Abstract
Cancer cells may evade immune surveillance as a result of defective antigen processing and presentation. In this study, we demonstrate that CD40 ligation overcomes this defect through the coordinated action of the transcription factors NF-κB and interferon regulatory factor 1 (IRF-1). We show that unlike interferon signaling, which triggers the STAT1-mediated transcriptional activation of IRF-1, the ligation of CD40 in carcinomas induces the rapid upregulation of IRF-1 in a STAT1-independent but NF-κB-dependent manner. The transcriptional activation of IRF-1 is controlled largely by the recruitment of p65 (RelA) NF-κB to the IRF-1 promoter following the engagement of a TAK1/IκB kinase β/IκBα signaling pathway downstream of CD40. NF-κB and de novo-synthesized IRF-1 converge to regulate the expression of genes involved in antigen processing and transport, as evident from the sequential recruitment of NF-κB and IRF-1 to the promoters of the genes encoding transporter for antigen processing 1 (TAP1), TAP2, tapasin, and low-molecular-mass polypeptides LMP2 and LMP10. Moreover, the RNA interference-mediated knockdown of IRF-1 reduced, whereas the inhibition of NF-κB abolished, the effects of CD40 on TAP1 and LMP2 upregulation in carcinoma cells. Collectively, these data reveal a novel “feed-forward” mechanism induced by NF-κB which ensures that acutely synthesized IRF-1 operates in concert with NF-κB to amplify the immunoproteasome and antigen-processing functions of CD40.
Among the nine known members of the interferon regulatory factor (IRF) family of transcription factors, IRF-1 has attracted significant attention as a master regulator of genes involved in the development of innate and adaptive immunity (reviewed in reference 59). Indeed, studies of mice with a null mutation in the irf-1 alleles (irf-1−/−) have revealed that IRF-1 plays a crucial role in interferon (IFN)-induced antiviral and antibacterial responses. When challenged with pathogens, irf-1−/− mice display compromised Th1 cell differentiation associated with defects in interleukin-12 p35 subunit (IL-12p35) and inducible nitric oxide synthase (iNOS) gene expression in macrophages and concomitant hyporesponsiveness of CD4+ T lymphocytes and natural killer cells to IL-12 (24, 37). In line with this observation, the promoters of the IL-12p35 and iNOS genes possess functional IRF-1 binding motifs (42). IRF-1 also plays an important role in the transcriptional control of the transporter for antigen processing TAP1 and the immunoproteasome component LMP2 in response to IFN-γ (41, 68), thereby influencing the presentation of viral and tumor antigens to CD8+ T cells. Moreover, IRF-1 participates in an autoregulatory loop in the context of type I IFN signaling in which IRF-1 is both a target and a transcriptional activator of IFN-β (19, 20, 72).
In addition to its role in regulating the immune response to pathogens, IRF-1 has been proposed to function as a tumor suppressor (reviewed in reference 59) and to influence p53 activity (10). Thus, the loss of IRF-1 dramatically exacerbates susceptibility to oncogenic lesions in vivo (44), whereas its overexpression inhibits the proliferation and survival of carcinoma cells in vitro and in vivo (33, 35, 47).
Intriguingly, the spectrum of functions of IRF-1 displays similarities to that of CD40, a tumor necrosis factor (TNF) receptor family member with a pivotal role in the generation of adaptive and innate immune responses (reviewed in reference 62). Thus, the stimulation of CD40 in macrophages and dendritic cells triggers the production of cytokines and other mediators of the immune response, including IL-12p35 and iNOS (30), as well as the antigen-processing and presentation capacities of the cells. Humans with mutations in the CD40 ligand (CD40L) gene develop a rare immune disorder called X-linked hyper-immunoglobulin M (hyper-IgM) syndrome, which is characterized by impaired macrophage, dendritic, and B-cell function and is clinically manifested by recurrent viral and bacterial infections and increased susceptibility to malignancy (3, 28).
The widespread expression of CD40 in various types of carcinoma and lymphoma has recently attracted much attention as a potential target for cancer therapy (reviewed in references 14 and 61). Similar to IRF-1 overexpression, the ligation of CD40 inhibits tumor cell proliferation and survival in vitro and in vivo (4, 12, 21, 25). Moreover, CD40L stimulates a dendritic cell-mediated Th1 antitumor immune response which can suppress cancer growth, even in the absence of CD40 expression on the malignant cells (32, 38, 57). Recent work has shown that CD40L may fulfill additional functions by directly enhancing the immunogenicity of CD40-positive malignant cells, resulting in their enhanced recognition and killing by antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) (29, 31). Of note, we have recently demonstrated that the upregulation of TAP1 expression by CD40L is required for the generation of CTL responses to at least some tumor antigens (29). However, the precise mechanism by which CD40 ligation induces the expression of genes involved in antigen transport and processing remains unknown.
The aforementioned functional similarities provide a theoretical link between CD40 and IRF-1 and prompted us to evaluate the hypothesis that IRF-1 is a physiological target of the CD40 pathway. Data presented in this study confirm this hypothesis and demonstrate that the IRF-1 gene is acutely induced by CD40L in human carcinoma cells. Whereas type I and type II IFNs stimulate IRF-1 synthesis through Janus kinase-STAT signaling which triggers the STAT1-mediated transcriptional activation of IRF-1 (5), CD40L induces IRF-1 in a STAT1-independent manner. We show that the alternative pathway utilized by CD40L to regulate IRF-1 gene expression involves the activation of NF-κB and its recruitment to a promoter element proximal to the IRF-1 gene transcription start site. Moreover, we demonstrate that de novo-synthesized IRF-1 acts in concert with NF-κB to regulate the expression of genes responsible for antigen processing and transport, such as the TAP1, TAP2, tapasin, LMP2, and LMP10 genes. This mechanism of sequential mobilization of NF-κB and IRF-1 by CD40L thus ensures effective transactivation of components of the antigen presentation machinery, the synchronous synthesis of which is required for the engagement of antitumor immune responses and for immune function.
MATERIALS AND METHODS
Cell culture, adenovirus constructs, and reagents.
The bladder carcinoma cell lines EJ and VM-CUB-1 (26) were maintained in RPMI medium supplemented with 10% fetal calf serum (FCS). The cervical cancer line HeLa and the human embryonic kidney (HEK) line 293 were cultured in Dulbecco's modified Eagle medium supplemented with 10% FCS (GIBCO). HeLa clones transfected with CD40 constructs were established as described previously (8). AGE60 is a carcinoma cell line established in our laboratory from the ascitic fluid of a patient with ovarian cancer (L. Vardouli, C. Lindqvist, K. Vlahov, A. Loskog, and A. Eliopoulos, unpublished data) and is maintained in RPMI medium supplemented with 10% FCS. Human recombinant soluble CD40L was purchased from Bender MedSystems, Austria. Chemical kinase inhibitors were purchased from Calbiochem and dissolved in dimethyl sulfoxide prior to use. To generate recombinant adenovirus (RAd) expressing transdominant IκBα (Μ-IκBα), a Ser32→Ala/Ser36→Ala double mutation (7) was first introduced into the corresponding IκBα cDNA by site-directed mutagenesis using the QuikChange kit from Stratagene. The product was then subcloned into the AdEasyT XL adenoviral vector system (Stratagene), and a replication-deficient adenovirus was generated by homologous recombination in AdEasier-1 cells according to the manufacturer's instructions. The virus produced was expanded in HEK 293 cells, and a passage 4 virus was collected, purified, titrated, and used to infect cells.
Antibodies and immunoblotting.
Phospho-specific antibodies were diluted in 5% bovine serum albumin in Tris-buffered saline-0.1% (vol/vol) Tween 20 (TBS-T); all other antibodies were diluted in 5% milk in TBS-T. Phospho-Jun N-terminal protein kinase (phospho-JNK; phosphorylated at Ser473), phospho-p42/p44 mitogen-activated protein kinase (phospho-p42/p44 MAPK; phosphorylated at Thr202 and Tyr204), phospho-p38 MAPK (phosphorylated at Thr180 and Tyr182), phospho-STAT1 (phosphorylated at Tyr701), phospho-TAK1 (phosphorylated at Thr187), and the corresponding antibodies which recognize both the phosphorylated and unphosphorylated forms were purchased from Cell Signaling Technology, and the antibodies were used at dilutions of 1:500 to 1:1,000. The IκBα/MAD3 (C-21), IRF-1 (C-20), IRF-3, p65 (C-20), and RNA polymerase II (N-20) antibodies were purchased from Santa Cruz Biotechnology. The tubulin antibody was purchased from Sigma and used at a 1:1,000 dilution. Anti-rabbit IgG-horseradish peroxidase (HRP; 1:2,000) was purchased from Cell Signaling Technology, and anti-mouse IgG-HRP (1:1,000) was purchased from Sigma. For immunoblotting, 15 to 40 μg of protein was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride membrane (0.45 μM; Millipore), and blocked for 45 min at room temperature with 5% nonfat milk dissolved in TBS-T. Following three washes with TBS-T, membranes were incubated overnight at 4°C with primary antibody and 1 h at room temperature with the appropriate secondary antibody and then subjected to enhanced chemiluminescence analysis using an ECL kit (Amersham).
Preparation of nuclear extracts and EMSAs.
Cells were resuspended in 5 volumes of homogenization buffer (10 mM HEPES-KOH [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT]) supplemented with protease inhibitors (0.1 mM sodium orthovanadate, 10 mM sodium glycerophosphate, 2 μg of leupeptin/ml, and 2 μg of aprotinin/ml) and centrifuged at 800 × g for 10 min. The pellet was resuspended in 3 volumes of ice-cold homogenization buffer containing 0.05% NP-40, and cells were homogenized with 15 to 20 strokes of a tight-fitting Dounce homogenizer on ice. Following centrifugation for 10 min at 800 × g at 4°C, the cytoplasmic fraction was collected. The nuclei were washed once with homogenization buffer and then resuspended in buffer containing 40 mM HEPES-KOH (pH 7.9), 0.4 M KCl, 1 mM DTT, and 10% glycerol with protease inhibitors. NaCl was added at a final concentration of 300 mM, and the suspension was mixed and incubated on ice for 30 min and then centrifuged at 80,000 × g for 30 min. The supernatant was removed and snap-frozen. Electrophoretic mobility shift assays (EMSAs) were performed as described previously (13). The probes used were designated IRF-1 κB (5′-AGGGCTGGGGAATCCCGCTAA-3′) and IRF-1 M-κB (5′-AGGGCTGCGTAATAGCGCTAA-3′) and were annealed with their complementary oligonucleotides prior to radiolabeling.
Chromatin immunoprecipitation (ChIP) assay.
