Abstract
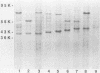
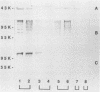
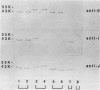
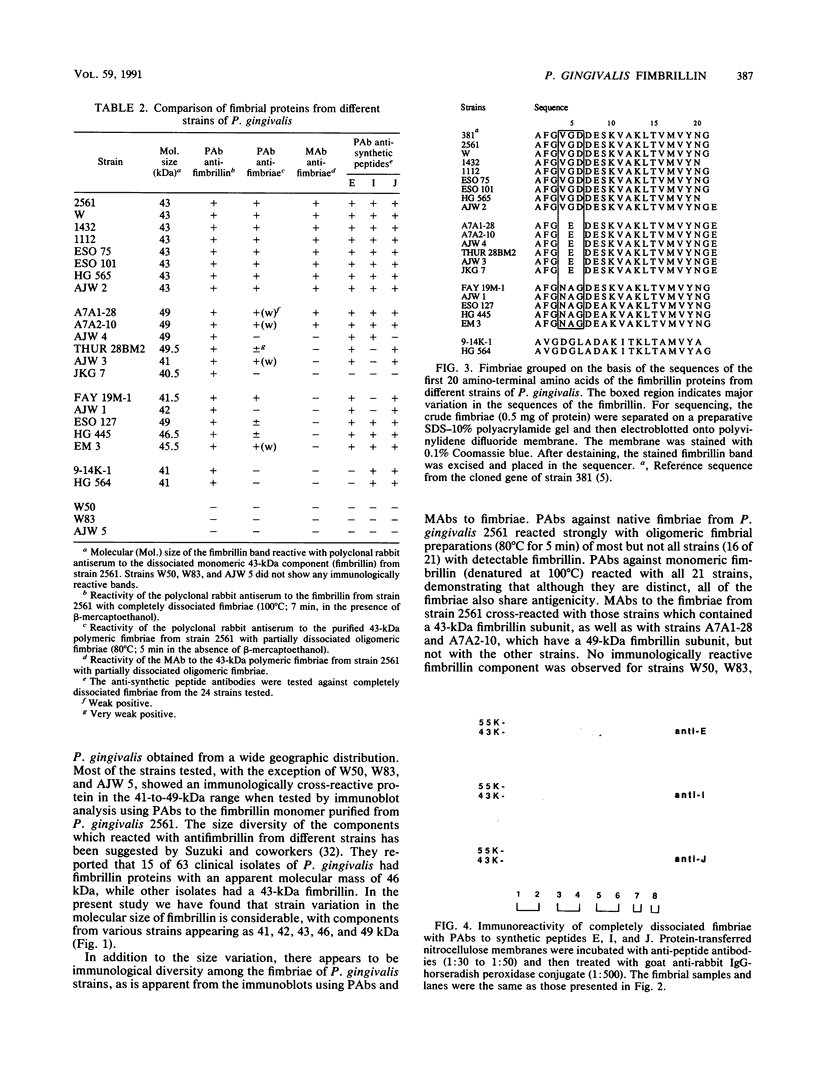
Bacterial fimbriae mediate cell adhesion and are important in colonization. Fimbrial proteins from strains of Porphyromonas (Bacteroides) gingivalis isolated from different individuals were compared for their size, amino-terminal sequence, and antigenic diversity. Two major protein components of the crude fimbrial preparations differed in apparent molecular mass, ranging from 41 to 49 kDa for the fimbrillin monomer and from 61 to 78 kDa for the other major protein. The amino-terminal sequence of the antigenically related group of proteins of the fimbrillin monomer in the 41- to 49-kDa range showed significant homology; however, minor sequence heterogeneity was observed, mainly in residues 4 to 6. One of the observed amino-terminal sequences, AFGVGDDESKVAKLTVMVYNG, resembled the deduced sequence of P. gingivalis 381 (D.P. Dickinson, M. K. Kubiniec, F. Yoshimura, and R.J. Genco, J. Bacteriol. 170:1658-1665, 1988). Fimbriae from all the strains of P. gingivalis showing this sequence contained a fimbrillin monomer of 43 kDa and showed a strong reaction with both polyclonal and monoclonal antibodies directed to the fimbriae from P. gingivalis 2561 (381). Fimbriae from strains showing amino-terminal sequence variations in residues 4 to 6 (i.e., substitution of VGD with either E or NAG) were more diverse in their molecular sizes. Most of these variant fimbriae showed weak reactions with the polyclonal antibodies and no reaction with the monoclonal antibodies induced to the fimbriae of strain 2561. No correlation could be established between the molecular size and immunological reactivity of the fimbrillin monomer of P. gingivalis strains. Strains 9-14K-1 and HG 564 not only showed markedly different sequences from the other three amino-terminal sequences but also did not react with either polyclonal or monoclonal antibodies to the fimbriae of strain 2561. Strains W50, W83, and AJW 5 failed to show any immunological reactivity with the antibodies to fimbrillin or fimbriae of strain 2561. Fimbriae from different strains revealed different immunologic reactions with rabbit antisera to each of the synthetic peptides of residues 59-78 (peptide I), 79-100 (peptide J), and 91-108 (peptide E) of strain 381. These results suggest that P. gingivalis fimbrillin subunits have size, sequence, and antigenic heterogeneity among the strains and that these differences may be important in the function and immune reactivities of the fimbriae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bedi G. S., Back N. Production and characterization of monoclonal antibodies to rat plasma kininogen. Hybridoma. 1987 Oct;6(5):521–526. doi: 10.1089/hyb.1987.6.521. [DOI] [PubMed] [Google Scholar]
