Abstract
Objective
To investigate the association between muscle‐specific creatine kinase (CKMM) gene polymorphism and the effects of endurance training on running economy.
Methods
102 biologically unrelated male volunteers from northern China performed a 5000‐m running programme, with an intensity of 95–105% ventilatory threshold. The protocol was undertaken three times per week and lasted for 18 weeks. Running economy indexes were determined by making the participants run on a treadmill before and after the protocol, and the A/G polymorphism in the 3′ untranslated region of CKMM was detected by polymerase chain reaction‐restricted fragment length polymorphism (NcoI restriction enzyme).
Results
Three expected genotypes for CKMM‐NcoI (AA, AG and GG) were observed in the participants. After training, all running economy indexes declined markedly. Change in steady‐state consumption of oxygen, change in steady‐state consumption of oxygen by mean body weight, change in steady‐state consumption of oxygen by mean lean body weight and change in ventilatory volume in AG groups were larger than those in AA and GG groups.
Conclusions
The findings indicate that the CKMM gene polymorphism may contribute to individual running economy responses to endurance training.
The muscle‐specific creatine kinase (CKMM) enzyme is bound specifically to the M line of the myofibril subfragment,1 one of the heavy meromyosin in the vicinity of the myosin ATPase and to the outer membrane and vesicles of the sarcoplasmic reticulum,2 which might change the Ca2+ uptake3 and power of muscle. Type I (slow‐twitch) skeletal muscle fibres and type II (fast‐twitch) fibres have been shown to differ in their CKMM activities, with type I fibres showing at least a twofold lower CKMM activity.4 Skeletal muscles of athletes involved in endurance sports are characterised by a high proportion of type I fibres, as well as by high activity levels of marker enzymes of aerobic oxidative metabolism.5 Therefore, the lower activity of CKMM might be essential to endurance athletes.
Running economy is typically defined as the energy demand for a given velocity of submaximal running, and is determined by measuring the steady‐state consumption of oxygen (VO2) and the respiratory exchange ratio.6 Taking body mass into consideration, runners with good running economy use less energy and therefore less oxygen than runners with poor running economy at the same velocity.7 There is a strong association between running economy and distance running performance.8,9,10
The CKMM gene has been mapped to the q13.2–q13.3 region of chromosome 19.11 Now, several lines of evidence suggest that the CKMM‐NcoI polymorphism in the 3′ untranslated region might contribute to the individual differences in VO2max responses to endurance training.12,13 Lucia et al,14 however, detected CKMM‐NcoI polymorphisms in 50 top‐level Spanish professional cyclists and 119 sedentary controls and found no significant differences for CKMM‐NcoI polymorphisms between athletes and controls. Recently, we found that the CKMM‐Nco polymorphism in the 3′ untranslated region was A/G variant by sequencing, and there were considerable differences in the allelic frequencies and genotypic frequencies between the Chinese Han population and those in Europe and America.15 Moreover, less is known about the association between CKMM‐NcoI polymorphism and individual running economy responses to endurance training. The purpose of this study, therefore, was to explore the potential relationship between the CKMM gene A/G polymorphism in Chinese and running economy responses to an 18‐week 5000‐m training programme.
Participants and methods
Participants
The sample consisted of 102 biologically unrelated healthy male volunteers from northern China, who had no experience of endurance training. The mean (standard deviation (SD) age was 18.8 (0.9) years, the height was 171.8 (5.8) cm and the weight was 60.2 (6.6) kg. Written informed consent was obtained from each participant.
Training protocol
The participants underwent a distance running programme, which consisted of 5000 m running three times per week lasting for 18 weeks. Heart rate sporttesters (S810, Finland) were used to maintain constant training heart rates corresponding to the ventilatory threshold. During the initial 10 weeks, the participants were trained at a heart rate associated with an intensity of 95% ventilatory threshold. Then they were trained at a heart rate associated with an intensity of 105% ventilatory threshold during 11–18 weeks. The participants had not experienced any endurance training other than this.