EJ cells were treated with CD40L at a final concentration of 0.5 μg/ml for various time intervals and exposed to 1% formaldehyde for 10 min at room temperature. Cross-linking was stopped by the addition of glycine to a final concentration of 0.137 M. The cells were washed with cold phosphate-buffered saline (PBS), harvested in PBS containing 0.5% NP-40 and 0.5 μM phenylmethylsulfonyl fluoride (PMSF), and centrifuged at 800 × g for 5 min at 4°C. The pellets were resuspended in swelling buffer (25 mM HEPES, pH 7.8, 1.5 mM MgCl2, 10 mM KCl, 0.5% NP-40, 1 mM DTT, 0.5 μM PMSF, 2 μg of aprotinin/ml) and left on ice for 10 min. Following Dounce homogenization (20 strokes with pestle A), the nuclei were collected, resuspended in sonication buffer (50 mM HEPES, pH 7.9, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, and protease inhibitors), and sonicated on ice to obtain an average nucleic acid strand length of 200 to 1,000 bp. The samples were cleared by centrifugation at 20,000 × g for 15 min at 4°C. The supernatant was collected and precleared by rotation at 4°C for 2 h with protein G-Sepharose beads. One-tenth of the volume of the supernatant was used as input, and the remaining volume was immunoprecipitated with anti-p65, anti-IRF-1, or anti-RNA polymerase II antibodies overnight at 4°C. Immunocomplexes were collected by adsorption to protein G-Sepharose beads for 1 h at 4°C, and the beads were washed twice with sonication buffer; twice with sonication buffer containing 500 mM NaCl; twice with buffer containing 20 mM Tris-Cl (pH 8), 1 mM EDTA, 250 mM LiCl, 0.5% sodium deoxycholate, 0.5 mM PMSF, and 2 μg of aprotinin/ml; and once with TE (10 mM Tris-Cl [pH 8], 1 mM EDTA, 0.5 mM PMSF, and 2 μg of aprotinin/ml). The immunocomplexes were eluted with buffer including 50 mM Tris (pH 8.0), 1 mM EDTA, and 1% SDS at 65°C for 10 min and then adjusted to contain 200 mM NaCl and incubated at 65°C for 5 h to reverse the cross-links. After successive treatments with RNase A and proteinase K, the samples were extracted with phenol-chloroform and precipitated with ethanol. Precipitated chromatin was analyzed by PCR using primers corresponding to the promoter regions of the IRF-1, TAP1, TAP2, tapasin, LMP10, and IL-8 genes (primer sequences are shown in Table 1). The products of the PCR amplifications were analyzed by agarose gel electrophoresis, and the quantification of the results was performed by measuring the intensities of the bands using the Tinascan version 2 software.
TABLE 1.
Primers used in ChIP assays
| Primera | Sequence |
|---|---|
| IRF-1 (forw) | 5′-GAGCAGGGGTGGATTGG-3′ |
| IRF-1 (rev) | 5′-GCACGTCTTGCCTCGACTA-3′ |
| TAP1 (forw) | 5′-AACTGGTGCAAGTGGAAAGG-3′ |
| TAP1 (rev) | 5′-GGGACACCTAGAGCTAGCCATT-3′ |
| TAP2 (forw) | 5′-CCTGTACAGTGCGAACCAGA-3′ |
| TAP2 (rev) | 5′-TGGAGTGGGTAGTCACTTGG-3′ |
| LMP10 (forw) | 5′-AGCTATGCCTGATGCTACTGG-3′ |
| LMP10 (rev) | 5′-GATGAGTCGGCCAGACAAG-3′ |
| Tapasin (forw) | 5′-GGAAGCCGGAGTAATGGTTT-3′ |
| Tapasin (rev) | 5′-TGAAGCCTCCTCTTCCTCCT-3′ |
| IL-8 (forw) | 5′-GTTGTAGTATGCCCCTAAGAG-3′ |
| IL-8 (rev) | 5′-CTCAGGGCAAACCTGAGTCATC-3′ |
forw, forward primer; rev, reverse primer.
Immunofluorescence microscopy.
For immunofluorescence microscopy, EJ cells were plated onto slides and stimulated 24 h later with 0.5 μg of CD40L/ml for various time intervals. The slides were then washed in PBS, and the cells were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. Following extensive washing in PBS (10 min at room temperature), the cells were permeabilized in 0.1% Triton X-100 in PBS for exactly 3 min and then washed again in PBS for 5 min at room temperature. The cells were then incubated with primary antibody anti-p65 or anti-IRF-1 at a 1:50 dilution in PBS supplemented with 10% heat-inactivated goat serum for 1 h at 37°C in a humidified chamber. Following extensive washing in PBS, the cells were incubated for 45 min with Alexa Fluor 594-anti-rabbit IgG (Molecular Probes, Leiden, The Netherlands) at a 1:500 dilution in PBS supplemented with 10% heat-inactivated goat serum. Following a 10-min wash in PBS, slides were mounted with DABCO antifade reagent and red fluorescence was visualized on a Nikon E600 digital microscope.
Reporter assays.
A sequence of approximately 600 bp of the IRF-1 promoter was PCR amplified from genomic DNA isolated from EJ cells by using the forward primer 5′-TACCCTCGAGCTTTCTGCCTCCTTCACTTCC-3′ and the reverse primer 5′-ACGTAAGATCTGCCAGGGCAGCGGCCCCACCGA-3′. The product was cloned first into the pCR2.1 plasmid (Invitrogen) and then into the KpnI/BglII sites of the pGL2-Basic vector (Promega), upstream of the luciferase gene. The QuikChange site-directed mutagenesis kit (Stratagene) was used to mutate the κB element of the IRF-1 promoter. The cloned products were sequenced and used for reporter assays. These assays were performed using 105 HEK 293 or EJ cells per well in a 24-well dish. HEK 293 cells were transfected with 50 ng of the NF-κB-luciferase reporter construct 3Enh.κB-ConALuc, containing three tandem repeats of the NF-κB binding element of the Ig(κ) promoter, or 50 ng of the IRF-1 promoter-luciferase reporter construct and 50 ng of a Renilla plasmid by using Lipofectamine (Invitrogen) in Opti-MEM medium (Invitrogen) according to the manufacturer's instructions. EJ cells were transfected with 500 ng of each reporter construct. Following 10 h of incubation with the transfection cocktail, the medium was changed to Dulbecco's modified Eagle medium supplemented with 10% FCS, and 12 h later, cells were stimulated with CD40L for 6 h prior to lysis. Luciferase and Renilla values were measured as described previously (8).
RNA interference (RNAi).
For the delivery of small interfering RNAs (siRNAs), 105 EJ cells were plated into each well of a 24-well plate (Costar) and the next day the cells were transfected with siRNA duplexes by using the siIMPORTER transfection reagent according to the instructions of the manufacturer (Upstate Biotechnology). IRF-1 was targeted with the siRNA duplex consisting of the sequence GGGCUCAUCUGGAUUAAUAUU and its antisense strand (Dharmacon). The control siRNA duplex comprised the sequence CGUACGCGGAAUACUUCGAUU and the corresponding antisense strand. The p65 (RelA) siRNA was purchased from Cell Signaling Technology. Cells were transfected overnight with IRF-1 and control siRNAs. The transfection cocktail was then removed, and fresh culture medium with or without CD40L was added. For the delivery of p65 siRNA, cells were cultured with the transfection cocktail for 6 h and then a volume of medium equal to that of the cocktail was added. Cells were left to recover for 10 h and were replated into 24-well dishes. The next day, the cells were subjected to a second round of transfection to increase the siRNA-mediated gene suppression. Twenty-four hours later, cultures were stimulated with CD40L, before lysis and further analysis.
RT-PCR.
RNA was isolated using Trizol according to the instructions of the manufacturer (Invitrogen). Two micrograms of RNA was then used for cDNA synthesis with avian myeloblastosis virus reverse transcriptase (RT) and a reverse transcription system from Promega (13). PCRs were performed using 1/10 of the cDNA reaction mixture. The sequences of the primers used are available upon request.
RESULTS
CD40 ligation induces the rapid upregulation of IRF-1 expression in carcinoma cells.
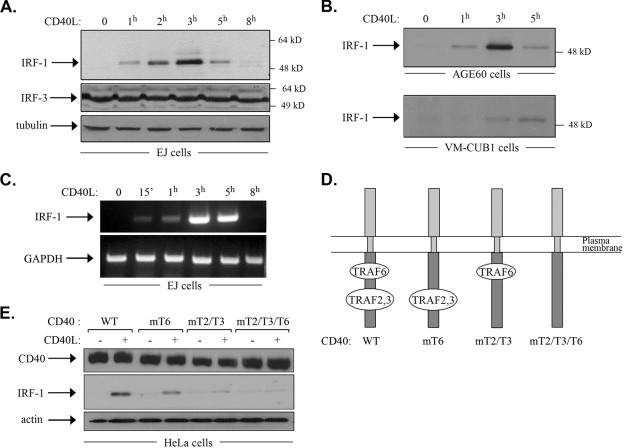
The ectopic expression of the transcription factor IRF-1 in tumor cells has been reported previously to confer antiproliferative, proapoptotic, and/or immunoregulatory properties, similar to those manifested upon the engagement of CD40. This observation provided a theoretical link between CD40 and IRF-1 and prompted us to examine the effects of CD40 ligation on IRF-1 expression. To this end, EJ bladder carcinoma cells, which naturally express CD40 (56), were stimulated with recombinant soluble CD40L for various time intervals (0, 1, 2, 3, 5, or 8 h) prior to lysis and the assessment of IRF-1 levels by immunoblotting. The results showed that whereas IRF-1 was absent in unstimulated cells, treatment with CD40L induced the upregulation of IRF-1 protein expression, which was evident as early as 1 h poststimulation, peaked at 3 h, and declined thereafter such that only very low levels could be detected after 8 h of treatment (Fig. 1A). A similar pattern of IRF-1 induction was observed in CD40L-stimulated AGE60 ovarian carcinoma cells and, to some extent, VM-CUB-1 bladder carcinoma cells (Fig. 1B). Unlike IRF-1, the levels of IRF-3, another IRF family member which is known to be constitutively expressed in a variety of different cell types (59), and those of β-tubulin remained unchanged following CD40 stimulation (Fig. 1A). To determine whether the marked upregulation of IRF-1 protein expression reflects changes at the RNA level, RT-PCR was performed using RNA isolated from EJ cells exposed to CD40L or from untreated controls. As shown in Fig. 1C, CD40 stimulation induced the rapid upregulation (within 15 min) of IRF-1 RNA expression, which peaked after 3 h of treatment with CD40L and declined thereafter.
FIG. 1.
CD40 stimulation induces the upregulation of IRF-1 expression. (A) CD40 ligation upregulates IRF-1 protein expression in EJ bladder carcinoma cells. EJ cells were stimulated with recombinant soluble CD40L at 0.5 μg/ml for the indicated times, and lysates were analyzed for IRF-1, IRF-3, or β-tubulin levels by immunoblotting. (B) Generality of the effect of CD40 stimulation on IRF-1 upregulation. AGE60 and VM-CUB-1 cells were treated as described in the legend to panel A, and IRF-1 levels were determined by immunoblotting. (C) CD40 stimulation induces the rapid induction of IRF-1 RNA expression. EJ cells were stimulated with recombinant soluble CD40L at 0.5 μg/ml for the indicated times before RNA was isolated. cDNA was synthesized and used as a template for semiquantitative PCR with primers specific for IRF-1 or the GAPDH housekeeping gene, which served as a loading and amplification control. The results shown are representative of results from three independent experiments. 15′, 15 min. (D) Graphical representation of CD40 and its TRAF binding domains. TRAF6 interacts with a membrane-proximal region, whereas TRAF2 and TRAF3 associate with a membrane-distal domain at the cytoplasmic C terminus of CD40. A double Q234E235→AA mutation, yielding CD40mT6 (mT6), selectively abolishes the interaction of TRAF6 with CD40, whereas a T254→A mutation, yielding CD40mT2/T3 (mT2/T3), inhibits TRAF2 and TRAF3 binding but leaves TRAF6-CD40 interactions intact. The CD40mT2/T3/T6 construct (mT2/T3/T6) contains a triple Q234E235 T254→AAA mutation which perturbs the binding of all TRAFs. These constructs are described in detail in reference 8. WT, wild type. (E) CD40L-induced IRF-1 synthesis depends on TRAF binding to the cytoplasmic C terminus of CD40. HeLa cervical carcinoma cells, which do not express CD40, were transfected with the CD40 constructs depicted in panel D. Selected stable clones were stimulated with CD40L for 3 h, and lysates were analyzed for CD40 or IRF-1 levels by immunoblotting. The results shown are representative of results from three independent experiments. +, present; −, absent.