- Brook I., Gober A. E. Bacteroides melaninogenicus. Its recovery from tonsils of children with acute tonsillitis. Arch Otolaryngol. 1983 Dec;109(12):818–820. doi: 10.1001/archotol.1983.00800260040010. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Haapasalo M., Ranta H., Ranta K., Shah H. Black-pigmented Bacteroides spp. in human apical periodontitis. Infect Immun. 1986 Jul;53(1):149–153. doi: 10.1128/iai.53.1.149-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. S., Tipler L. S. An electron microscope survey of the surface structures and hydrophobicity of oral and non-oral species of the bacterial genus Bacteroides. Arch Oral Biol. 1986;31(5):325–335. doi: 10.1016/0003-9969(86)90047-6. [DOI] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Alexander B., McGowan B. Purification, characterization, and serologic characteristics of Bacteroides nodosus pili and use of a purified pili vaccine in sheep. Am J Vet Res. 1983 Sep;44(9):1676–1681. [PubMed] [Google Scholar]
- Loos B. G., Mayrand D., Genco R. J., Dickinson D. P. Genetic heterogeneity of Porphyromonas (Bacteroides) gingivalis by genomic DNA fingerprinting. J Dent Res. 1990 Aug;69(8):1488–1493. doi: 10.1177/00220345900690080801. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Baskerville A., Dowsett A. B., Ellwood D. C., Marsh P. D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986 May;52(2):349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Haemagglutinating activity of Bacteroides melaninogenicus. Arch Oral Biol. 1974 May;19(5):415–416. doi: 10.1016/0003-9969(74)90184-8. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Wang L., Teng N. N., Schoolnik G. K. Antibodies to peptides corresponding to a conserved sequence of gonococcal pilins block bacterial adhesion. Proc Natl Acad Sci U S A. 1985 Feb;82(3):915–919. doi: 10.1073/pnas.82.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. A., O'Hanley P., Schoolnik G. K. Gal-Gal pyelonephritis Escherichia coli pili linear immunogenic and antigenic epitopes. J Exp Med. 1985 Apr 1;161(4):705–717. doi: 10.1084/jem.161.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Tai J. Y., Gotschlich E. C. A pilus peptide vaccine for the prevention of gonorrhea. Prog Allergy. 1983;33:314–331. [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Yoshimura F., Takahashi K., Tani H., Suzuki T. Detection of fimbriae and fimbrial antigens on the oral anaerobe Bacteroides gingivalis by negative staining and serological methods. J Gen Microbiol. 1988 Oct;134(10):2713–2720. doi: 10.1099/00221287-134-10-2713. [DOI] [PubMed] [Google Scholar]
- Woo D. D., Holt S. C., Leadbetter E. R. Ultrastructure of Bacteroides species: Bacteroides asaccharolyticus, Bacteroides fragilis, Bacteroides melaninogenicus subspecies melaninogenicus, and B. melaninogenicus subspecies intermedius. J Infect Dis. 1979 May;139(5):534–546. doi: 10.1093/infdis/139.5.534. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takasawa T., Yoneyama M., Yamaguchi T., Shiokawa H., Suzuki T. Fimbriae from the oral anaerobe Bacteroides gingivalis: physical, chemical, and immunological properties. J Bacteriol. 1985 Aug;163(2):730–734. doi: 10.1128/jb.163.2.730-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkelhoff A. J., Carlee A. W., de Graaff J. Bacteroides endodontalis and other black-pigmented Bacteroides species in odontogenic abscesses. Infect Immun. 1985 Sep;49(3):494–497. doi: 10.1128/iai.49.3.494-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]