Summary
Interest in development of therapeutics targeting brain neuropeptide systems for treatment of cocaine addiction (e.g., κ opioid agonists) is based on animal data showing interactions between the neuropeptides, brain dopamine, and cocaine. In this autopsied brain study, our major objective was to establish by radioimmunoassay whether levels of dynorphin and other neuropeptides (e.g. metenkephalin, neurotensin and substance P) are increased in the dopamine-rich caudate, putamen, and nucleus accumbens of human chronic cocaine users (n=12) vs. matched control subjects (n=17) as predicted by animal findings. Changes were limited to markedly increased dynorphin immunoreactivity in caudate (+92%), decreased caudate neurotensin (−49%), and a trend for increased dynorphin (+75%) in putamen. In other examined subcortical/cerebral cortical areas dynorphin levels were normal with the striking exception of the ventral pallidum (+346% ), whereas cerebral cortical metekephalin levels were generally decreased and neurotensin variably changed. Our finding that, in contradistinction to animal data, the other striatal neuropeptides were not increased in human cocaine users could be explained by differences in pattern and contingency between human drug users and the animal models. However, the human dynorphin observations parallel well animal findings and suggest that the dynorphin system is upregulated manifested as elevated neuropeptide levels after chronic drug exposure in striatum and ventral pallidum. Our postmortem brain data suggest involvement of striatal dynorphin systems in human cocaine users and should add to the interest in the testing of new dynorphin-related therapeutics for the treatment of cocaine addiction.
Keywords: dynorphin, human, cocaine, metenkephalin, neurotensin, drug abuse
1. Introduction
Because a primary action of cocaine (COC) and several other drugs of abuse is elevation of striatal (caudate and putamen) and limbic extracellular levels of the neurotransmitter dopamine (DA) in the human (Leyton, 2007), much effort has been devoted to develop drugs acting on the brain DA system that might help in treatment of COC addiction. However, this focus on DA has not yet led to a clinically useful therapeutic and the actual role of DA proposed for the transition from COC “liking” to “wanting“ or “craving” continues to be revised (Salamone et al., 2007).
A different, but related, pharmacological strategy involves development of therapeutics targeted to one or more brain neuropeptide pathways that interact with the brain DA system. The clinical impetus supporting this approach is derived from preliminary human studies showing that a non-selective opioid antagonist (naltrexone) can partially block some of the subjective effects of cocaine (Kosten et al., 1992; Kuzmin et al., 1997; Kiyatkin and Brown, 2003; Oslin et al., 1999; Schmitz et al, 2001). The argument in the experimental preclinical literature for neuropeptide involvement stems from animal findings showing that COC can act in a DA-dependent manner on basal ganglia- and limbic-related neurons containing the neuropeptides dynorphin (DYN), metenkephalin (MENK), neurotensin (NT), and substance P (SP). According to this argument, altered functioning of the neuropeptide systems (e.g. DYN) might have either a feedback effect on the originating DA system itself, or cause downstream changes in pathways creating clinically important functional outcomes (for review see Shippenberg et al., 2007).
In the preclinical studies, COC increased tissue levels of DYN (+70–101%; Smiley et al., 1990), NT (+100–200%; Hanson et al., 1989) and SP (+30%; Alburges et al., 2000) have been reported in striatum while similar increases in DYN (+150%) and NT (+85%; Hanson et al., 1989), but not SP (Alburges et al., 2000) have been reported for nucleus accumbens. Likewise, in similar studies, COC-induced activation of the neuropeptide pathways has been demonstrated by changes in the expression of their precursor mRNA (Adams et al., 2001; Fagergren et al., 2003; Hurd et al., 1992). Thus, based upon the aforementioned animal studies, it would be anticipated that the human neuropeptide systems linked to DA should be “altered” by COC. However, in our previous study of neuropeptides in striatum and nucleus accumbens of human chronic users of the psychostimulant METH (Frankel et al., 2007), we found that, with the exception of a trend for increased DYN in caudate, levels of the above-mentioned neuropeptides were not increased as observed in animal studies.