Measurement of running economy and ventilatory threshold
Before and after the training programme, running economy and ventilatory threshold were measured with an automated metabolic measurement cart (Erich Jaeger, Hochberg, Germany). The protocol was that the participants ran at a speed of 7.5 km/h (grade 0%) for 2 min, after warming up for 5 min on the treadmill. Then the load increased gradually to 10 km/h for 2 min and 12 km/h for 5 min. After that, the speed increased by 0.5 km/h and 2% grade every minute until volitional exhaustion was reached. The stable VO2 during the 5‐min run at the speed of 12 km/h was used for running economy (VO2RE). The minute ventilatory volume (VE), oxygen uptake (VO2) and carbon dioxide volume output (VCO2) were used to determine the ventilatory threshold during running, when the ventilatory volume, VCO2 and VE/VO2 appeared as the ventilatory breakpoint16 (Δ = ((after training−before training)/before training)×100%).
Genotype analysis
DNA was extracted from blood cells by a protocol recommended by the kit manufacturer (Promega, Madison, Wisconsin, USA). Primer pairs for polymerase chain reaction (PCR)‐restricted fragment length polymorphism of the CKMM gene polymorphism were designed by Primer V.5.0 software: forward: 5′‐GGG ATG CTC AGA CTC ACA GA‐3′; reverse: 5′‐AAC TTG AAT TTA GCC CAA CG‐3′.
The amplification protocol was (1) one cycle of denaturation at 94°C for 5 min; (2) 30 cycles of denaturation at 94°C for 40 s, annealing at 50°C for 40 s and extension at 72°C for 60 s; and (3) one final elongation cycle at 72°C for 10 min. Preventive contamination measures were taken by the inclusion of PCR mixture without DNA (negative control). After each amplification, the PCR product was digested by 8 U restriction enzyme NcoI (Takara, Kyoto, Japan) for 5 h at 37° C. The allele without the NcoI restriction site was designated as 359 bp, whereas the allele with the NcoI site was designated as 206+153 bp. The resulting fragments were separated by horizontal electrophoresis on 2% agarose gels. Each gel was run for 40 min at 100 V, stained with ethidium bromide and photographed under ultraviolet light. The PUC19 DNA/MspI (HpaII) Marker 23 (MBI Fermentas, Amherst, New York, USA) was used as a length marker to estimate the size of the digested DNA fragments.
Statistical analysis
Pearson's χ2 was used to determine whether the observed genotype frequencies were in the Hardy–Weinberg equilibrium. The paired t test was used to examine the differences in variables before and after training. Differences in variables between the three genotypes were tested by one‐way analysis of variance and retrospective multiple comparisons. These tests were performed with SPSS software version 11.5 for Windows. p Values <0.05 were considered significant. The results are presented as mean (standard error (SE)).
Results
The three expected genotypes for CKMM‐NcoI (AA, AG and GG) were obtained (fig 1). The observed genotypic frequencies of CKMM‐NcoI were 72% (AA), 26% (AG) and 2% (GG), in agreement (p>0.05) with those expected under the Hardy–Weinberg equilibrium.
Figure 1 The polymerase chain reaction‐restricted fragment length polymorphism products of muscle‐specific creatine kinase (CKMM) gene polymorphism. Marker is PUC19 DNA/MspI (HpaII) Marker 23; lanes 3–5, AA; lanes 2, 7–9, AG; lanes 1, 6, GG.
Table 1 shows that all running economy variables decreased significantly after training.
Table 1 Changes in running economy variables after training.
| RE variables | Unit | Before training | After training |
|---|---|---|---|
| VO2RE | ml/min | 2689.45 (26.19) | 2477.11 (25.07)* |
| VO2RE/w | ml/kg/min | 44.86 (0.34) | 39.57 (0.27)* |
| VO2RE/lbw | ml/kg/min | 72.12 (0.53) | 66.03 (0.45)* |
| HRRE | beats/min | 168.38 (1.00) | 163.36 (1.12)* |
| VERE | l/min | 75.97 (1.00) | 64.47 (0.81)* |
| VCO2RE | ml/min | 2611.67 (29.04) | 2345.18 (24.21)* |
Values are mean (SE).