CD40 triggers signal transduction and phenotypic changes through the recruitment of adapter molecules of the TNF receptor-associated factor (TRAF) family to its cytoplasmic C terminus. Specifically, yeast two-hybrid and coprecipitation experiments have shown previously that a membrane-proximal CD40 domain binds TRAF6 and that a membrane-distal region directly recruits TRAF2 and TRAF3 (14, 48, 61) (Fig. 1D). To determine the relative contributions of these domains to the regulation of IRF-1 expression, we stably transfected cells of the CD40-negative cervical carcinoma line HeLa with vectors expressing wild-type CD40 or mutated forms which were unable to bind directly to TRAF6 (CD40mT6) (Fig. 1D), TRAF2 and TRAF3 (CD40mT2/mT3), or all TRAFs (CD40mT2/T3/T6). These mutant forms have been evaluated previously for their TRAF binding capacities in the same cell line (8). Stable clones, expressing similar levels of CD40 (Fig. 1E), were then exposed to CD40L for 3 h or left untreated prior to lysis and the assessment of IRF-1 levels by immunoblotting. The results showed that the CD40L-induced upregulation of IRF-1 was partly reduced by CD40 mutations which disrupted TRAF6 binding and was severely affected by mutations which abolished CD40 interactions with TRAF2 and TRAF3 (Fig. 1E). CD40mT2/T3/T6 failed altogether to respond to CD40L by the upregulation of IRF-1 expression (Fig. 1E). We conclude that both the TRAF2/TRAF3 and TRAF6 binding domains of CD40 contribute to the CD40L-induced upregulation of IRF-1, with the former domain conferring the major regulatory effects.
CD40 ligation induces MAPK and NF-κB but not STAT1 signaling prior to the induction of IRF-1 expression.
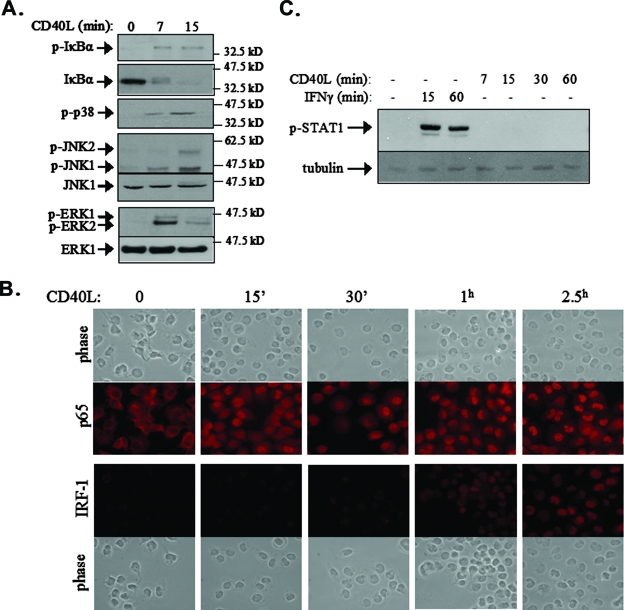
CD40 ligation in carcinoma cells triggers the TRAF-dependent activation of multiple signaling pathways, including the NF-κB and the JNK, p38, and extracellular signal-regulated kinase (ERK) MAPK pathways. In light of the observation that IRF-1 mRNA is induced within 15 min of CD40 ligation (Fig. 1C), we assessed NF-κB and MAPK signal activation at early time points following stimulation with CD40L. To this end, EJ cell cultures were treated with CD40L for 7 or 15 min prior to lysis and the evaluation of IκBα levels and phosphorylation status by immunoblotting. IκBα is a negative regulator of NF-κB. The stimulus-induced phosphorylation of IκBα at Ser32 and Ser36 triggers its ubiquitination and proteasomal degradation, resulting in the release of associated p65 (RelA) NF-κB, which translocates to the nucleus and transactivates a variety of target genes (27). As shown in Fig. 2A, CD40 ligation resulted in rapid phosphorylation and significant degradation of IκBα, which was evident at 7 min and proceeded at 15 min poststimulation.
FIG. 2.
CD40 ligation induces MAPK and NF-κB but not STAT1 signaling prior to the induction of IRF-1 expression. (A) EJ cells were stimulated with 0.5 μg of CD40L/ml for 7 or 15 min or left untreated, and cell lysates were analyzed for the expression of IκBα and the phosphorylated, active forms of p38 (p-p38), JNK, and ERK by immunoblotting. The levels of total (phosphorylated and unphosphorylated) JNK1 and ERK1 were also assessed as loading and expression controls. (B) The nuclear translocation of p65 NF-κB precedes the synthesis and nuclear localization of IRF-1. EJ cells were stimulated with 0.5 μg of CD40L/ml for the indicated times and immunostained with anti-p65 or anti-IRF-1 antibody as described in Materials and Methods. 15′ and 30′, 15 and 30 min. (C) CD40L does not induce the phosphorylation of STAT1. EJ cells were stimulated with CD40L for the indicated times, and lysates were analyzed for the expression of STAT1 phosphorylated at Tyr701, a residue critical for STAT1 activation. As a control, cell lysates from IFN-γ-treated EJ cells were used. −, absent.
The subcellular localization of the p65 NF-κB subunit was monitored by immunofluorescence staining at various time points (0, 15, and 30 min and 1 and 2.5 h) following CD40 stimulation. Untreated EJ cells demonstrated predominantly cytoplasmic p65 staining (Fig. 2B). Within 15 min of treatment with CD40L, a significant proportion of the cells displayed nuclear p65, consistent with the IκBα degradation observed at the same time point (Fig. 2A). The mobilization of p65 to the nucleus further increased at later time points and was still prominent after 2.5 h of treatment. In parallel to those of p65, we examined the expression and subcellular localization of IRF-1. IRF-1 was undetectable in untreated cells or in cells stimulated with CD40L for 15 min (Fig. 2B). Low-level nuclear expression of IRF-1 could be detected at 30 min, and this expression increased significantly at later time points of treatment, suggesting that NF-κB activation precedes IRF-1 induction. This observation was confirmed by immunoblot analysis of nuclear versus cytoplasmic protein extracts from CD40L-stimulated EJ cells (data not shown).
The engagement of the ERK, JNK, and p38 MAPK pathways was evaluated by immunoblotting using antibodies specific for the phosphorylated, active forms of these kinases. It was found that MAPK activation also precedes IRF-1 upregulation, as the phosphorylation of all three MAPKs was detected within 7 min of CD40 stimulation (Fig. 2A). IFN-γ is known to induce the expression of IRF-1 through the Janus kinase-mediated phosphorylation and activation of STAT1, which binds to an IFN-γ-activated sequence element on the IRF-1 promoter (51). Theoretically, CD40 ligation may also mediate IRF-1 upregulation through STAT1. To confirm or refute this hypothesis, we analyzed the levels of STAT1 phosphorylated at Tyr701, a residue critical for its activation, before and 7, 15, 30, and 60 min after CD40 stimulation. As a control, cell lysates from IFN-γ-treated EJ cells were used. The results of this analysis showed that IFN-γ but not CD40L induced the phosphorylation of STAT1 (Fig. 2C). Taken together, the aforementioned data suggest that CD40 ligation activates MAPK and NF-κB but not STAT1 signals prior to or concomitantly with the upregulation of IRF-1 expression.
CD40-mediated upregulation of IRF-1 in carcinomas depends on NF-κB signaling.
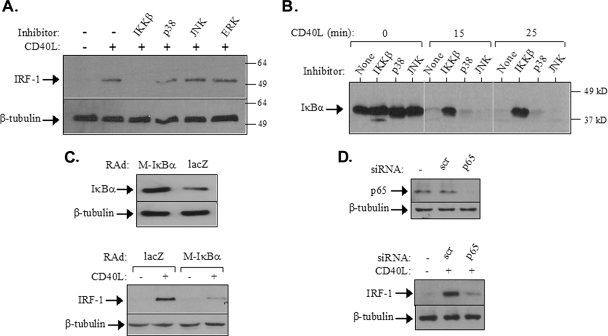
On the basis of the data shown in Fig. 2, we proceeded to identify the signaling pathway(s) responsible for the CD40-mediated de novo synthesis of IRF-1. To this end, EJ cells were pretreated with chemical inhibitors targeting JNK (SP600125) (2), p38 (SB203580) (36), and MEK, the ERK kinase (PD98059) (11), and then exposed to CD40L for 2 h prior to lysis and the assessment of IRF-1 expression by immunoblotting. The inhibitor concentrations used in this evaluation have been shown previously to impair CD40L-induced JNK, p38, and ERK activation, respectively, in carcinoma lines, including EJ (7, 9). Interestingly, none of these MAPK inhibitors were found to affect the CD40L-induced upregulation of IRF-1 (Fig. 3A).
FIG. 3.
CD40 ligation induces IRF-1 expression via NF-κB. (A) EJ cells were pretreated with chemical inhibitors targeting IKKβ (34), JNK (SP600125) (2), p38 (SB203580) (36), or MEK, the ERK kinase (PD098059) (11), at a final concentration of 20 μM and then exposed to CD40L for 2 h prior to lysis and the assessment of IRF-1 expression by immunoblotting. Reblotting with anti-β-tubulin was used to evaluate the levels of protein loading. Numbers to the right are molecular sizes in kilodaltons. +, present; −, absent. (B) The IKKβ but not the JNK or p38 inhibitor blocks the CD40L-induced IκBα degradation. EJ cells were pretreated with the chemical inhibitors listed in the legend to panel A and then stimulated with 0.5 μg of CD40L/ml for the indicated times. Cell lysates were then evaluated for the levels of IκBα expression by immunoblotting. (C) The overexpression of transdominant IκBα suppresses CD40-mediated IRF-1 upregulation. EJ cells were infected with a RAd expressing either Μ-IκBα, carrying a Ser32→Ala/Ser36→Ala double mutation, or as a control, lacZ at a multiplicity of infection of 50. (Lower panel) Thirty-six hours postinfection, cells were either treated with CD40L for 2 h or left untreated and then examined for the expression of IRF-1 or β-tubulin by immunoblotting. (Upper panel) Cell lysates from untreated infected cultures were also assessed for IκBα or tubulin levels. (D) The RNAi-mediated knockdown of p65 (RelA) NF-κB inhibits the effects of CD40L on IRF-1 upregulation. EJ cells were transfected with siRNA against p65 or with scramble siRNA (scr) as a control as described in Materials and Methods. (Upper panel) Lysates were evaluated for p65 or tubulin levels by immunoblotting. (Lower panel) Parallel cultures were treated with CD40L for 2 h and then examined for the expression of IRF-1 or β-tubulin by immunoblotting.
To suppress CD40 signaling on the NF-κB axis, a chemical inhibitor which blocks the activity of IκB kinase β (IKKβ) (34), a kinase that phosphorylates IκBα at the critical Ser32 and Ser36 residues, was first utilized. As shown in Fig. 3B, this reagent efficiently blocked the CD40L-triggered degradation of IκBα, whereas treatment with MAPK inhibitors had no effect. Immunofluorescence staining demonstrated that the IKKβ inhibitor also blocked the CD40-mediated nuclear mobilization of p65 NF-κB (data not shown). Interestingly, the inhibition of IKKβ activity abolished the effects of CD40L on IRF-1 expression (Fig. 3A).