Thus, the major objective of the present report was to establish whether, as predicted by animal models, levels of DYN, MENK, NT and SP systems are increased in the DA-rich striatum and nucleus accumbens of human chronic COC users. A secondary exploratory objective was to extend this study to include measurement of the neuropeptides in DA-poor subcortical and cerebral cortical areas in which the function of the peptides appears to be more hypothetical.
2. Materials and Methods
2.1. Subjects and brain material
This study was approved by Institutional Review Board of the Centre for Addiction and Mental Health at Toronto. Postmortem brains from a total of 17 controls (15 males and 2 females) and 12 chronic users of COC (10 males and 2 females) were obtained from medical examiner offices in the United States and Canada using a standardized protocol. Drug histories, brain neuropathological findings and other subject information have been previously reported in detail (Wilson et al., 1996). Striatal levels of DA, the DA transporter, and the vesicular monoamine transporter also have been reported previously for all of the COC cases (Wilson et al., 1996). There were no statistical differences in age (control, 32.7 ± 1.7; COC, 35.9 ± 3.6 years) or postmortem intervals (PMI, interval between death and freezing of the brain; control, 13.6 ± 1.6 hours; COC 18.0 ± 1.9 hours) between the control and COC users (Student’s two-tailed t-tests). At autopsy, one half of each brain was fixed in formalin fixative for neuropathological analysis, whereas the other half was immediately frozen until dissection for neurochemical analysis. Blood samples were obtained from all of the COC users and the control subjects for drug screening. Scalp hair samples for drug analyses were obtained from 14 of 17 controls and 7 of 12 COC users. Levels of drugs of abuse in blood and other bodily fluids were measured by the local medical examiner whereas drug analyses in brain and hair samples were conducted at the Armed Forces Institute of Pathology (Washington, DC, USA). All COC users met the following selection criteria: (a) presence of COC and/or metabolites on toxicology screens in blood or urine, brain and, where available, hair; (b) absence of other drugs of abuse in bodily fluids with the exception of ethanol (three cases) and absence of other drugs of abuse in brain; (c) evidence from the case records of COC as the primary drug of abuse for at least 1 year prior to death; (d) absence of neurological illness or brain pathology unrelated to COC use (as determined at autopsy). Hair analysis of the 7 COC users for which hair was available also disclosed cocaethylene in five subjects (indicating some past use of alcohol) and, in one case, an opiate metabolite. All control subjects were neurologically normal and had no evidence of brain pathology on neuropathological examination. All control subjects tested negative for the presence of drugs of abuse (including COC and COC metabolites) in blood, urine and where available, hair (Wilson et al., 1996). The control subjects were selected so that most had experienced, like the drug users, a sudden death. The suspected or known causes of death of the controls were: drowning (n=1); leukemia (n=1); electrocution (n=1); accidental death/trauma (n=3); myocardial infarction (n=1); atherosclerotic cardiovascular disease (n=3); pulmonary embolism (n=2); valvular disease (n=1); hypertensive cardiovascular disease (n=1); massive cardiomegaly (n=1); compressional asphyxia (n=1); and slit throat (n=1). Details of the known or suspected causes of death of the COC users have also been previously reported (Wilson et al., 1996) and were COC intoxication (n = 7). multiple stab wounds to the chest (n = 1); gunshot wound to the chest (n =1); ruptured carotid artery aneurysm with COC abuse a contributing factor (n =1); hypertensive cardiac disease with metoprolol/diltiazem intoxication (n=1); and hypertensive cardiac disease with COC abuse a contributing factor (n = 1).