*Significant difference compared with that before training in the same genotype group.
HR, heart rate; RE, response economy; lbw, mean lean body weight; VCO2 carbon dioxide volume output; VE, minute ventilatory volume; VO2, oxygen uptake; w, mean body weight. The subscript RE is when RE was determined.
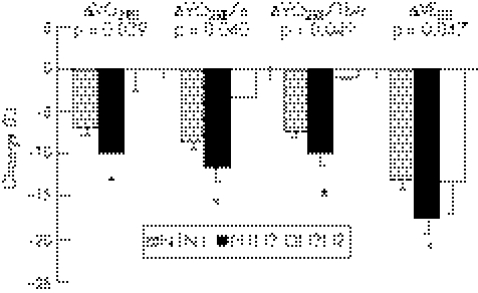
Table 2 summarises the association between the CKMM‐NcoI genotype and running economy variables. No significant differences were found in baseline running economy variables among the three genotypes before training, but the ΔVO2RE, ΔVO2RE/w, ΔVO2RE/lbw and ΔVERE in AG all decreased more significantly than those in AA and GG after training.
Table 2 Running economy variables in three genotypes before and after training.
| p Values | p1 Values | p2 Values | p3 Values | ||||
|---|---|---|---|---|---|---|---|
| AA | AG | GG | |||||
| (AA/AG) | (AA1/GG) | (AG/GG) | |||||
| Number | 73 | 27 | 2 | ||||
| Before training VO2RE | 2693 (31) | 2551 (49) | 2685 (244) | NS | NS | NS | NS |
| VO2RE/w | 44.52 (0.43) | 45.56 (0.59) | 44.73 (1.14) | NS | NS | NS | NS |
| VO2RE/lbw | 71.76 (0.65) | 72.59 (0.84) | 65.61 (2.23) | NS | NS | NS | NS |
| VERE | 75.14 (1.26) | 78.1 (1.74) | 71.35 (1.85) | NS | NS | NS | NS |
| VCO2RE | 2604 (35) | 2613 (54) | 2530 (330) | NS | NS | NS | NS |
| ΔVO2RE | −6.92 (0.66) | −9.82 (1.43)* | 0.01 (2.60) | <0.05 | <0.05 | NS | <0.05 |
| ΔVO2RE/w | −8.71 (0.65) | −11.67 (1.41)* | −3.42 (0.00) | <0.05 | <0.05 | NS | NS |
| ΔVO2RE/lbw | −7.40 (0.68) | −9.98 (1.38)* | −0.95 (0.25) | <0.05 | NS | NS | <0.05 |
| ΔVERE | −12.99 (1.22) | −17.64 (1.84)* | −13.55 (3.55) | NS | <0.05 | NS | NS |
| ΔVCO2RE | −9.16 (0.84) | −11.41 (1.59) | −4.25 (2.75) | NS | NS | NS | NS |
*Running economy variables in AG decreased more significantly than those in AA or GG after training.
Values are mean (SE) for variables.
lbw, mean lean body weight; RE, running economy; VCO2, carbon dioxide volume output; VE, minute ventilatory volume; VO2, oxygen uptake; w, mean body weight.
Discussion
This study was the first to investigate the association between CKMM gene polymorphism and the effects of endurance training on running economy, and showed that all running economy variables decreased significantly after training compared with before training, hence running economy was improved. The most important finding of this study was the significant association between the polymorphism in the 3′ untranslated region of the CKMM gene and running economy response to endurance training. Heterozygotes with the AG genotype showed larger ΔVO2RE, ΔVO2RE/w, ΔVO2RE/lbw and ΔVERE than those with the other two genotypes (fig 2).
Figure 2 Changes in running economy variables in three genotypes after training. *Running economy variables in AG decreased more significantly than those in AA or GG after training. ΔVO2RE, change in steady‐state consumption of oxygen; ΔVO2RE/w, change in steady state consumption of oxygen by mean body weight; ΔVO2RE/lbw, change in steady state consumption of oxygen by mean lean body weight; ΔVERE, change in ventilatory volume.
