Abstract
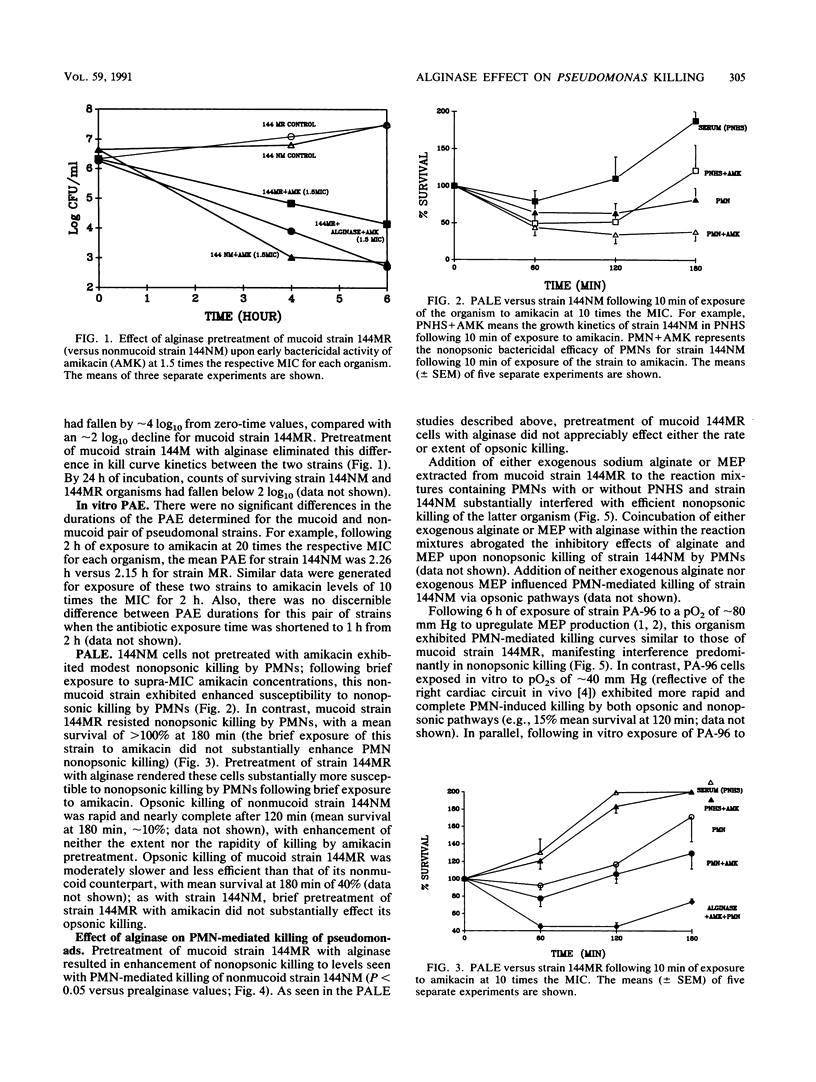
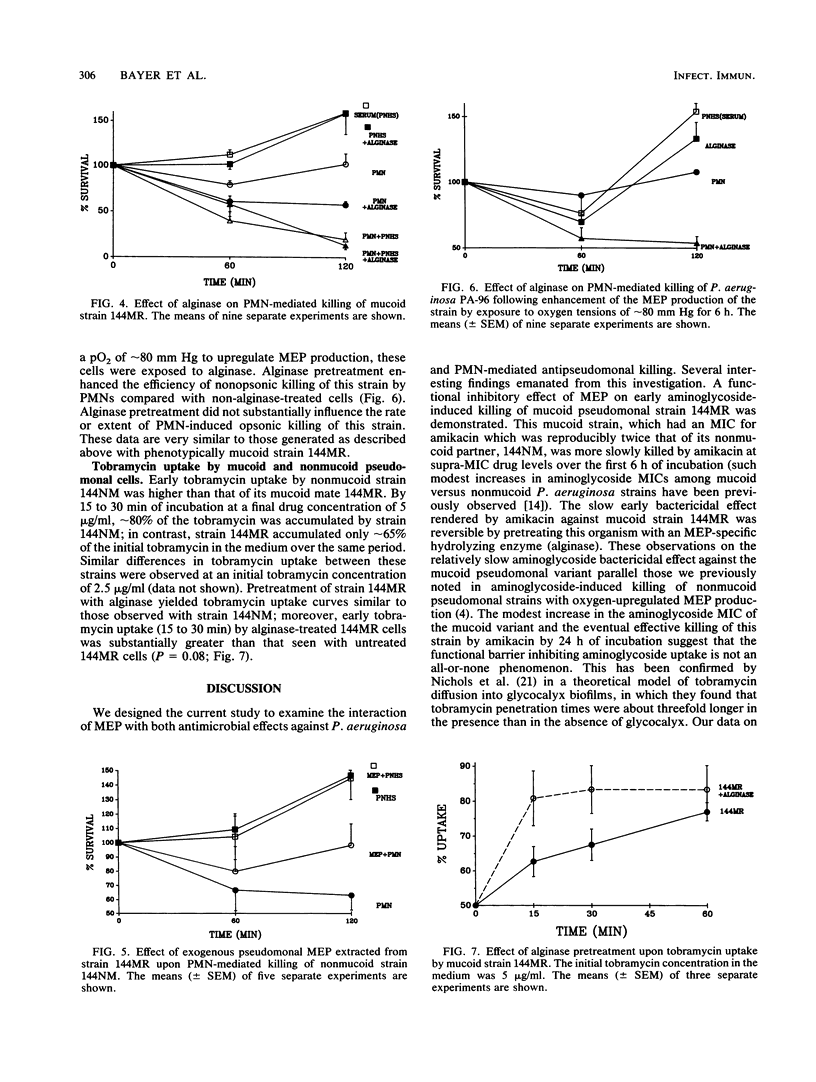
We evaluated in vitro the functional role of mucoid exopolysaccharide (MEP) of Pseudomonas aeruginosa in blocking antibiotic-induced and polymorphonuclear leukocyte (PMN)-mediated pseudomonal killing. The serum-resistant P. aeruginosa isolates used were mucoid strain 144MR and its nonmucoid revertant, strain 144NM. By timed kill curves, early bacterial effects of amikacin against mucoid strain 144MR were substantially less than those observed with nonmucoid strain 144NM; this effect was reversible with enzymatic hydrolysis of MEP of strain 144MR by alginase. Also, early tobramycin uptake (15 to 30 min) by mucoid 144MR cells was less than that seen with nonmucoid strain 144NM; pretreatment of 144MR cells with alginase substantially enhanced early tobramycin uptake compared with untreated 144MR cells (P = 0.08). In strain 144NM (but not in strain 114MR) there was a notable postantibiotic leukocidal enhancement effect manifested by increased nonopsonic killing following brief exposure of these cells to supra-MIC amikacin; pretreatment of strain 144MR with alginase rendered these cells more susceptible to amikacin-induced postantibiotic leukocidal enhancement. Similarly, direct PMN-mediated nonopsonic killing of mucoid strain 144MR was significantly less than that observed with strain 144NM (P less than 0.05); pretreatment of 144MR cells with alginase rendered this strain equal to strain 144NM in susceptibility to nonopsonic killing. In addition, exogenous sodium alginate or extracted MEP of strain 144MR interfered with effective nonopsonic killing of strain 144NM by PMNs. Studies also indicated that mucoid strain 144MR was phagocytosed significantly less well than its nonmucoid mate (P less than 0.00001), an effect reversed by pretreatment of the mucoid cells with alginase. These data confirm that P. aeruginosa MEPs functionally decrease the uptake and early bactericidal effect of aminoglycosides in vitro and interfere with effective PMN-mediated nonopsonic phagocytosis and killing of mucoid strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., van Biesen T., Dasgupta M., Lam K., Costerton J. W. Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother. 1989 Oct;33(10):1824–1826. doi: 10.1128/aac.33.10.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Crowell D. J., Yih J., Bradley D. W., Norman D. C. Comparative pharmacokinetics and pharmacodynamics of amikacin and ceftazidime in tricuspid and aortic vegetations in experimental Pseudomonas endocarditis. J Infect Dis. 1988 Aug;158(2):355–359. doi: 10.1093/infdis/158.2.355. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Eftekhar F., Tu J., Nast C. C., Speert D. P. Oxygen-dependent up-regulation of mucoid exopolysaccharide (alginate) production in Pseudomonas aeruginosa. Infect Immun. 1990 May;58(5):1344–1349. doi: 10.1128/iai.58.5.1344-1349.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., O'Brien T., Norman D. C., Nast C. C. Oxygen-dependent differences in exopolysaccharide production and aminoglycoside inhibitory-bactericidal interactions with Pseudomonas aeruginosa--implications for endocarditis. J Antimicrob Chemother. 1989 Jan;23(1):21–35. doi: 10.1093/jac/23.1.21. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Yih J., Chiu C. Y., Nast C. C. Pathogenic effects of monocytopenia, granulocytopenia and dexamethasone on the course of experimental Pseudomonas aeruginosa endocarditis in rabbits. Chemotherapy. 1989;35(4):278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- Bosso J. A., Black P. G., Matsen J. M. Ciprofloxacin versus tobramycin plus azlocillin in pulmonary exacerbations in adult patients with cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):180–184. [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M., Carter R. E. Mucoid Pseudomonas aeruginosa in patients with chronic illnesses. Lancet. 1971 Jan 30;1(7692):236–237. doi: 10.1016/s0140-6736(71)90973-1. [DOI] [PubMed] [Google Scholar]
- Eftekhar F., Speert D. P. Alginase treatment of mucoid Pseudomonas aeruginosa enhances phagocytosis by human monocyte-derived macrophages. Infect Immun. 1988 Nov;56(11):2788–2793. doi: 10.1128/iai.56.11.2788-2793.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- Gordon C. A., Hodges N. A., Marriott C. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J Antimicrob Chemother. 1988 Nov;22(5):667–674. doi: 10.1093/jac/22.5.667. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn D. B., Brestel E. P., Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun. 1987 Aug;55(8):1813–1818. doi: 10.1128/iai.55.8.1813-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker A., Evans L. R. Isolation and characterization of an alginase from mucoid strains of Pseudomonas aeruginosa. J Bacteriol. 1984 Sep;159(3):958–964. doi: 10.1128/jb.159.3.958-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker A., Jones R. S. A new polysaccharide resembling alginic acid isolated from pseudomonads. J Biol Chem. 1966 Aug 25;241(16):3845–3851. [PubMed] [Google Scholar]
- McDonald P. J., Craig W. A., Kunin C. M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977 Feb;135(2):217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Wetherall B. L., Pruul H. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981 Jan-Feb;3(1):38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Dorrington S. M., Slack M. P., Walmsley H. L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. 1988 Apr;32(4):518–523. doi: 10.1128/aac.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Wolff S. M., Puziss M. Summary of a workshop on infections in patients with cystic fibrosis. J Infect Dis. 1979 Aug;140(2):252–256. doi: 10.1093/infdis/140.2.252. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Alazard M. J., Borowski R. S. Serum sensitivity of a Pseudomonas aeruginosa mucoid strain. Infect Immun. 1984 Sep;45(3):748–755. doi: 10.1128/iai.45.3.748-755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. Antibiotic penetration through bacterial capsules and exopolysaccharides. J Antimicrob Chemother. 1982 Nov;10(5):368–372. doi: 10.1093/jac/10.5.368. [DOI] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. The penetration of antibiotics through sodium alginate and through the exopolysaccharide of a mucoid strain of Pseudomonas aeruginosa. Lancet. 1981 Sep 5;2(8245):502–503. doi: 10.1016/s0140-6736(81)90885-0. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver H. G., Zachidniak K., Speert D. P. Inhibition of polymorphonuclear leukocyte chemotaxis by the mucoid exopolysaccharide of Pseudomonas aeruginosa. Clin Invest Med. 1988 Aug;11(4):247–252. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]