To further substantiate the contribution of NF-κB to IRF-1 synthesis, we utilized two additional approaches. First, we infected EJ cells with a RAd expressing Μ-IκBα, carrying a Ser32→Ala/Ser36→Ala double mutation (7) which prevents IκBα phosphorylation and stimulus-dependent degradation (67). Elevated levels of IκBα could be detected in lysates from cultures infected with the Μ-IκBα RAD relative to those in lysates from cells infected with the lacZ control RAD, which demonstrated only comigrating endogenous, wild-type IκBα (Fig. 3C, upper panel). When cells infected with RAD expressing Μ-IκBα were exposed to CD40L, a significant reduction in IRF-1 synthesis compared to that in cells infected with control virus was observed (Fig. 3C, lower panel), implicating IκBα in CD40-mediated IRF-1 regulation.
Second, we utilized RNAi to knock down p65 NF-κB in EJ cells. As shown in Fig. 3D, upper panel, a p65-specific siRNA significantly reduced the endogenous expression of p65 whereas a scramble control had no effect. We therefore applied this RNAi approach to evaluate the role of p65 in IRF-1 synthesis. To this end, EJ cells transfected with either p65 siRNA or the scramble control were treated for 3 h with CD40L prior to lysis and the evaluation of IRF-1 levels by immunoblotting. The results showed that the knockdown of p65 markedly reduced the CD40-mediated upregulation of IRF-1 (Fig. 3D, lower panel). Taken together, the aforementioned data demonstrate the involvement of the IKKβ/IκBα/p65 NF-κB cascade in the induction of IRF-1 expression following CD40 stimulation.
The p65 NF-κB is recruited to and regulates the activity of the IRF-1 promoter in CD40-stimulated tumor cells.
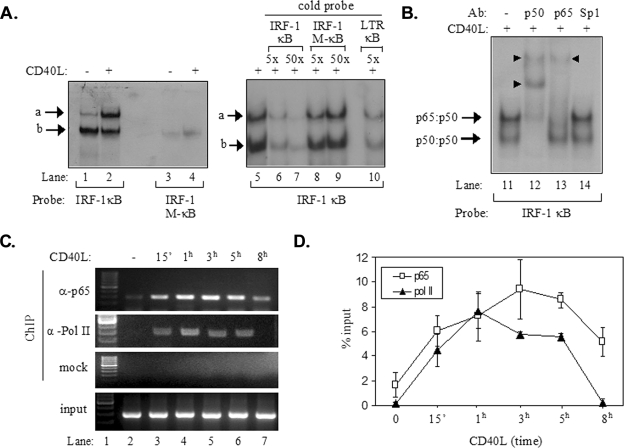
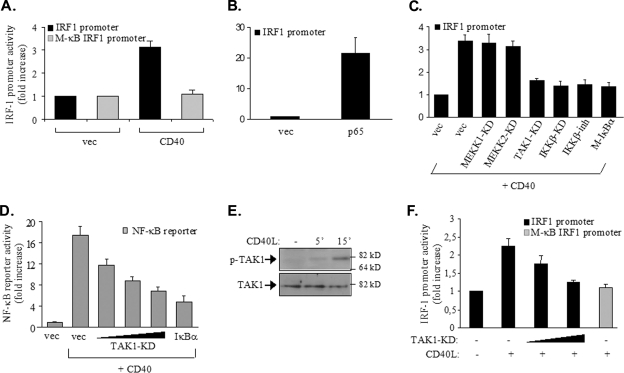
On the basis of the results shown in Fig. 3, we evaluated the hypothesis that CD40L-induced NF-κB directly regulates the human IRF-1 promoter. In support of this hypothesis, we identified a putative NF-κB binding element, GGGGAATCCC, approximately 30 bp upstream of the transcription start site of the IRF-1 gene by using the TRANSFAC database. A radiolabeled oligonucleotide probe containing this putative NF-κB binding site (probe IRF-1 κB) was used in EMSAs to evaluate interactions with nuclear proteins isolated from EJ cells treated with CD40L or from untreated controls. As shown in Fig. 4A, this analysis identified two DNA-protein complexes, one of which (complex a) was found to be significantly induced following CD40 stimulation (Fig. 4A, lanes 1 and 2). In contrast, an oligonucleotide probe in which four nucleotides of the putative NF-κB binding element were mutated (GCGTAATAGC, where underlining indicates the mutated nucleotides; probe IRF-1 M-κB) failed to form similar complexes (Fig. 4A, lanes 3 and 4).
FIG. 4.
Recruitment of p65 NF-κB to the IRF-1 promoter following CD40 ligation. (A) Results from EMSAs showing the in vitro binding of nuclear proteins to a radiolabeled probe containing the NF-κB binding site of IRF-1, forming complexes a and b (lanes 1 to 2 and 5 to 10). Binding was not observed when a radiolabeled probe containing a mutated NF-κB binding site (IRF-1 M-κB) was used (lanes 3 and 4). Excess cold IRF-1 κB or HIV LTR κB but not IRF-1 M-κB oligonucleotide effectively competed with complexes a and b for binding to the radiolabeled IRF-1 κB probe (lanes 5 to 10). +, present; −, absent. (B) Results from EMSAs showing the effects of antibodies (Ab) to p65 and p50 NF-κB or Sp1 on the electrophoretic mobilities of complexes a and b (lanes 11 to 14). The supershift in lanes 12 and 13 (marked with arrowheads) suggests that complex a contained p65-p50 heterodimers. (C and D) Results from a representative ChIP assay (C) and collective data from four experiments (D) showing the kinetics of the in vivo recruitment of p65 and of RNA polymerase II (Pol II) to the IRF-1 promoter. ChIP assays were performed as described in detail in Materials and Methods. One-tenth of the volume of the chromatin obtained was used for PCR as input, and the remaining volume was immunoprecipitated with anti-p65 (α-p65) or anti-RNA polymerase II (α-Pol II) antibodies or without antibody (mock). Precipitated DNA encompassing the IRF-1 promoter was then assayed by PCR. The quantification of the results was performed by measuring the intensities of the bands using the Tinascan version 2 software, and the levels of recruitment are expressed as percentages of recruited protein relative to the input (D). 15′, 15 min.
To further analyze the nature of the bound proteins, competition experiments with a 5- or 50-fold excess of unradiolabeled (“cold”) IRF-1 κB or IRF-1 M-κB oligonucleotide were performed. In parallel, a fivefold excess of a cold probe containing the human immunodeficiency virus (HIV) long terminal repeat (LTR) κB element, which is known to bind NF-κB (43), was used. The results obtained from the EMSAs demonstrated that both cold IRF-1 κB and the HIV LTR κB probe reduced the interaction of nuclear proteins with the radiolabeled IRF-1 κB oligonucleotide probe and that an excess of unlabeled IRF-1 M-κB did not (Fig. 4A, lanes 5 to 10). Additionally, the incubation of nuclear proteins isolated from CD40L-stimulated EJ cells with a p65 NF-κB antibody supershifted complex a, whereas an antibody against transcription factor Sp1 had no effect (Fig. 4B). However, both complex a and complex b were supershifted following incubation with anti-p50 NF-κB antibody, suggesting that complex a contained p65-p50 heterodimers and that complex b contained p50-p50 homodimers. We conclude that p65 binds to the IRF-1 gene promoter NF-κB binding site in vitro and that this binding increases significantly following CD40 stimulation.
To verify that p65 is also recruited to the IRF-1 promoter in vivo, ChIP experiments were performed. Chromatin from EJ cells treated with CD40L for various time intervals or from untreated controls was used for immunoprecipitation with an anti-p65 or anti-RNA polymerase II antibody, and precipitated DNA encompassing the IRF-1 gene promoter NF-κB binding site was assayed by PCR. The results (Fig. 4C) showed small amounts of p65 associating with the IRF-1 promoter in unstimulated cells. The recruitment of p65 dramatically increased within 15 min of CD40 stimulation, reached a peak at 3 h, and declined thereafter (Fig. 4C and D). Similarly, the recruitment of RNA polymerase II increased rapidly within 15 min of stimulation, reached a peak at 1 h, and returned to background levels at 8 h poststimulation (Fig. 4C and D).
On the basis of the aforementioned observations demonstrating the recruitment of p65 NF-κB to the IRF-1 promoter, we determined whether the NF-κB binding site functions to promote IRF-1 gene transcription upon CD40 signal activation. To this end, we constructed a reporter plasmid containing 600 bp of the IRF-1 promoter upstream of the luciferase gene. In addition, we used site-directed mutagenesis to mutate the NF-κB binding site of the IRF-1 promoter to match the sequence of IRF-1 M-κB, which failed to bind NF-κB (Fig. 4A). HEK 293 cells were transiently transfected with these constructs, together with a CD40 expression vector and a Renilla plasmid to correct for transfection efficiency. We have used HEK 293 cells because they lack CD40 and can be transfected with a high level of efficiency, allowing us to arrive at clear conclusions about the effects of various kinase inhibitors on CD40-mediated IRF-1 transactivation. As shown previously (49, 50), the overexpression of CD40 in 293 cells results in signal activation through the transient formation of receptor multimers and the recruitment of TRAFs in a ligand-independent manner. Consistent with these observations, the transfection of cells with the CD40 expression vector resulted in an approximately threefold increase in IRF-1 promoter activity relative to that in cells transfected with the control vector, and this activity was abolished by the IRF-1 M-κB mutation (Fig. 5A). As a control, the overexpression of p65 stimulated reporter activity by approximately 20-fold (Fig. 5B).
FIG. 5.
p65 NF-κB regulates the activity of the IRF-1 promoter in CD40-stimulated tumor cells. (A) The NF-κB binding site of the IRF-1 promoter is required for promoter activity in response to CD40: HEK 293 cells (105) were transfected with 50 ng of a luciferase reporter construct containing approximately 600 bp of the IRF-1 promoter or a reporter construct in which the NF-κB binding site was mutated to impair the binding of NF-κB (M-κB IRF1 promoter), according to the results shown in Fig. 4A. Cells were cotransfected with 500 ng of a CD40 expression vector (CD40) or a control empty plasmid (vec). The transfection efficiency was monitored by using a Renilla reporter. Luciferase and Renilla activities were assessed at 30 h posttransfection and expressed as the increase in reporter activity (measured as the ratio of luciferase to Renilla values) in relation to the activity in cells transfected with the control vector expressing no CD40. The mean values ± standard deviations (SD) from three independent experiments are shown. (B) The ectopic expression of p65 induces IRF-1 transactivation: HEK 293 cells were transfected with 50 ng of the IRF-1-luciferase reporter construct, 50 ng of a Renilla reporter, and 100 ng of p65 RelA, and reporter activities were assessed as described in the legend to panel A. The y axis depicts the increase (n-fold) in IRF-1 promoter activity. (C) TAK1 and IKKβ participate in CD40-mediated IRF-1 transactivation: cells were transfected with reporter plasmids as described in the legend to panel A, 250 ng of CD40, and 100 ng of kinase-dead (KD) mutants of TAK1, MEKK1, MEKK2, or IKKβ prior to lysis and the assessment of reporter activities. In parallel experiments, cells transfected with CD40 and reporter constructs were treated with an IKKβ chemical inhibitor (IKKβ-inh) (34) for 6 h prior to the assessment of reporter activity. As a control, the effects of Μ-IκBα, carrying a Ser32→Ala/Ser36→Ala double mutation, on CD40-induced IRF-1 transactivation were determined. Mean values ± SD from at least four independent experiments are shown. The y axis depicts the increase (n-fold) in IRF-1 promoter activity. (D) TAK1 participates in CD40-mediated NF-κB transactivation: HEK 293 cells were transfected with 50 ng of an NF-κB-luciferase reporter construct, 50 ng of a Renilla reporter construct, 250 ng of CD40, and 50, 100, or 200 ng of kinase-dead mutant form of TAK1 or, as a control, 100 ng of transdominant IκBα prior to lysis and the assessment of reporter activities as described in the legend to panel A. Mean values ± SD from three independent experiments are shown. Immunoblotting of lysates from the transfected cultures confirmed equal levels of CD40 expression (data not shown). (E) CD40 ligation induces the phosphorylation of TAK1: EJ cells were stimulated with 0.5 μg of CD40L/ml for the indicated times or left untreated (−) prior to lysis and the analysis of the levels of Thr187-phosphorylated, active TAK1 (p-TAK1) or total (phosphorylated and unphosphorylated) TAK1 by immunoblotting. The data shown are representative of results from three independent experiments. 5′, 5 min. (F) EJ cells were transfected with 500 ng of each Renilla reporter and a luciferase reporter containing either the IRF-1 promoter or the IRF-1 M-κB form of the promoter, along with 200 or 500 ng of kinase-dead TAK1. Cells were treated with CD40L for 6 h prior to lysis and the assessment of reporter activities as described in the legend to panel A. Mean values ± SD from three independent experiments are shown. +, present; −, absent.