Previous research has indicated that the A/G polymorphism in the 3′ untranslated region of the CKMM gene was associated with △VO2max to endurance training. Rivera et al12 analysed the CKMM gene A/G polymorphism among Caucasian participants from the heritage family study (160 parents and 80 adult offspring), and found significant association between the CKMM gene polymorphism and VO2max in the sedentary state, as well as its response (△VO2max) to a standardized 20‐week endurance training programme. Heterozygotes with the AG genotype showed a larger △VO2max than those with the GG genotype. After that a significant linkage was found between CKMM gene polymorphism and △VO2max in response to 20 weeks of endurance training in 277 full sib pairs from 98 nuclear families of the heritage family study.13
The strong relationship between response economy and distance running performance has been shown by many independent reports.8,9,10 Conley and Krahenbuhl8 showed that response economy was a good predictor of performance in runners of comparable ability. In their study, 12 highly trained male distance runners (VO2max ∼72 ml/kg/min and 10 km performance ∼32 min) were tested 3–6 days after they had competed in a 10‐km race. There were significant correlations between submaximal VO2 and performance at running speeds of 14, 16 and 18 km/h. About 65% of the variation in race performance could be attributed to differences in response economy, with the more economical runners performing the best. The more economical runners were able to run at a lower percentage of their VO2max, resulting in lower blood lacate concentration at a given speed. The lower blood lacate concentration is closely associated with the pace a runner is able to maintain for races >15 min.17 Several studies reported that endurance training improves response economy in untrained and moderately trained subjects, with trained runners having a better response economy than their untrained or less trained counterparts.18,19,20
What is already known on this topic
The muscle‐specific creatine kinase‐NcoI polymorphism may contribute to the individual differences in maximal oxygen uptake responses to endurance training.
There are considerable differences in the allelic frequencies and genotypic frequencies between the Chinese Han population and those in Europe and America.
What this study adds
This study is the first to explore the potential relationship between the muscle‐specific creatine kinase‐NcoI polymorphism in the Chinese and response economy responses to an endurance training programme.
The genotypic effects underlying individual differences in trainability may arise from DNA sequence polymorphisms that translate into gene product polymorphism or variation in gene expression. The CKMM gene polymorphism examined in this study lies in the 3′ untranslated region,21 which influences the intracellular localisation of its mRNA, and may affect its stability and the rate of transcription, leading to differences in its expression.22 Also, endurance performance is influenced by type I fibres, which are known for their high level of oxidative enzyme activity, along with a low CKMM activity.5 In addition, research on transgenic mice indicates an improved endurance performance in CKMM knock‐out mice compared with that seen in control animals.23 Thus, there was some indirect support for the hypothesis that CKMM activity plays a part in limiting endurance performance and its responsiveness to endurance training.
The homozygote for GG was the less often observed (2%) homozygote, which was different from the Europeans or the Americans (p<0.05), but was little different from the Japanese (p<0.05). We found racial and regional differences in the A/G polymorphism in the 3′ untranslated region of the CKMM gene between Asian and other ethnic groups.15
Conclusion
The findings show that the A/G polymorphism in the CKMM gene might contribute to individual responses to endurance training. VO2RE, VO2RE/w, VO2RE/lbw and VERE declined more significantly in participants with the AG genotype compared with those with AA or GG after endurance training.
Abbreviations
CKMM - muscle‐specific creatine kinase
PCR - polymerase chain reaction
Footnotes
Funding: This work was supported by Department of Science and Technology of China.
Competing interests: None declared.
