treated with doxycycline in vitro. These results show that tumors that arose in mice treated with doxycycline fail to express the AP-1 inhibitor. Such results suggest that AP-1 blockade prevents tumor formation and that tumors only arise in cells in which Tam67 is somehow inactivated. This lack of expression of Tam67 could occur through inactivation of the Tam67 promoter (e.g., through methylation of the promoter). We are currently investigating the mechanism by which Tam67 is inactivated.
Fig. 6.
Mammary cell lines derived from trigenic erbB2 tumors respond similarly to doxycycline treatment. A, we transplanted the tumors that arose in trigenic mice with or without receiving doxycycline into tissue culture dishes. We then treated these cell lines in the presence of doxycycline. The tumor cell lines derived from the control trigenic mice (no prior doxycycline exposure) show expression of induced Flag-tagged Tam67 and unaffected erbB2 and rtTA. B and C, the cell lines were plated in 24-well tissue culture plates and cell growth assays were done. The number of live cells form triplicate wells were averaged and plotted on different days of culture.
Discussion
In this study, we determined whether in vivo AP-1 blockade achieved by inducing an AP-1 inhibitor, Tam67, in the mammary gland can prevent the development of erbB2-induced ER-negative mammary carcinomas. At a time when 100% control erbB2 mice developed mammary tumors, only approximately half of the AP-1-blocked mice developed tumors. In addition, 25% of the AP-1-blocked mice survived more than 72 weeks of age without developing mammary tumors. Thus, ER-negative mammary tumor formation in adult MMTV-erbB2 mice was delayed in all mice and prevented in 25% of mice by AP-1 blockade. In addition, in six of seven AP-1-blocked mice in which tumors did arise, Tam67 was no longer expressed in these tumors, suggesting that tumors cannot arise in the presence of AP-1 blockade. This long-term induction of AP-1 blockade in the experimental mice was without any obvious toxicity. We further showed that AP-1 blockade by Tam67 effectively blocks the development of pre-malignant lesions. These data show that blockade of the AP-1 transcription factor suppresses erbB2-induced mammary tumorigenesis, and support the idea that targeting the AP-1 transcription factor may be an effective strategy to prevent ER-negative breast cancer.
The AP-1 transcription factor is uniquely positioned in the mitogenic signal transduction cascades. It transduces multiple signals from cellular stress, growth factors, steroid hormones, and inflammatory cytokines. All of these various signals may be involved in mammary tumorigenesis. Thus, blockade of this transcription factor interferes with multiple signal transduction pathways at a distal point, potentially avoiding resistance that can occur when signal transduction is blocked at the cell membrane or cytoplasm. Our studies support a concept that blockade of transcription factors can be an effective strategy to be used in future for preventing ER-negative breast cancer.
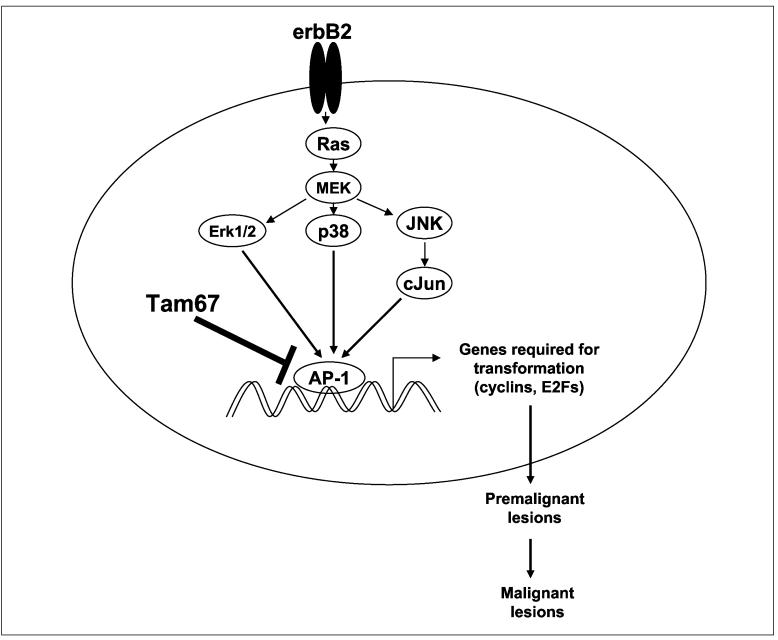
AP-1 has been shown to regulate AP-1-dependent genes such as cell cycle regulator cyclin D1, cyclin E, cyclin-dependent kinases, Rb, and p27 (27, 33). It is also shown to regulate E2F-dependent genes such as cyclins and cell proliferation regulators (28, 35). We recently showed that AP-1 cross-talks with ER (2, 36). Cyclin D1 and E2F factors are certainly downstream effectors of erbB2 signaling. Thus, we speculate that AP-1 blockade suppresses erbB2-induced mammary tumorigenesis and the formation of premalignant lesions mainly by inhibiting cell cycle progression and cell growth through several mechanisms: (a) suppression of cell cycle regulators resulting in cell cycle arrest and inhibited proliferation of deregulated mammary cells; (b) suppression of E2F-dependent genes by down-regulating E2F dimerizing partners; and (c) reduction of cyclin-dependent kinase activities, increase in p27 expression, and hypophosphorylation of Rb (Fig. 7). These changes then block pathways required for the formation of premalignant lesions and the evolution to invasive breast cancer.