To confirm the role of the CD40-activated NF-κB signaling pathway in the regulation of IRF-1 transcription, HEK 293 cells were transfected with an Μ-IκBα expression plasmid, along with CD40 and the IRF-1 promoter-driven luciferase reporter construct. It was found that Μ-IκBα suppressed the ability of CD40 to activate the IRF-1 promoter (Fig. 5C). In parallel, we assessed the effects of various NF-κB signaling components on CD40-mediated IRF-1 promoter activity. To this end, cells were cotransfected with the luciferase and Renilla reporter plasmids and a CD40 expression vector, in the absence or presence of kinase-inactive mutant forms of IKKβ, TAK1, MEK kinase 1 (MEKK1), and MEKK2 and a chemical inhibitor targeting IKKβ. TAK1 and MEKKs were chosen because they have been shown previously to function upstream of IKKβ in response to various NF-κB-inducing stimuli (27, 43). Moreover, MEKK1 has been implicated in CD40-induced JNK and p38 signaling (23). We found that the overexpression of a kinase-inactive mutant form of either TAK1 or IKKβ (66) inhibited CD40-induced IRF-1 promoter and NF-κB-dependent luciferase reporter activities (Fig. 5C and D). In contrast, kinase-defective MEKK1 or MEKK2 had no effect (Fig. 5C and data not shown).
The role of TAK1 in CD40-mediated IRF-1 promoter activity was also evaluated by using EJ cells stimulated with CD40L. We found that Thr187 phosphorylation at the activation loop of TAK1 increased within 5 min of CD40 stimulation (Fig. 5E) and that kinase-inactive TAK1 inhibited the CD40L-induced IRF-1 promoter activity also in these cells (Fig. 5F). The aforementioned data, coupled with the results shown in Fig. 4, demonstrate that p65 NF-κB is recruited to the IRF-1 promoter and suggest that CD40-induced IRF-1 promoter activity depends on the engagement of a TAK1/IKKβ/IκBα/p65 NF-κB pathway.
Coordinated induction of antigen transport and immunoproteasome gene expression in response to CD40 ligation.
Antigen processing and presentation play a crucial role in the antitumor immune response. Peptides derived from tumor-related proteins are generated in the cytoplasm by the action of a large multicatalytic protease complex, the immunoproteasome, which contains the low-molecular-mass polypeptides LMP2 and LMP10, among others. The TAP1 and TAP2 transporters associated with antigen processing function by transporting these peptides from the cytoplasm to the lumen of the endoplasmic reticulum, where each peptide forms a ternary complex with the major histocompatibility complex (MHC) class I heavy chain and β2-microglobulin, a process promoted by chaperone proteins such as tapasin, ERp57, and calreticulin (15, 69). These complexes are then transported to the cell surface and recognized by CTLs, which destroy cells that present them. We have recently reported that the stimulation of CD40 in carcinoma cells enhances their susceptibility to CTL-mediated killing via a mechanism which depends on the upregulation of TAP1 protein levels (29).
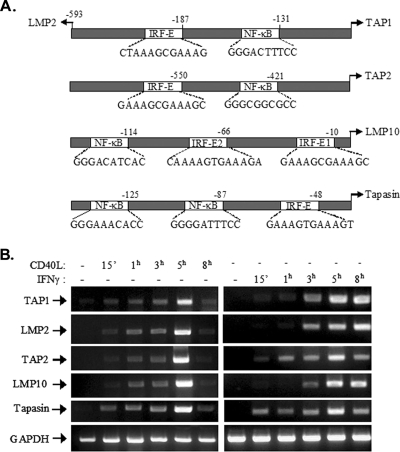
TAP1 and the immunoproteasome component LMP2 are regulated by a shared, bidirectional promoter which contains a functional NF-κB binding motif approximately 130 bp upstream of the transcription start site of the TAP1 gene (Fig. 6A) (70). Other studies have identified an IRF-1 binding element (IRF-E) proximal to the LMP2 gene transcription start site which is important for the transcriptional control of the LMP2 and TAP1 genes in response to IFN-γ/STAT signaling (68). Together, these reported findings raise the possibility that, in the context of CD40 signaling, NF-κB and IRF-1 may cooperate to regulate TAP1 and LMP2 expression. On this premise, we searched for NF-κB and IRF-1 binding motifs in the promoter regions of other genes involved in antigen processing and transport. Indeed, using the TRANSFAC database, we identified putative NF-κB and IRF-1 binding motifs in the promoter regions of the LMP10, TAP2, and tapasin genes (Fig. 6A). In line with this finding, the human LMP10 promoter and the mouse TAP2 promoter, which is highly homologous to the human TAP2 promoter, have been reported to possess functional IRF-E sequences that dictate transcriptional activation in response to IFN-γ (1, 17).
FIG. 6.
Coordinated induction of antigen transport and immunoproteasome gene expression in response to CD40 ligation. (A) Schematic representation of the TAP1/LMP2 bidirectional promoter and of the promoter regions of the TAP2, tapasin, and LMP10 genes. The sequences and relative positions of putative IRF-Es and NF-κB binding elements in relation to the transcription start sites are depicted. The consensus motif for IRF-1 is (G/C)(A)AAA(N)2-3AAA(G/C)(T/C) (60), and that for NF-κB is GGGRNNYYCC, where R represents purine, Y represents pyrimidine, and N indicates any base (45). Numbers above the diagrams indicate nucleotide positions relative to the transcription start sites. (B) Induction of antigen transporter and immunoproteasome gene expression following CD40 stimulation. EJ cells were treated with CD40L or, as a control, IFN-γ for the indicated times, and isolated RNA was subjected to RT-PCR using primers specific for the TAP1, LMP2, TAP2, tapasin, or LMP10 gene or the GAPDH housekeeping gene. Data are representative of results from five independent experiments for TAP1 and three experiments for LMP2, TAP2, tapasin, and LMP10. −, absent; 15′, 15 min.
On the basis of the aforementioned observations, we reasoned that CD40L-induced NF-κB and de novo-synthesized IRF-1 could act in a coordinated manner to orchestrate the transcription of genes involved in antigen processing and transport. To confirm or refute this hypothesis, we first examined the expression of TAP1, TAP2, LMP2, LMP10, and tapasin mRNAs in EJ bladder carcinoma cells before and after CD40 stimulation by using RT-PCR. This analysis revealed strikingly similar kinetics of gene upregulation following exposure to CD40L, with maximal induction occurring at 5 h poststimulation (Fig. 6B). As a control stimulus, we used IFN-γ, the prototypic inducer of IRF-1, to stimulate EJ cells at the same time points as those for CD40L. The results demonstrated that the pattern of IFN-γ-mediated TAP and LMP upregulation differs from that of CD40L-mediated upregulation, showing prolonged induction compared to that by CD40L and kinetics of upregulation which differ partly among the evaluated genes (Fig. 6B).
Both NF-κB and IRF-1 are recruited to the promoter regions of genes involved in antigen processing and transport.
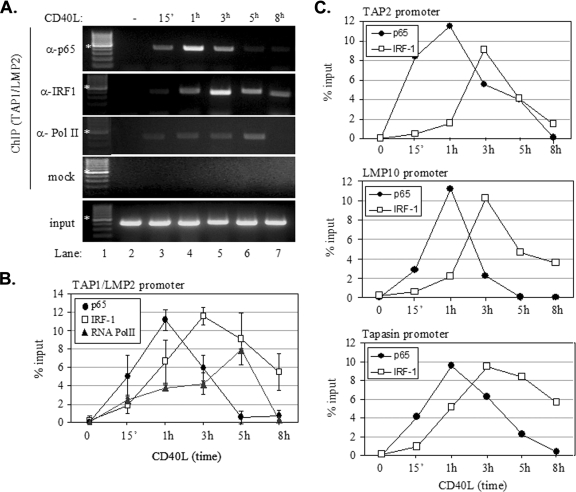
On the basis of the data shown in Fig. 6, we examined the recruitment of the transcription factors NF-κB and IRF-1, as well as that of RNA polymerase II, to the TAP1/LMP2 promoter by ChIP assays. Chromatin from EJ cells treated with CD40L for various times or untreated controls was used for immunoprecipitation with anti-p65, anti-IRF-1, or anti-RNA polymerase II antibody, and precipitated DNA encompassing the TAP1/LMP2 promoter was assayed by PCR. Results from a representative assay are shown in Fig. 7A, and collective data from four independent experiments are presented in Fig. 7B. The recruitment of p65 NF-κB to the TAP1/LMP2 promoter occurred as early as 15 min poststimulation with CD40L, peaked at 1 h, slightly decreased at 3 h, and declined thereafter. Thus, the maximum recruitment of p65, at 1 h, was equivalent to 11% of the input chromatin, and the minimum recruitment, after 5 to 8 h of CD40 ligation, was equivalent to 1% (Fig. 7B). The recruitment of IRF-1 occurred with delayed kinetics relative to that of p65. Thus, IRF-1 was detectable at 1 h poststimulation, peaked at 3 h, slightly decreased at 5 h, and declined thereafter. The recruitment of the RNA polymerase II gradually increased following CD40 ligation, reaching a peak at 5 h, but declined to background levels by 8 h poststimulation (Fig. 7A and B). The pattern of RNA polymerase II recruitment to the TAP1 promoter correlates with the levels of TAP1 RNA expressed and the kinetics of expression following CD40 stimulation (Fig. 6B).
FIG. 7.
Both NF-κB and IRF-1 are recruited to the promoter regions of genes involved in antigen processing and transport. (A and B) Results from a representative ChIP assay (A) and collective data from four experiments (B) showing the kinetics of the in vivo recruitment of p65, IRF-1, and RNA polymerase II to the bidirectional TAP1/LMP2 promoter following CD40 stimulation. ChIP assays were performed as described in detail in Materials and Methods. One-tenth of the volume of the chromatin obtained was used for PCR as input, and the remaining volume was immunoprecipitated with anti-p65 (α-p65), anti-IRF-1, or anti-RNA polymerase II (α-Pol II) antibody or subjected to mock (i.e., without antibody) precipitation. Precipitated DNA encompassing the TAP1/LMP2 promoter was then assayed by PCR. The quantification of the results was performed by measuring the intensities of the bands using the Tinascan version 2 software (B), and the levels of recruitment were expressed as percentages of recruited protein relative to the input. Lane 1 in panel A is a DNA molecular size marker, with the bands indicated by the asterisks corresponding to 500 bp. −, absent; 15′, 15 min. (C) Kinetics of the in vivo recruitment of p65 and IRF-1 to the promoter regions of the TAP2, LMP10, and tapasin genes. The data shown are the means of results from two independent experiments.