References
- 1.Turner D C, Wallimann T, Eppenberger H M. A protein that binds specifically to the M‐line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci USA 197370702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korge P, Camphcll K B. Local ATP regeneration is important for sarcoplasmic rcticulum Ca2+‐pump function. Am J Physiol 1994267(Pt1)C357–C366. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A M, Eppenberger H M, Volpe P.et al Muscle‐type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem 19902655258–5266. [PubMed] [Google Scholar]
- 4.Yamashita K, Yoshioka T. Profiles of creatine kinase isoenzyme compositions in single muscle fibers of different types. J Muscle Res Cell Motil 19911237–44. [DOI] [PubMed] [Google Scholar]
- 5.Holloszy J O, Coyle E F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 198456831–838. [DOI] [PubMed] [Google Scholar]
- 6.Saunders P U, Pyne D B, Telford R D.et al Factors affecting running economy in trained distance runners. Sports Med 200434465–485. [DOI] [PubMed] [Google Scholar]
- 7.Thomas D Q, Fernhall B, Grant H. Changes in running economy during a 5 km run in trained men and women runners. J Strength Cond Res 199913162–167. [Google Scholar]
- 8.Conley D L, Krahenbuhl G S. Running economy and distance running performance of highly trained athletes. Med Sci Sports Exerc 198012357–360. [PubMed] [Google Scholar]
- 9.Jones A M, Carter H. The effect of endurance training on parameters of aerobic fitness. Sports Med 200029373–386. [DOI] [PubMed] [Google Scholar]
- 10.Conley D L, Krahenbuhl G S, Burkett L N.et al Physiological correlates of female road racing performance. Res Q Exerc Sport 198152441–448. [DOI] [PubMed] [Google Scholar]
- 11.Nigro J M, Schweinfest C W, Rajkovic A.et al cDNA cloning and mapping of the human creatine kinase M gene to 19q13. Am J Hum Genet 198740115–125. [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera M A, Dionne F T, Simoneau J A.et al Muscle‐specific creatine kinase gene polymorphism and VO2max in the Heritage Family Study. Med Sci Sports Exerc 1997291311–1317. [DOI] [PubMed] [Google Scholar]
- 13.Rivera M A, Perusse L, Simoneau J A.et al Linkage between a muscle‐specific CK gene marker and VO2max in the Heritage Family Study. Med Sci Sports Exerc 199931698–701. [DOI] [PubMed] [Google Scholar]
- 14.Lucia A, Gallego G F, Chicharro J L.et al Is there an association between ACE and CKMM polymorphisms and cycling performance status during 3‐week races? Int J Sports Med 200526442–447. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D Q, Hu Y, Liu G.et al An A/G polymorphism in muscle‐specific creatine kinase gene in Han population in northern China. Yi Chuan 200527535–538. [PubMed] [Google Scholar]
- 16.Caiozzo V J, Davis J A, Ellis J F.et al A comparison of gas exchange indices used to detect the anaerobic threshold. J Appl Physiol 1982531184–1189. [DOI] [PubMed] [Google Scholar]
- 17.Farrell P A, Wilmore J H, Coyle E F. Plasma lactate accumulation and distance running performance. Med Sci Sports Exerc 1993251091–1097. [DOI] [PubMed] [Google Scholar]
- 18.Daniels J T. A physiologist's view of running economy. Med Sci Sports Exerc 198517332–338. [PubMed] [Google Scholar]
- 19.Dolgener F. Oxygen cost of walking and running in untrained, sprint trained, and endurance trained females. J Sports Med Phys Fitness 19822260–65. [PubMed] [Google Scholar]
- 20.Krahenbuhl G S, Pangrazi R P. Characteristics associated with running performance in young boys. Med Sci Sports Exerc 198315486–490. [PubMed] [Google Scholar]
- 21.Coerwinkel D M, Schepens M J, Van P Z.et al NcoI RFLP at the creatine kinase‐muscle type gene locus (CKMM, chromosome 19). Nucleic Acids Res 1988168743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson I A, Brindle K M, Fulton A M. Differential localization of the mRNA of the M and B isoforms of creatine kinase in myoblasts. Biochem J 1995308(Pt 2)599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van D J, Heerschap A, Oerlemans F. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 199374621–631. [DOI] [PubMed] [Google Scholar]