2.2. Measurement of Neuropeptides by Radioimmunoassay
The radioimmunoassays used to analyze the neuropeptide levels in this study were previously described (Frankel et al., 2007). Following tissue homogenization the samples were boiled, centrifuged, aliquoted and lyophilized overnight and stored at −80°C until the radioimmunoassay was performed (Frankel et al., 2007). The concentrations of neuropeptides were determined with a modified solid-phase radioimmunoassay technique described for NT by Maidment et al. (1991). Lyophilized samples were reconstituted in 300 µl phosphate-buffered saline (pH 7.4) containing 0.1% (w/v/) gelatin and 0.1% (v/v) Triton X-100. Nunc-Immunoplates (ISC BioExpress, Kaysville, UT) were incubated overnight at 4°C with 40 µl of protein G solution (50 ng/100 µl in 0.1 mol/l sodium bicarbonate; pH 9.0). After washing the wells three times with wash buffer (0.15 mol/l K2HPO4, 0.02 mol/l NaH2PO4, 0.2 mmol/l ascorbic acid, 0.2% (v/v) Tween-20 and 0.1% (w/v) sodium azide; pH 7.5), 25 µl of a highly selective antiserum for one of the following were diluted in assay buffer (same as wash buffer containing 0.1% (w/v) gelatin): DYN diluted to 1:10,000; MENK diluted to 1:1,000; NT diluted to 1:20,000; SP diluted to 1:200,000. Following addition of DYN or MENK antisera, wells were incubated overnight at room temperature. Wells for NT and SP assays were incubated with the respective antisera for 2h at room temperature in order to allow the attachment of antibody to the protein G-coated surface. After incubation, wells were washed three times and 25 µl of sample or standard(s) were added to each well and incubated for 2 h at room temperature. After incubation, 25 µl of the labeled peptide (125I-MENK, 125I-NT, or 125I-SP) diluted with assay buffer to approximately 6500 dpm per 25 µl, were added to the wells and incubated for 2 h at room temperature. Unlike the other labeled peptides, 125I-DYN was added immediately after the samples (Hanson et al., 1988). After incubation, wells were washed, separated and placed in 12X75-mm polypropylene tubes and counted in a five-channel Packard Cobra II Auto-Gamma counter (Packard Instrument Co., Meriden, CT). The total and nonspecific binding were defined by adding 25 µl of the labeled peptide to protein G-untreated and –treated wells, respectively. Quantities of neuropeptide immunoreactivity were determined by comparing bound to free [125I]-DYN or –MENK in each sample to a standard curve (from 8 to 1000 pg/assay tube). For the quantities of immunoreactivity for [125I] -NT and –SP, bound to free comparisons were made using a standard curve from 1 to 125 pg/assay tube. The reproducibility of the assay was evaluated using cerebellum tissue spiked with 62 and 250 pg of each peptide. This technique has been demonstrated to be very reproducible, resulting in less than 10% variability between assays and less than 5% between sample and standard duplicates (Hanson et al., 1997).
2.3. Antisera
The MENK antiserum employed in this study was raised by Zymed Laboratories (South San Francisco, CA) using the single-point, site-directed peptide conjugation, antipeptide technique (Posnett et al., 1988). This highly selective MENK antibody displayed less than 1% cross-reactivity with leuenkephalin or DYN and did not significantly cross-react with 1000-fold excess concentration of NT and has been used for other studies by this laboratory (Alburges et al., 2001). The DYN, NT and SP anstisera were raised in New Zealand White rabbits as previously described (Hanson et al., 1988). These antisera recognize the DYN, NT or the SP carboxy terminus and are highly selective, expressing no cross reactivity with 1000-fold excess concentrations of other endogenous neuropeptides such as DYN (for NT or SP antiserum), MENK, cholecystockinin, SP (for DYN or NT antiserum) or substance K.
2.4. Statistical Analyses
Correlations between neuropeptides and postmortem time for samples of controls and COC users were determined using the Pearson correlation test. Correlations between neuropeptides and brain drug levels of striatal dopamine levels in COC users were also determined using the Spearman rank correlation test. As this was an exploratory study only, differences in neuropeptide levels (DYN, MENK, NT, SP) between the control and the COC groups were analyzed using the Bonferroni t-test. The criterion for statistical significance for all comparisons was p < 0.05.
3. Results
3.1. Selection of brain areas
As described previously (Frankel et al., 2007) subcortical brain regions to be analyzed were dissected using the atlas of Riley (Riley, 1943) whereas cerebral cortical areas were identified using Brodmann classification. The portion of the nucleus accumbens taken for examination was that described in Figure 1 (slice #2, lateral) in Tong et al., 2006. The ventral pallidum, located just ventral to the external globus pallidus and the anterior commissure was excised as described in Heimer (2003, Figure 8b) and in Riley (1943; plate T4-2066). Note that the ventral pallidum is reported as the “substantia innominata: in Riley and in our earlier study of neuropeptides in METH users (Frankel et al., 2007).