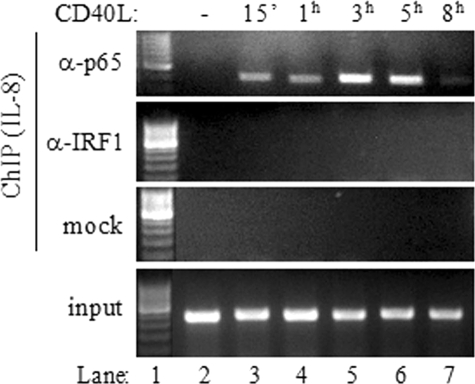
Similar to the TAP1/LMP2 promoter, the promoter regions of the LMP10, TAP2, and tapasin genes were found to recruit both p65 and IRF-1 (Fig. 7C). Importantly, IRF-1 recruitment occurred with delayed kinetics relative to that of p65 recruitment, probably reflecting the requirement for NF-κB-dependent de novo synthesis of IRF-1 prior to binding to IRF-E sequences. As a control, p65 and IRF-1 association with the IL-8 promoter, which is known to respond to CD40 stimulation (52), was assessed by using ChIP assays. In agreement with the results in a previous report (52), we found that p65 was rapidly recruited to the IL-8 promoter and that its binding increased with time, reaching a maximum at 3 to 5 h poststimulation. In marked contrast, however, no IRF-1 recruitment was observed throughout this course of CD40L treatment (Fig. 8).
FIG. 8.
IRF-1 is not recruited to the IL-8 promoter following the exposure of carcinoma cells to CD40L. EJ cells were stimulated with CD40L for the indicated times prior to ChIP analysis as described in detail in Materials and Methods. One-tenth of the volume of the chromatin obtained was used for PCR as input, and the remaining volume was immunoprecipitated with anti-p65 (α-p65) or anti-IRF-1 antibody or subjected to mock (i.e., without antibody) precipitation. Precipitated DNA encompassing the IL-8 promoter was then assayed by PCR. Lane 1 corresponds to a DNA molecular size marker. −, absent; 15′, 15 min.
On the basis of the aforementioned data, we conclude that both NF-κB and IRF-1 are recruited to the promoters of genes involved in antigen processing and transport, but with different kinetics. Moreover, elevated recruitment of IRF-1 to immunoproteasome and TAP promoters correlates with the maximal induction of gene expression.
The recruitment of IRF-1 and that of NF-κB have differential impacts on the regulation of TAP1 and LMP2 gene expression in response to CD40 stimulation.
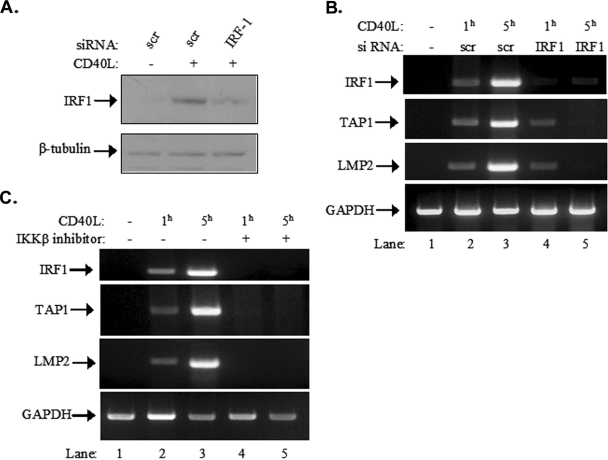
To determine the relative contribution of IRF-1 to the CD40-mediated upregulation of TAP1 and LMP2 expression, IRF-1 was knocked down in EJ cells by RNAi. As shown in Fig. 9A, upper panel, an IRF-1-specific siRNA significantly reduced the inducible expression of IRF-1 compared to that in cells transfected with the scramble control. In parallel, RNA was isolated from siRNA-transfected cultures before and 1 and 5 h after stimulation with CD40L and used in RT-PCR with primers specific for the IRF-1, TAP1, and LMP2 genes and the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene. It was found that the knockdown of IRF-1 had little impact on CD40L-mediated TAP1 or LMP2 induction at the earlier time point of 1 h but diminished gene expression at 5 h poststimulation (Fig. 9B).
FIG. 9.
The recruitment of IRF-1 and NF-κB regulates different stages of TAP1 and LMP2 gene expression in response to CD40 stimulation. (A and B) Effects of IRF-1 knockdown on CD40-mediated TAP1 and LMP2 upregulation. EJ cells were transfected with either IRF-1 siRNA (IRF-1) or control scramble siRNA (scr), stimulated with CD40L, and lysed. The levels of IRF-1 expression were assessed by immunoblotting (A). Alternatively, RNA was isolated and subjected to RT-PCR using primers specific for the IRF-1, TAP1, or LMP2 gene or the GAPDH housekeeping gene, which served as an amplification control (B). The data shown are representative of results from three independent experiments. +, present; −, absent. (C) Effects of NF-κB inhibition on CD40-mediated TAP1 and LMP2 upregulation. EJ cells were pretreated with an IKKβ inhibitor (lanes 4 and 5) or a vehicle control (dimethyl sulfoxide; lanes 1 to 3) and then stimulated with CD40L for 1 or 5 h, as indicated. RNA was isolated and subjected to RT-PCR using primers specific for the IRF-1, TAP1, or LMP2 gene or the GAPDH housekeeping gene. The data are representative of results from four independent experiments.
To assess the contribution of NF-κB to the CD40L-induced TAP1 expression, EJ cells were pretreated with the IKKβ inhibitor or left untreated and then exposed to CD40L for 1 or 5 h. RNA was isolated from these cultures, and RT-PCR was performed using primers specific for the IRF-1, TAP1, and LMP2 genes and the GAPDH housekeeping gene. As expected based on the results shown in Fig. 3B, treatment with the IKKβ inhibitor suppressed the CD40-mediated upregulation of IRF-1 mRNA (Fig. 9C). Importantly, the inhibitor also abolished the CD40L-inducible expression of TAP1 and LMP2 at both time points but did not influence the GAPDH RNA levels (Fig. 9C). Taken together, these observations suggest that the recruitment of NF-κB participates predominantly in the early stages of TAP1 and LMP2 induction by CD40L and that IRF-1 dictates the maximal gene expression at the later stages.
DISCUSSION
The data presented in this report establish CD40L as a novel regulator of IRF-1 gene expression in various carcinoma cells. We have shown that unlike IFN signaling, which triggers the STAT1-mediated transcriptional activation of IRF-1, the interaction of CD40 with its ligand induces the upregulation of IRF-1 in a STAT-independent but NF-κB-dependent manner. This conclusion is supported by a number of observations. First, the upregulation of IRF-1 occurred predominantly via signals transduced by the TRAF2/TRAF3-interacting domain of CD40 and, to a lesser extent, the TRAF6 binding region (Fig. 1), a finding which is reminiscent of the relative contributions of these CD40 domains to NF-κB but not MAPK signal activation in carcinoma cells (8). Second, the CD40L-induced degradation of IκBα and the nuclear mobilization of p65 NF-κB preceded the induction of IRF-1 RNA and protein levels, whereas the stimulation of CD40 did not affect STAT1 phosphorylation (Fig. 2). Finally, the inhibition of CD40-mediated NF-κB activation by the overexpression of nondegradable IκBα, treatment with a chemical inhibitor which targets IKKβ, or the depletion of p65 NF-κB by RNAi severely impaired de novo IRF-1 synthesis (Fig. 3).
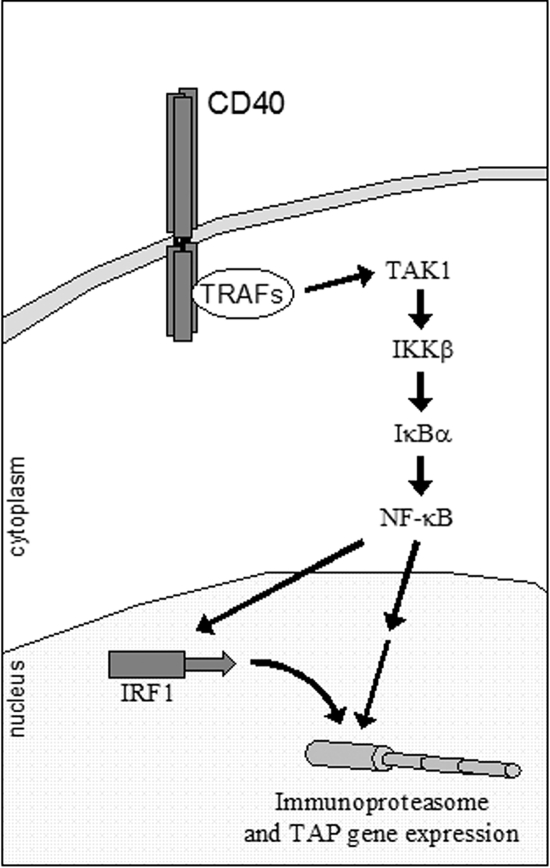
In line with these findings, we have characterized an NF-κB binding element proximal to the IRF-1 gene transcription start site and shown that it binds p65 in vitro and in vivo (Fig. 4). This domain is required for transcriptional activation because the introduction of mutations that impair the binding of NF-κB to this element abolished the responsiveness of the IRF-1 promoter to CD40 (Fig. 5A). Moreover, interference with IKKβ/IκBα or the upstream, TRAF-interacting kinase TAK1 led to significant reduction of CD40-induced IRF-1 promoter activity (Fig. 5C). The data presented in this study have also provided the first evidence for the involvement of TAK1 in CD40-stimulated NF-κB signaling. Thus, CD40 ligation was shown to induce TAK1 phosphorylation, whereas a mutated, kinase-inactive TAK1 suppressed NF-κB transactivation (Fig. 5). On the basis of the aforementioned findings, we conclude that CD40, through its association with TRAFs, triggers the engagement of a TAK1/IKKβ/IκBα/p65 NF-κB cascade which leads to the mobilization of p65 to the IRF-1 promoter and the stimulation of IRF-1 transcriptional activation (Fig. 10).
FIG. 10.
Proposed model in which NF-κB orchestrates antigen transport and immunoproteasome gene expression via the de novo synthesis of IRF-1. The data presented in this study demonstrate that stimulated CD40, through its association with TRAFs, triggers the engagement of a TAK1/IKKβ/IκBα cascade which leads to the nuclear translocation of p65, recruitment to the IRF-1 promoter, and the rapid stimulation of IRF-1 synthesis. NF-κB and de novo-synthesized IRF-1 converge to regulate the expression of genes involved in antigen transport and processing, thus ensuring efficient upregulation of the antigen-processing machinery in CD40-stimulated tumor cells.
