Abstract
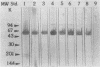
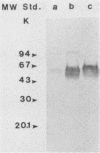
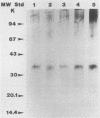
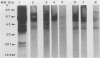
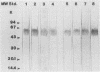
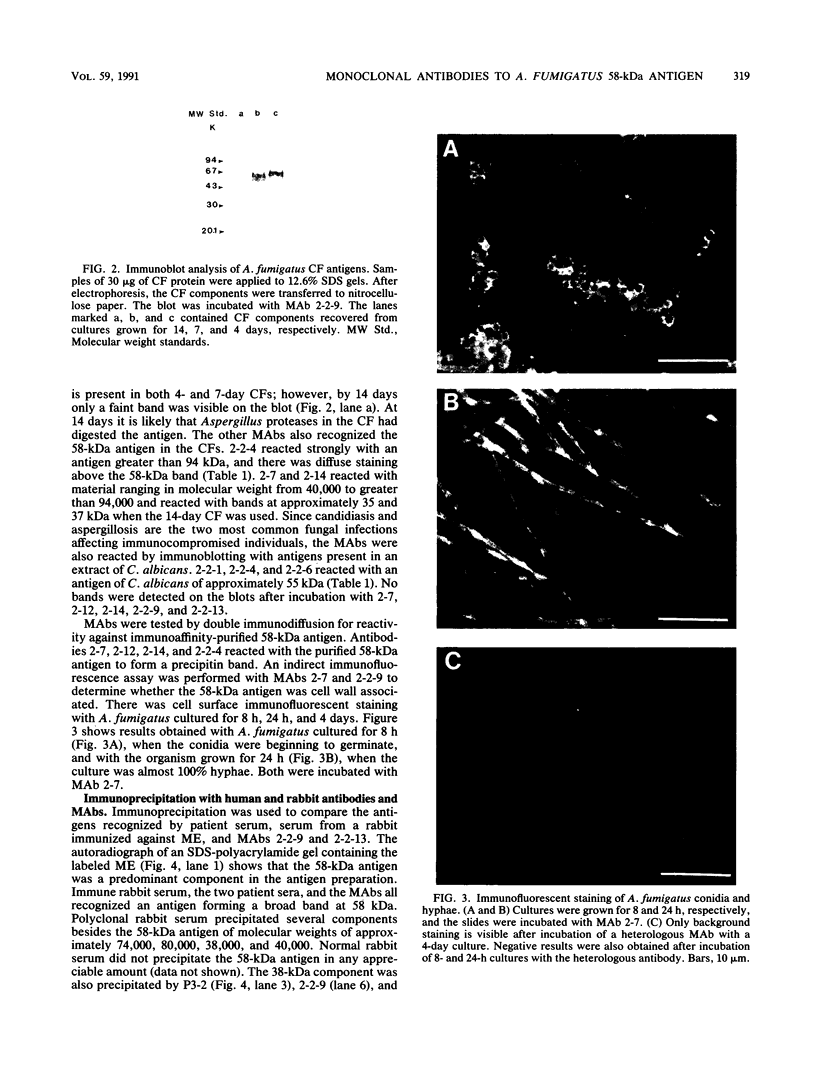
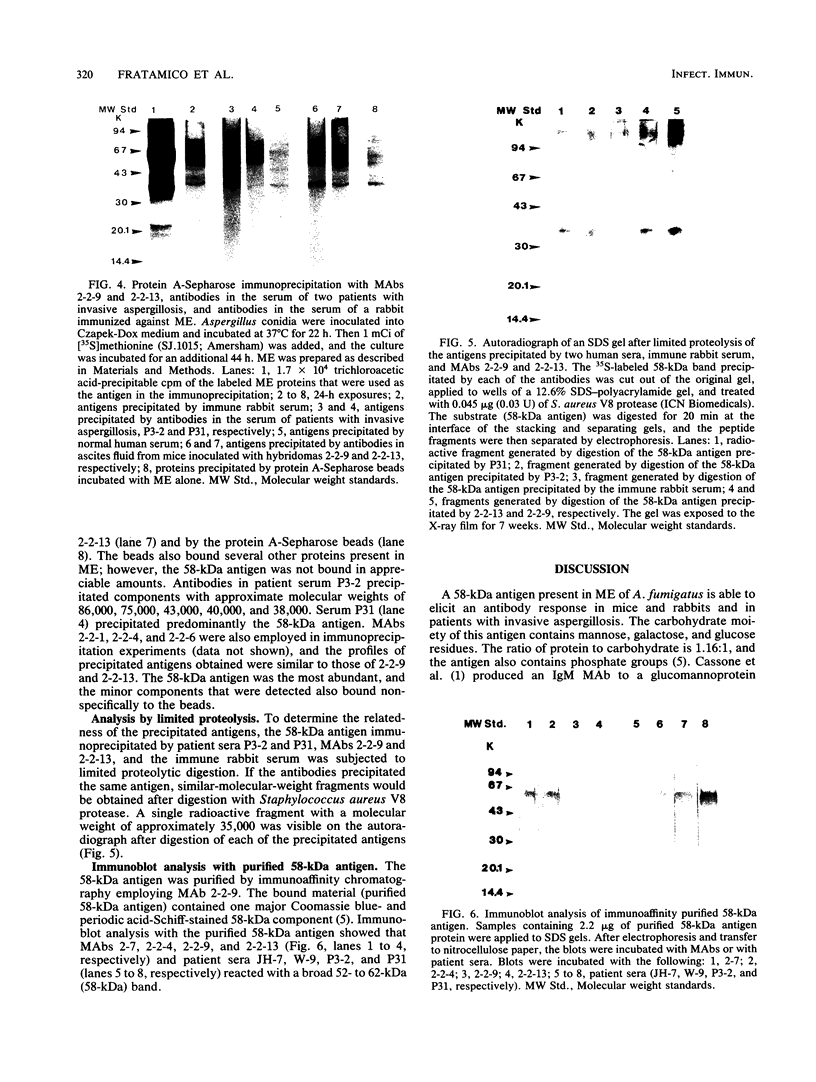
Eight monoclonal antibodies that recognize a serodiagnostically important 58-kDa antigen of Aspergillus fumigatus were produced and partially characterized. 2-7, 2-12, and 2-14 are of the immunoglobulin M class, and 2-2-1, 2-2-4, 2-2-6, 2-2-9, and 2-2-13 are all immunoglobulin G1(kappa) antibodies. Immunoblot analysis with A. fumigatus mycelial extract demonstrated that all of the monoclonal antibodies recognize a major 58-kDa antigen. The antigen was also detected by immunoblot analysis of 4- and 7-day culture filtrate preparations. 2-2-1, 2-2-4, and 2-2-6 cross-reacted with an antigen of approximately 55 kDa from an extract of Candida albicans. 2-7, 2-12, 2-14, and 2-2-4 formed a precipitin band with immunoaffinity-purified 58-kDa antigen by immunodiffusion. Results from indirect immunofluorescence assays with 2-7 and 2-2-9 showed fluorescent staining mainly on the surfaces of conidia and hyphae, indicating that the 58-kDa antigen may be cell wall associated. 2-2-9 and 2-2-13 and antibodies in patient and immune rabbit sera precipitated the [35S]methionine-labeled 58-kDa antigen. The 58-kDa antigen immunoprecipitated by each of the antibodies was enzymatically cleaved by Staphylococcus aureus V8 protease; one cleavage product, a 35-kDa fragment, was generated, indicating that the precipitated antigens share primary structure. Immunoblot analysis with an immunoaffinity-purified 58-kDa antigen showed that sera from patients with invasive aspergillosis reacted with the same antigen as that recognized by the monoclonal antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassone A., Torosantucci A., Boccanera M., Pellegrini G., Palma C., Malavasi F. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J Med Microbiol. 1988 Dec;27(4):233–238. doi: 10.1099/00222615-27-4-233. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Elder B. L., Fives-Taylor P. Characterization of monoclonal antibodies specific for adhesion: isolation of an adhesin of Streptococcus sanguis FW213. Infect Immun. 1986 Nov;54(2):421–427. doi: 10.1128/iai.54.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratamico P. M., Buckley H. R. Identification and characterization of an immunodominant 58-kilodalton antigen of Aspergillus fumigatus recognized by sera of patients with invasive aspergillosis. Infect Immun. 1991 Jan;59(1):309–315. doi: 10.1128/iai.59.1.309-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Ingemarson R., Lankinen H. The herpes simplex virus type 1 ribonucleotide reductase is a tight complex of the type alpha 2 beta 2 composed of 40K and 140K proteins, of which the latter shows multiple forms due to proteolysis. Virology. 1987 Feb;156(2):417–422. doi: 10.1016/0042-6822(87)90422-3. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koga J., Chatterjee S., Whitley R. J. Studies on herpes simplex virus type 1 glycoproteins using monoclonal antibodies. Virology. 1986 Jun;151(2):385–389. doi: 10.1016/0042-6822(86)90059-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Phillips P., Weiner M. H. Invasive aspergillosis diagnosed by immunohistochemistry with monoclonal and polyclonal reagents. Hum Pathol. 1987 Oct;18(10):1015–1024. doi: 10.1016/s0046-8177(87)80218-6. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Largen M. T., Buckley H. R. Production and characterization of three monoclonal antibodies to Candida albicans proteins. Infect Immun. 1984 Mar;43(3):1012–1018. doi: 10.1128/iai.43.3.1012-1018.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. V., De Magaldi S. W., Hearn V. M. Preparative isoelectric focusing of immunologically reactive components of Aspergillus fumigatus mycelium. J Gen Microbiol. 1984 Apr;130(4):919–925. doi: 10.1099/00221287-130-4-919. [DOI] [PubMed] [Google Scholar]
- de Wit M. Y., Klatser P. R. Purification and characterization of a 36 kDa antigen of Mycobacterium leprae. J Gen Microbiol. 1988 Jun;134(6):1541–1548. doi: 10.1099/00221287-134-6-1541. [DOI] [PubMed] [Google Scholar]