Abstract
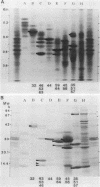
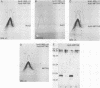
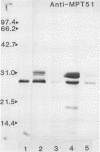
Five actively secreted proteins (MPT32, MPT45, MPT51, MPT53, and MPT63) and the MPT46 protein were purified to homogeneity from Mycobacterium tuberculosis culture fluid and compared with proteins previously purified by ourselves and other investigators. Antisera were obtained by immunization of rabbits with all of the newly isolated proteins identified to be immunogenic. Two-dimensional electrophoresis of culture fluids obtained each week for 2 to 10 weeks of culturing of M. tuberculosis revealed characteristic changes, permitting identification of two distinct groups of proteins being actively secreted from the mycobacterial cells or appearing later in the culture fluids as a result of the release of soluble proteins from the cytosol after lysis of bacteria. The N-terminal amino acid sequences of five MPTs were shown to be identical to those of proteins previously isolated by other investigators and given different designations, and five new sequences are given. These sequences and the use of the antisera may serve to identify these proteins with mycobacterial constituents isolated by other investigators. The previously identified but not isolated MPT45 protein was shown to correspond to the C component of the antigen 85 complex. The 27-kDa MPT51 protein was demonstrated to cross-react with the three components of the antigen 85 complex, and the N-terminal amino acid sequences of MPT51 and MPT59 showed 60% homology. This finding and the extensive cross-reactivity between the components of the antigen 85 complex may indicate that there is a family of closely related secreted proteins in mycobacteria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. A major antigen from Mycobacterium tuberculosis which is homologous to the heat shock proteins groES from E. coli and the htpA gene product of Coxiella burneti. Nucleic Acids Res. 1988 Sep 26;16(18):9047–9047. doi: 10.1093/nar/16.18.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Ferguson L. E. Purification and Characterization of Two Proteins from Culture Filtrates of Mycobacterium tuberculosis H(37)Ra Strain. Infect Immun. 1970 Feb;1(2):164–168. doi: 10.1128/iai.1.2.164-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Fifis T., Plackett P., Corner L. A., Wood P. R. Purification of a major Mycobacterium bovis antigen for the diagnosis of bovine tuberculosis. Scand J Immunol. 1989 Jan;29(1):91–101. doi: 10.1111/j.1365-3083.1989.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Hirai T., Uchida T., Yoneda M. Extracellular proteins of tubercle bacilli. IV. Alpha and beta antigens as major extracellular protein products and as cellular components of a strain (H37Rv) of Mycobacterium tuberculosis. Biken J. 1965 Dec;8(4):189–199. [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjorvatn B., Kronvall G., Axelsen N. H. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect Immun. 1977 Dec;18(3):792–805. doi: 10.1128/iai.18.3.792-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984 Mar;129(3):444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- Harboe M., Nagai S., Patarroyo M. E., Torres M. L., Ramirez C., Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986 Apr;52(1):293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Wiker H. G., Duncan J. R., Garcia M. M., Dukes T. W., Brooks B. W., Turcotte C., Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990 May;28(5):913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasløv K., Andersen A. B., Ljungqvist L., Weis Bentzon M. Comparison of the immunological activity of five defined antigens from Mycobacterium tuberculosis in seven inbred guinea pig strains. The 38-kDa antigen is immunodominant. Scand J Immunol. 1990 Apr;31(4):503–514. doi: 10.1111/j.1365-3083.1990.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Chase M. W. Antibodies to mycobacteria in human tuberculosis. II. Response to nine defined mycobacterial antigens with evidence for an antibody common to tuberculosis and lepromatous leprosy. J Infect Dis. 1980 Dec;142(6):835–843. doi: 10.1093/infdis/142.6.835. [DOI] [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nagai S., Matsumoto J., Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981 Mar;31(3):1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Nagasuga T., Matsumoto J., Koda K. Isolation of tuberculin skin test reactive proteins from heated culture filtrate of Mycobacterium tuberculosis H37Rv. Am Rev Respir Dis. 1974 Jan;109(1):17–28. doi: 10.1164/arrd.1974.109.1.17. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Plikaytis B. B., Hyche A. D., Van Landingham R. M., Walker L. L. The Mycobacterium tuberculosis BCG-a protein has homology with the Escherichia coli GroES protein. Nucleic Acids Res. 1989 Feb 11;17(3):1254–1254. doi: 10.1093/nar/17.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka K., Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Yamada T. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FEMS Microbiol Lett. 1989 Apr;49(2-3):273–276. doi: 10.1016/0378-1097(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turneer M., Van Vooren J. P., De Bruyn J., Serruys E., Dierckx P., Yernault J. C. Humoral immune response in human tuberculosis: immunoglobulins G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J Clin Microbiol. 1988 Sep;26(9):1714–1719. doi: 10.1128/jcm.26.9.1714-1719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Bennedsen J., Closs O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis. Comparison with a reference system for Mycobacterium bovis, BCG. Scand J Immunol. 1988 Feb;27(2):223–239. doi: 10.1111/j.1365-3083.1988.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Lea T. E. Purification and characterization of two protein antigens from the heterogeneous BCG85 complex in Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):298–306. doi: 10.1159/000234153. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Sletten K., Nagai S., Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990 Jan;58(1):272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Abe C., Nagai S., Terasaka K., Yamada T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect Immun. 1989 Jan;57(1):283–288. doi: 10.1128/iai.57.1.283-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Terasaka K., Yamada T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FEBS Lett. 1988 Nov 21;240(1-2):115–117. doi: 10.1016/0014-5793(88)80350-8. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]