Interestingly, however, we detected low levels of p65 associating with the IRF-1 promoter, even in the absence of CD40 stimulation (Fig. 4). Under these conditions, neither RNA polymerase II recruitment nor IRF-1 RNA expression was observed. We postulate that a threshold of p65 binding to the IRF-1 promoter may dictate transcription initiation. Alternatively, CD40 ligation may induce posttranslational modifications of the NF-κB transcription factor complex or relevant histones surrounding the NF-κB binding domain of the IRF-1 promoter which may contribute to transcription initiation. Indeed, IKKβ has been shown previously to mediate the Ser536 phosphorylation of p65 in response to TNF or lipopolysaccharide, resulting in enhanced NF-κB transcriptional activity (53, 71). The role of p65 posttranslational modifications in the regulation of IRF-1 gene expression is currently under investigation.
The CD40 pathway holds promise for the immunotherapy of various types of cancer, including non-Hodgkin's lymphoma (18), chronic lymphocytic leukemia (58), and carcinoma (32, 38), and CD40L has recently entered clinical trials, with promising results (63). Clinical efforts to target CD40 in advanced solid tumors and non-Hodgkin's lymphoma have accelerated in the past years with the development of anti-CD40 monoclonal antibodies. Like those with CD40L, these trials have confirmed that CD40 therapies are well tolerated and associated with antitumor activity (16, 64). The therapeutic potential of the CD40 pathway has been attributed largely to its effects on dendritic cell maturation and the induction of CTL responses independent of the expression of CD40 on tumor cells (18, 32, 40, 57).
The processing and presentation of tumor antigens to CD8+ CTLs depends on the expression of the TAP1 and TAP2 transporters for antigen processing and on a large multicatalytic protease complex, the immunoproteasome, which contains the low-molecular-mass polypeptides LMP2 and LMP10, among others (69). The chaperone protein tapasin also contributes to this process by promoting the assembly of the MHC class I loading complex and functions to bridge the TAP peptide transporter to MHC class I molecules. A number of published reports have shown that components of the antigen presentation pathway are frequently impaired in human tumors (reviewed in reference 54). In particular, low or no basal expression of TAP1, TAP2, and tapasin is a feature common to many primary tumors and cancer cell lines (6, 22) and has been attributed in part to diminished transcription of the corresponding genes (55). As a result, carcinomas are not recognized by effector CTLs and evade immune surveillance (39).
Recent studies from our laboratory have demonstrated that CD40L may overcome the TAP deficiency and restore immune system recognition of carcinoma cells (29, 31). The data given in the present report demonstrate that CD40 ligation induces the expression of a spectrum of genes involved in antigen processing and transport, such as the TAP1, TAP2, LMP2, LMP10, and tapasin genes, with strikingly similar kinetics (Fig. 6B), suggesting similar modes of transcriptional regulation. In line with this notion, the promoters of these genes possess NF-κB binding elements and IRF-Es and sequentially recruit p65 and IRF-1 following stimulation with CD40L (Fig. 7). The pattern of transcription factor recruitment probably reflects the requirement for NF-κB-dependent de novo synthesis of IRF-1 prior to the binding of IRF-1 to IRF-E sequences. While the kinetics of RNA polymerase II recruitment is likely to be influenced by various transcription cofactors, we have shown that NF-κB and IRF-1 are critical determinants of TAP and LMP transcriptional upregulation. Moreover, the assessment of the relative contributions of NF-κB and IRF-1 to TAP1 and LMP2 expression demonstrated that whereas the recruitment of NF-κB is important in driving the early phase of gene induction, the NF-κB-induced IRF-1 dictates the later-phase and maximal expression of TAP1 and LMP2 (Fig. 9). Future work will assess the impacts of NF-κB and IRF-1 on, as well as the relative contributions of TAPs and LMPs to, the CD40-mediated enhancement of CTL responses.
The described role of NF-κB as a master regulator of TAP and LMP expression suggests that NF-κB may make a positive contribution to CD40L therapy by controlling the immunogenicity of tumor cells. This observation further highlights the dual nature of NF-κB, which can either support or antagonize cancer in a tissue- or stimulus-dependent manner (27, 46). Moreover, the CD40 gene is itself subject to regulation by IRF-1 following the treatment of epithelial and endothelial cells with IFN-γ (65, 73). It is therefore possible that the ligation of CD40 on carcinoma cells may also result in the upregulation of CD40 expression via IRF-1, thus further amplifying the multiple effects of CD40L on tumor cells.
Investigations into the signal transduction pathways triggered by CD40 ligation have typically focused on direct (i.e., one-step) events that stimulate gene expression. The data presented in this report reveal a novel, two-step mechanism by which CD40 signals can be propagated (Fig. 10). The sequential mobilization of NF-κB and de novo-synthesized IRF-1 thus ensures coordinated and enhanced transactivation of components of the antigen-processing machinery, the synthesis of which is required for the CD40L-induced antitumor immune response. The results of this study may therefore have broader implications in appreciating the significance of such “feed-forward” cascades for the regulation of gene expression.
Acknowledgments
We are grateful to Angelica Loskog, University of Uppsala, Sweden, for providing us the VM-CUB-1 cell line and to Zhenguo Wu, Hong Kong University of Science and Technology, for the TAK1 constructs.
This work was supported by an Association for Cancer Research (AICR, United Kingdom) grant and the European Commission FP6 funded program Apotherapy (EC contract number 037344; http://apotherapy.med.uoc.gr) to A.G.E.
Footnotes
Published ahead of print on 11 August 2008.
REFERENCES
- 1.Arons, E., V. Kunin, C. Schechter, and R. Ehrlich. 2001. Organization and functional analysis of the mouse transporter associated with antigen processing 2 promoter. J. Immunol. 1663942-3951. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 9813681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callard, R. E., R. J. Armitage, W. C. Fanslow, and M. K. Spriggs. 1993. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol. Today 14559-564. [DOI] [PubMed] [Google Scholar]
- 4.Challa, A., A. G. Eliopoulos, M. J. Holder, A. S. Burguete, J. D. Pound, A. Chamba, G. Grafton, R. J. Armitage, C. D. Gregory, H. Martinez-Valdez, L. Young, and J. Gordon. 2002. Population depletion activates autonomous CD154-dependent survival in biopsylike Burkitt lymphoma cells. Blood 993411-3418. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee-Kishore, M., R. Kishore, D. J. Hicklin, F. M. Marincola, and S. Ferrone. 1998. Different requirements for signal transducer and activator of transcription 1α and interferon regulatory factor 1 in the regulation of low molecular mass polypeptide 2 and transporter associated with antigen processing 1 gene expression. J. Biol. Chem. 27316177-16183. [DOI] [PubMed] [Google Scholar]
- 6.Cromme, F. V., J. Airey, M. T. Heemels, H. L. Ploegh, P. J. Keating, P. L. Stern, C. J. Meijer, and J. M. Walboomers. 1994. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J. Exp. Med. 179335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, C. C., D. Bem, L. S. Young, and A. G. Eliopoulos. 2005. NF-kappaB overrides the apoptotic program of TNF receptor 1 but not CD40 in carcinoma cells. Cell. Signal. 17729-738. [DOI] [PubMed] [Google Scholar]
- 8.Davies, C. C., T. W. Mak, L. S. Young, and A. G. Eliopoulos. 2005. TRAF6 is required for TRAF2-dependent CD40 signal transduction in nonhemopoietic cells. Mol. Cell. Biol. 259806-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, C. C., J. Mason, M. J. Wakelam, L. S. Young, and A. G. Eliopoulos. 2004. Inhibition of phosphatidylinositol 3-kinase- and ERK MAPK-regulated protein synthesis reveals the pro-apoptotic properties of CD40 ligation in carcinoma cells. J. Biol. Chem. 2791010-1019. [DOI] [PubMed] [Google Scholar]
- 10.Dornan, D., M. Eckert, M. Wallace, H. Shimizu, E. Ramsay, T. R. Hupp, and K. L. Ball. 2004. Interferon regulatory factor 1 binding to p300 stimulates DNA-dependent acetylation of p53. Mol. Cell. Biol. 2410083-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 927686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos, A. G., C. W. Dawson, G. Mosialos, J. E. Floettmann, M. Rowe, R. J. Armitage, J. Dawson, J. M. Zapata, D. J. Kerr, M. J. O. Wakelam, J. C. Reed, E. Kieff, and L. S. Young. 1996. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene 132243-2254. [PubMed] [Google Scholar]
- 13.Eliopoulos, A. G., C. C. Wang, C. D. Dumitru, and P. N. Tsichlis. 2003. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 223855-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., and L. S. Young. 2004. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr. Opin. Pharmacol. 4360-367. [DOI] [PubMed] [Google Scholar]
- 15.Elliott, T., and A. Williams. 2005. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol. Rev. 20789-99. [DOI] [PubMed] [Google Scholar]
- 16.Forero-Torres, A., R. R. Furman, J. D. Rosenblatt, A. Younes, K. Harrop, and J. G. Drachman. 2006. A humanized antibody against CD40 (SGN-40) is well tolerated and active in non-Hodgkin's lymphoma (NHL): results of a phase 1 study. J. Clin. Oncol. 24430s. [Google Scholar]
- 17.Foss, G. S., and H. Prydz. 1999. Interferon regulatory factor 1 mediates the interferon-gamma induction of the human immunoproteasome subunit multicatalytic endopeptidase complex-like 1. J. Biol. Chem. 27435196-35202. [DOI] [PubMed] [Google Scholar]
- 18.French, R. R., H. T. C. Chan, A. L. Tutt, and M. J. Glennie. 1999. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T cell help. Nat. Med. 5548-553. [DOI] [PubMed] [Google Scholar]
- 19.Fujita, T., Y. Kimura, M. Miyamoto, E. L. Barsoumian, and T. Taniguchi. 1989. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature 337270-272. [DOI] [PubMed] [Google Scholar]
- 20.Fujita, T., L. F. Reis, N. Watanabe, Y. Kimura, T. Taniguchi, and J. Vilcek. 1989. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc. Natl. Acad. Sci. USA 869936-9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funakoshi, S., D. L. Longo, M. Beckwith, D. K. Conley, G. Tsarfaty, I. Tsarfaty, R. J. Armitage, W. C. Fanslow, M. K. Spriggs, and W. J. Murphy. 1994. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood 832787-2794. [PubMed] [Google Scholar]
- 22.Gabathuler, R., G. Reid, G. Kolaitis, J. Driscoll, and W. A. Jefferies. 1994. Comparison of cell lines deficient in antigen presentation reveals a functional role for TAP-1 alone in antigen processing. J. Exp. Med. 1801415-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher, E., T. Enzler, A. Matsuzawa, A. Anzelon-Mills, D. Otero, R. Holzer, E. Janssen, M. Gao, and M. Karin. 2007. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat. Immunol. 857-63. [DOI] [PubMed] [Google Scholar]