Fig. 7.
Schematic model of the mechanisms by which in vivo AP-1 blockade with Tam67 prevents malignant transformation induced by oncogene erbB2.
Our results show that in MMTV-erbB2/rtTA/Tam67 trigenic mice, AP-1 blockade prevents breast cancer in ∼25% mice but does not totally prevent breast cancer in all mice. However, in tumors that did arise in the doxycycline-treated trigenic mice, Tam67 protein expression was lost or greatly reduced. Given that only 40% to 60% of the mammary epithelial cells express the Tam67 transgene (28), it is not surprising that the doxycycline-treated trigenic mice can still develop mammary tumors. These tumors may have developed from a mammary epithelial cell that does not experience AP-1 blockade. Alternatively, Tam67 expression could be actively repressed by gene silencing, thus allowing tumors to form in erbB2-expressing mice. In any event, the observation that the tumors that did arise in doxycycline-treated mice fail to express the AP-1 inhibitor supports our conclusion that erbB2-induced tumorigenesis is dependent on the activation of the AP-1 transcription factor.
Our results from the in vivo mammary tumor prevention experiments (Figs. 2 and 5) and tumor cell lines derived from mice expressing Tam67 (Fig. 6) indicate that AP-1 is required during erbB2-induced mammary tumorigenesis, and that the tumors and premalignant lesions that did arise in doxycycline-treated animals failed to express Tam67. The mechanism by which Tam67 is lost in these lesions is not yet known; however, the loss of Tam67 can occur early during tumorigenesis (at the time of DCIS lesions or possibly before). We are currently investigating whether this down-regulation of the Tam67 transgene occurs through epigenetic silencing.
Previous studies by Yu et al. (37) investigated the effect of ablation of cyclin D1 in MMTV-c-Myc, MMTV-Wnt1, MMTV-ras, and MMTV-erbB2 mice. Results from these studies showed that cyclin D1 is necessary for ras- and erbB2-induced mammary tumors, but not for c-Myc- and Wnt1-induced tumors in these mice. Cyclin D1 is an AP-1-dependent gene that is activated by many growth factors including steroid hormones, peptide growth factors, and cytokines. Thus, our current results are consistent with these previous in vivo studies reported by Yu et al. (37). Whether AP-1 blockade blocks all the cyclin D1-related pathways is an interesting question to examine in the future. We are now generating trigenic animals that express oncogenes other than erbB2 and will compare among these transgenic breast cancer models.
Our results indicate that in vivo AP-1 blockade suppresses ER-negative mammary tumor formation induced by erbB2 oncogene and suppresses the development of premalignant lesions. These results show that the AP-1 transcription factor is required for erbB2-induced ER-negative breast cancer formation. We are currently investigating the effect of AP-1 blockade on suppressing the growth of existing tumors and on invasion and metastasis, and whether AP-1 blockade will prevent ER-negative tumorigenesis induced by other oncogenes such as Wnt1 and c-Myc. The results from these studies provide the preclinical rationale to target the AP-1 transcription factor for the prevention of ER-negative breast cancer.
Acknowledgments
Grant support: Dan L. Duncan Cancer Center at Baylor College of Medicine and NIH R01 grant CA123246 (P.H. Brown).