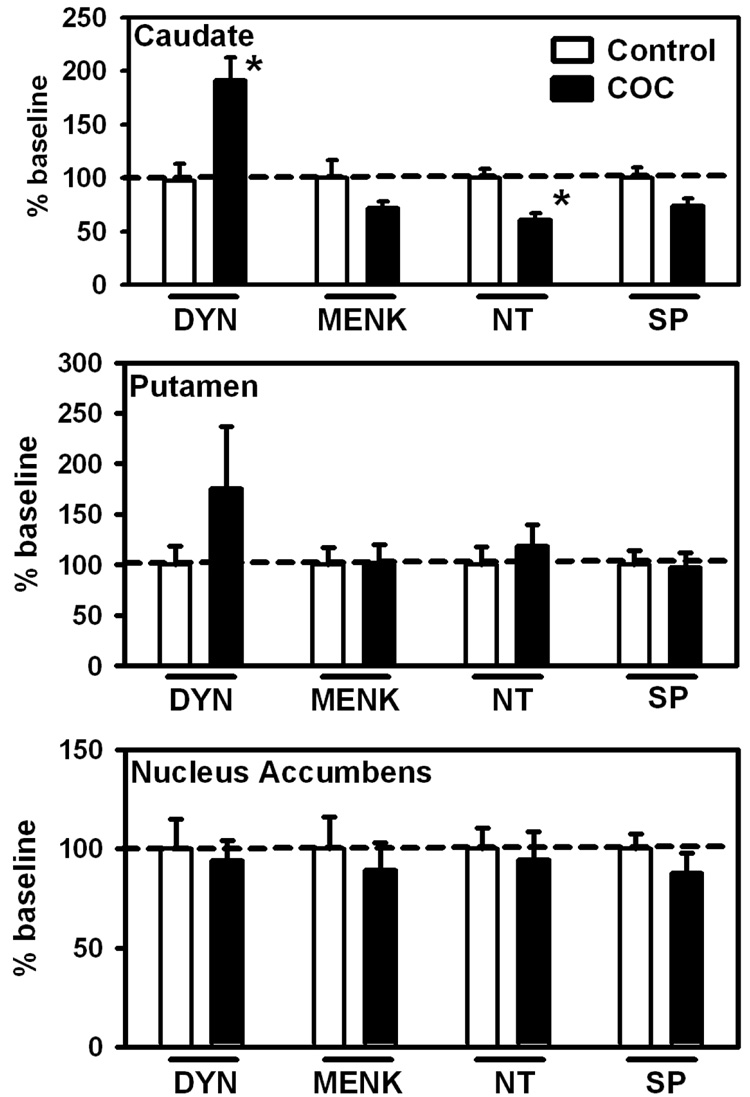
Figure 1.
Neuropeptide levels in the human caudate nucleus, putamen and nucleus accumbens in control (no drug history) or COC abusers. Baseline levels of peptides (ng/mg ± SEM) for these regions where significant differences were found are (Caudate: DYN: 0.42 ± 0.06; NT: 0.12 ± 0.01). Data (n = 12 – 17 per group) are expressed as percentage of baseline ± SEM *p < 0.05 versus control. Abbreviations: DYN, dynorphin; MENK, metenkephalin; NT, neurotensin; SP, substance P.
Baseline levels for the neuropeptides analyzed for the 10 brain regions have been previously published (Frankel et al., 2007). These regions included the DA-rich caudate, putamen and nucleus accumbens, as well as some relatively DA-poor brain regions [frontal cortex (Brodmann area 9); occipital cortex (Brodmann area 18); temporal cortex (Brodmann area 22); parietal cortex (Brodmann area 39); thalamus (nucleus lateralis and medial pulvinar); and the ventral pallidum].