- 24.Galon, J., C. Sudarshan, S. Ito, D. Finbloom, and J. J. O'Shea. 1999. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J. Immunol. 1627256-7262. [PubMed] [Google Scholar]
- 25.Ghamande, S., B. L. Hylander, E. Oflazoglu, S. Lele, W. Fanslow, and E. A. Repasky. 2001. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 617556-7562. [PubMed] [Google Scholar]
- 26.Grups, J. W., and H. G. Frohmuller. 1988. Antiproliferative effects of interferons against human bladder carcinoma cell lines in vitro. Urol. Int. 43265-268. [DOI] [PubMed] [Google Scholar]
- 27.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-κB. Genes Dev. 182195-2224. [DOI] [PubMed] [Google Scholar]
- 28.Hayward, A. R., J. Levy, F. Facchetti, L. Notarangelo, H. D. Ochs, A. Etzioni, J.-Y. Bonnefoy, M. Cosyns, and A. Weinberg. 1997. Cholangiopathy and tumours of the pancreas, liver and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 158977-983. [PubMed] [Google Scholar]
- 29.Hill, S. C., S. J. Youde, S. Man, G. R. Teale, A. J. Baxendale, A. Hislop, C. C. Davies, D. M. Luesley, A. M. Blom, A. B. Rickinson, L. S. Young, and A. G. Eliopoulos. 2005. Activation of CD40 in cervical carcinoma cells facilitates CTL responses and augments chemotherapy-induced apoptosis. J. Immunol. 17441-50. [DOI] [PubMed] [Google Scholar]
- 30.Kato, T., R. Hakamada, H. Yamane, and H. Nariuchi. 1996. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 1563932-3938. [PubMed] [Google Scholar]
- 31.Khanna, R., L. Cooper, N. Kienzle, D. J. Moss, S. R. Burrows, and K. K. Khanna. 1997. Engagement of CD40 antigen with soluble CD40 ligand up-regulates peptide transporter expression and restores endogenous processing function in Burkitt's lymphoma cells. J. Immunol. 1595782-5785. [PubMed] [Google Scholar]
- 32.Kikuchi, T., and R. G. Crystal. 1999. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum. Gene Ther. 101375-1387. [DOI] [PubMed] [Google Scholar]
- 33.Kim, P. K., M. Armstrong, Y. Liu, P. Yan, B. Bucher, B. S. Zuckerbraun, A. Gambotto, T. R. Billiar, and J. H. Yim. 2004. IRF-1 expression induces apoptosis and inhibits tumor growth in mouse mammary cancer cells in vitro and in vivo. Oncogene 231125-1135. [DOI] [PubMed] [Google Scholar]
- 34.Kishore, N., C. Sommers, S. Mathialagan, J. Guzova, M. Yao, S. Hauser, K. Huynh, S. Bonar, C. Mielke, L. Albee, R. Weier, M. Graneto, C. Hanau, T. Perry, and C. S. Tripp. 2003. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J. Biol. Chem. 27832861-32871. [DOI] [PubMed] [Google Scholar]
- 35.Kroger, A., A. Stirnweiss, J. E. Pulverer, K. Klages, M. Grashoff, J. Reimann, and H. Hauser. 2007. Tumor suppression by IFN regulatory factor-1 is mediated by transcriptional down-regulation of cyclin D1. Cancer Res. 672972-2981. [DOI] [PubMed] [Google Scholar]
- 36.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372739-746. [DOI] [PubMed] [Google Scholar]
- 37.Liu, J., S. Cao, L. M. Herman, and X. Ma. 2003. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 1981265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loskog, A., H. Dzojic, S. Vikman, C. Ninalga, M. Essand, O. Korsgren, and T. H. Totterman. 2004. Adenovirus CD40 ligand gene therapy counteracts immune escape mechanisms in the tumor microenvironment. J. Immunol. 1727200-7205. [DOI] [PubMed] [Google Scholar]
- 39.Lou, Y., T. Z. Vitalis, G. Basha, B. Cai, S. S. Chen, K. B. Choi, A. P. Jeffries, W. M. Elliott, D. Atkins, B. Seliger, and W. A. Jefferies. 2005. Restoration of the expression of transporters associated with antigen processing in lung carcinoma increases tumor-specific immune responses and survival. Cancer Res. 657926-7933. [DOI] [PubMed] [Google Scholar]
- 40.Mackey, M. F., J. R. Gunn, C. Maliszewski, H. Kikutani, R. J. Noelle, and R. J. Barth. 1998. Dendritic cells require maturation via CD40 to generate protective anti-tumour immunity. J. Immunol. 1612094-2098. [PubMed] [Google Scholar]
- 41.Min, W., J. S. Pober, and D. R. Johnson. 1996. Kinetically coordinated induction of TAP1 and HLA class I by IFN-gamma: the rapid induction of TAP1 by IFN-gamma is mediated by Stat1 alpha. J. Immunol. 1563174-3183. [PubMed] [Google Scholar]
- 42.Negishi, H., Y. Fujita, H. Yanai, S. Sakaguchi, X. Ouyang, M. Shinohara, H. Takayanagi, Y. Ohba, T. Taniguchi, and K. Honda. 2006. Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc. Natl. Acad. Sci. USA 10315136-15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398252-256. [DOI] [PubMed] [Google Scholar]
- 44.Nozawa, H., E. Oda, K. Nakao, M. Ishihara, S. Ueda, T. Yokochi, K. Ogasawara, Y. Nakatsuru, S. Shimizu, Y. Ohira, K. Hioki, S. Aizawa, T. Ishikawa, M. Katsuki, T. Muto, T. Taniguchi, and N. Tanaka. 1999. Loss of transcription factor IRF-1 affects tumor susceptibility in mice carrying the Ha-ras transgene or nullizygosity for p53. Genes Dev. 131240-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkins, N. D. 1997. Achieving transcriptional specificity with NF-κB. Int. J. Biochem. Cell Biol. 291433-1448. [DOI] [PubMed] [Google Scholar]
- 46.Perkins, N. D. 2004. NF-κB: tumor promoter or suppressor? Trends Cell Biol. 1464-69. [DOI] [PubMed] [Google Scholar]
- 47.Pizzoferrato, E., Y. Liu, A. Gambotto, M. J. Armstrong, M. T. Stang, W. E. Gooding, S. M. Alber, S. H. Shand, S. C. Watkins, W. J. Storkus, and J. H. Yim. 2004. Ectopic expression of interferon regulatory factor-1 promotes human breast cancer cell death and results in reduced expression of survivin. Cancer Res. 648381-8388. [DOI] [PubMed] [Google Scholar]
- 48.Pullen, S. S., T. T. Dang, J. J. Crute, and M. R. Kehry. 1999. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 27414246-14254. [DOI] [PubMed] [Google Scholar]
- 49.Pullen, S. S., M. E. Labadia, R. H. Ingraham, S. M. McWhirter, D. S. Everdeen, T. Alber, J. J. Crute, and M. R. Kehry. 1999. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 3810168-10177. [DOI] [PubMed] [Google Scholar]
- 50.Rothe, M., V. Sarma, V. M. Dixit, and D. V. Goeddel. 1995. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science (Washington, DC) 2691424-1427. [DOI] [PubMed] [Google Scholar]
- 51.Rothman, P., B. Kreider, M. Azam, D. Levy, U. Wegenka, A. Eilers, T. Decker, F. Horn, H. Kashleva, J. Ihle, et al. 1994. Cytokines and growth factors signal through tyrosine phosphorylation of a family of related transcription factors. Immunity 1457-468. [DOI] [PubMed] [Google Scholar]
- 52.Saccani, S., S. Pantano, and G. Natoli. 2002. p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 369-75. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai, H., S. Suzuki, N. Kawasaki, H. Nakano, T. Okazaki, A. Chino, T. Doi, and I. Saiki. 2003. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 27836916-36923. [DOI] [PubMed] [Google Scholar]
- 54.Seliger, B., M. J. Maeurer, and S. Ferrone. 1997. TAP off—tumors on. Immunol. Today 18292-299. [DOI] [PubMed] [Google Scholar]
- 55.Setiadi, A. F., M. D. David, S. S. Chen, J. Hiscott, and W. A. Jefferies. 2005. Identification of mechanisms underlying transporter associated with antigen processing deficiency in metastatic murine carcinomas. Cancer Res. 657485-7492. [DOI] [PubMed] [Google Scholar]
- 56.Stamenkovic, I., E. A. Clark, and B. Seed. 1989. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 81403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, Y., D. Peng, J. Lecanda, V. Schmitz, M. Barajas, C. Qian, and J. Prieto. 2000. In vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunity. Gene Ther. 71467-1476. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi, S., R. F. Rousseau, P. Yotnda, Z. Mei, G. Dotti, D. Rill, R. Hurwitz, F. Marini, M. Andreeff, and M. K. Brenner. 2001. Autologous antileukemic immune response induced by chronic lymphocytic leukemia B cells expressing the CD40 ligand and interleukin 2 transgenes. Hum. Gene Ther. 12659-670. [DOI] [PubMed] [Google Scholar]
- 59.Tamura, T., H. Yanai, D. Savitsky, and T. Taniguchi. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26535-584. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka, N., T. Kawakami, and T. Taniguchi. 1993. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol. Cell. Biol. 134531-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tong, A. W., and M. J. Stone. 2003. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 101-13. [DOI] [PubMed] [Google Scholar]
- 62.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 672-17. [DOI] [PubMed] [Google Scholar]
- 63.Vonderheide, R. H., J. P. Dutcher, J. E. Anderson, S. G. Eckhardt, K. F. Stephans, B. Razvillas, S. Garl, M. D. Butine, V. P. Perry, R. J. Armitage, R. Ghalie, D. A. Caron, and J. G. Gribben. 2001. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 193280-3287. [DOI] [PubMed] [Google Scholar]
- 64.Vonderheide, R. H., K. T. Flaherty, M. Khalil, M. S. Stumacher, D. L. Bajor, N. A. Hutnick, P. Sullivan, J. J. Mahany, M. Gallagher, A. Kramer, S. J. Green, P. J. O'Dwyer, K. L. Running, R. D. Huhn, and S. J. Antonia. 2007. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25876-883. [DOI] [PubMed] [Google Scholar]
- 65.Wagner, A. H., M. Gebauer, B. Pollok-Kopp, and M. Hecker. 2002. Cytokine-inducible CD40 expression in human endothelial cells is mediated by interferon regulatory factor-1. Blood 99520-525. [DOI] [PubMed] [Google Scholar]
- 66.Wan, J., L. Sun, J. W. Mendoza, Y. L. Chui, D. P. Huang, Z. J. Chen, N. Suzuki, S. Suzuki, W. C. Yeh, S. Akira, K. Matsumoto, Z. G. Liu, and Z. Wu. 2004. Elucidation of the c-Jun N-terminal kinase pathway mediated by Estein-Barr virus-encoded latent membrane protein 1. Mol. Cell. Biol. 24192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, C.-Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kB. Science 274784-787. [DOI] [PubMed] [Google Scholar]
- 68.White, L. C., K. L. Wright, N. J. Felix, H. Ruffner, L. F. Reis, R. Pine, and J. P. Ting. 1996. Regulation of LMP2 and TAP1 genes by IRF-1 explains the paucity of CD8+ T cells in IRF-1−/− mice. Immunity 5365-376. [DOI] [PubMed] [Google Scholar]
- 69.Williams, A., C. A. Peh, and T. Elliott. 2002. The cell biology of MHC class I antigen presentation. Tissue Antigens 593-17. [DOI] [PubMed] [Google Scholar]
- 70.Wright, K. L., L. C. White, A. Kelly, S. Beck, J. Trowsdale, and J. P. Ting. 1995. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J. Exp. Med. 1811459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, F., E. Tang, K. Guan, and C. Y. Wang. 2003. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 1705630-5635. [DOI] [PubMed] [Google Scholar]
- 72.Yarilina, A., K. H. Park-Min, T. Antoniv, X. Hu, and L. B. Ivashkiv. 2008. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9378-387. [DOI] [PubMed] [Google Scholar]
- 73.Zhao, Z., Y. Qian, D. Wald, Y. F. Xia, J. G. Geng, and X. Li. 2003. IFN regulatory factor-1 is required for the up-regulation of the CD40-NF-κB activator 1 axis during airway inflammation. J. Immunol. 1705674-5680. [DOI] [PubMed] [Google Scholar]