References
- 1.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 2.DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Mol Endocrinol. 2005;19:362–78. doi: 10.1210/me.2004-0267. [DOI] [PubMed] [Google Scholar]
- 3.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–57. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 4.Piu F, Aronheim A, Katz S, Karin M. AP-1 repressor protein JDP-2: inhibition of UV-mediated apoptosis through p53 down-regulation. Mol Cell Biol. 2001;21:3012–24. doi: 10.1128/MCB.21.9.3012-3024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 6.Aronheim A, Zandi E, Hennemann H, Elledge SJ, Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol Cell Biol. 1997;17:3094–102. doi: 10.1128/mcb.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt JT, Gopal TV, Moulton AD, Nienhuis AW. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986;83:4794–8. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–86. [PubMed] [Google Scholar]
- 9.Brown PH, Chen TK, Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 1994;9:791–9. [PubMed] [Google Scholar]
- 10.Szabo E, Preis LH, Brown PH, Birrer MJ. The role of jun and fos gene family members in 12-O-tetradecanoylphorbol-13-acetate induced hemopoietic differentiation. Cell Growth Differ. 1991;2:475–82. [PubMed] [Google Scholar]
- 11.Rodgers WH, Matrisian LM, Giudice LC, et al. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94:946–53. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ham J, Babij C, Whitfield J, et al. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–39. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 13.Schiff R, Reddy P, Ahotupa M, et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst. 2000;92:1926–34. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 14.Smith LM, Wise SC, Hendricks DT, et al. cJun overexpression in MCF-7 breast cancer cells produces a tumorigenic, invasive and hormone resistant phenotype. Oncogene. 1999;18:6063–70. doi: 10.1038/sj.onc.1202989. [DOI] [PubMed] [Google Scholar]
- 15.Schule R, Rangarajan P, Yang N, et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991;88:6092–6. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb P, Nguyen P, Valentine C, et al. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–85. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Xiao D, Kolluri SK, Zhang X. Unique anti-activator protein-1 activity of retinoic acid receptor β. Cancer Res. 2000;60:3271–80. [PubMed] [Google Scholar]
- 18.Bamberger AM, Methner C, Lisboa BW, et al. Expression pattern of the AP-1 family in breast cancer: association of fosB expression with a well-differentiated, receptor-positive tumor phenotype. Int J Cancer. 1999;84:533–8. doi: 10.1002/(sici)1097-0215(19991022)84:5<533::aid-ijc16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Chen TK, Smith LM, Gebhardt DK, Birrer MJ, Brown PH. Activation and inhibition of the AP-1 complex in human breast cancer cells. Mol Carcinog. 1996;15:215–26. doi: 10.1002/(SICI)1098-2744(199603)15:3<215::AID-MC7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Gee JM, Barroso AF, Ellis IO, Robertson JF, Nicholson RI. Biological and clinical associations of c-jun activation in human breast cancer. Int J Cancer. 2000;89:177–86. doi: 10.1002/(sici)1097-0215(20000320)89:2<177::aid-ijc13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Cooper SJ, MacGowan J, Ranger-Moore J, Young MR, Colburn NH, Bowden GT. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res. 2003;1:848–54. [PubMed] [Google Scholar]
- 22.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci U S A. 1994;91:609–13. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JJ, Rhim JS, Schlegel R, Vousden KH, Colburn NH. Expression of dominant negative Jun inhibits elevated AP-1 and NF-κB transactivation and suppresses anchorage independent growth of HPV immortalized human keratinocytes. Oncogene. 1998;16:2711–21. doi: 10.1038/sj.onc.1201798. [DOI] [PubMed] [Google Scholar]
- 24.Young MR, Li JJ, Rincon M, et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci U S A. 1999;96:9827–32. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan Y, Kim S, Yasumoto H, Namba M, Miyazaki H, Iwao H. Effects of dominant-negative c-Jun on platelet-derived growth factor-induced vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2002;22:82–8. doi: 10.1161/hq0102.101821. [DOI] [PubMed] [Google Scholar]
- 26.Ludes-Meyers JH, Liu Y, Munoz-Medellin D, Hilsenbeck SG, Brown PH. AP-1 blockade inhibits the growth of normal and malignant breast cells. Oncogene. 2001;20:2771–80. doi: 10.1038/sj.onc.1204377. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Ludes-Meyers J, Zhang Y, et al. Inhibition of AP-1 transcription factor causes blockade of multiple signal transduction pathways and inhibits breast cancer growth. Oncogene. 2002;21:7680–9. doi: 10.1038/sj.onc.1205883. [DOI] [PubMed] [Google Scholar]
- 28.Shen Q, Zhang Y, Uray IP, et al. The AP-1 transcription factor regulates postnatal mammary gland development. Dev Biol. 2006;295:589–603. doi: 10.1016/j.ydbio.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Gunther EJ, Belka GK, Wertheim GB, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–92. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- 30.Wu K, DuPre E, Kim H, et al. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat. 2006;96:147–57. doi: 10.1007/s10549-005-9071-1. [DOI] [PubMed] [Google Scholar]
- 31.Wu K, Zhang Y, Xu XC, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–80. [PubMed] [Google Scholar]
- 32.Liu MM, Albanese C, Anderson CM, et al. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–60. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Lu C, Shen Q, Munoz-Medellin D, Kim H, Brown PH. AP-1 blockade in breast cancer cells causes cell cycle arrest by suppressing G1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene. 2004;23:8238–46. doi: 10.1038/sj.onc.1207889. [DOI] [PubMed] [Google Scholar]
- 34.Campiglio M, Normanno N, Menard S. Re: Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2004;96:715–716. doi: 10.1093/jnci/djh126. 715author reply. [DOI] [PubMed] [Google Scholar]
- 35.Shen Q, Uray IP, Li Y, et al. The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors. Oncogene. 2008;27:366–77. doi: 10.1038/sj.onc.1210643. [DOI] [PubMed] [Google Scholar]
- 36.DeNardo DG, Kim HT, Wu K, Lee A, Brown PH. Estrogen receptor stimulated breast cancer cell growth independent of DNA binding activity. Proc Am Assoc Cancer Res. 2005;46:217. [Google Scholar]
- 37.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]