3.2. Postmortem time and neuropeptide levels
The possible influence of postmortem time on neuropeptide levels was examined in all cases using the Pearson correlation test. Only one statistically significant correlation was observed in the control group: MENK in the medial pulvinar nucleus of the thalamus (−0.69, p<0.01). Two statistically significant correlations were observed in the COC user group: NT and SP in temporal cortex (Brodmann’s cortical area 22) (−0.68, p = 0.16 and 0.74, p = 0.006, respectively).
3.3. Brain COC vs. neuropeptide levels
The levels of COC and its major metabolites in autopsied brain of the COC users have been previously reported (Wilson et al., 1996; Kalasinsky et al., 2000). In the current study, Spearman rank correlations were performed in order to determine if there was a relationship between the amount of COC plus metabolites measured in post mortem brain and peptide levels. Molar sum totals of COC (COC plus benzoylecgonine, ecgonine methylester, norcocaine and cocaethylene) from occipital cortex were used for these determinations of “recent” drug use. No significant correlations were found in the COC-user group.
3.4. Neuropeptide levels in COC users
All neuropeptide levels are represented as a percentage of baseline by region. Statistically significant differences between COC users and control subjects in the striatum and nucleus accumbens were limited to the caudate (Figure 1) in which DYN levels were markedly increased (+92%) and NT concentration decreased (−39%). Additionaly, non-significant trends were observed for decreased SP (−26%) and MENK (−28%) in caudate and increased DYN (+75%) in putamen.
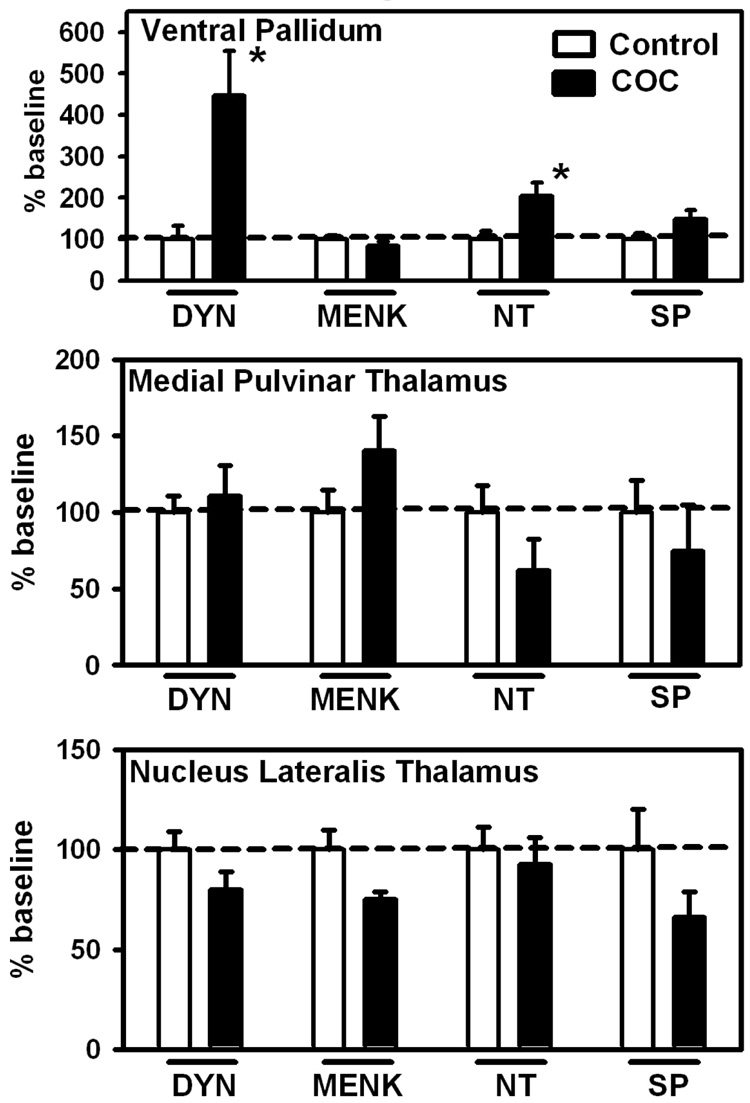
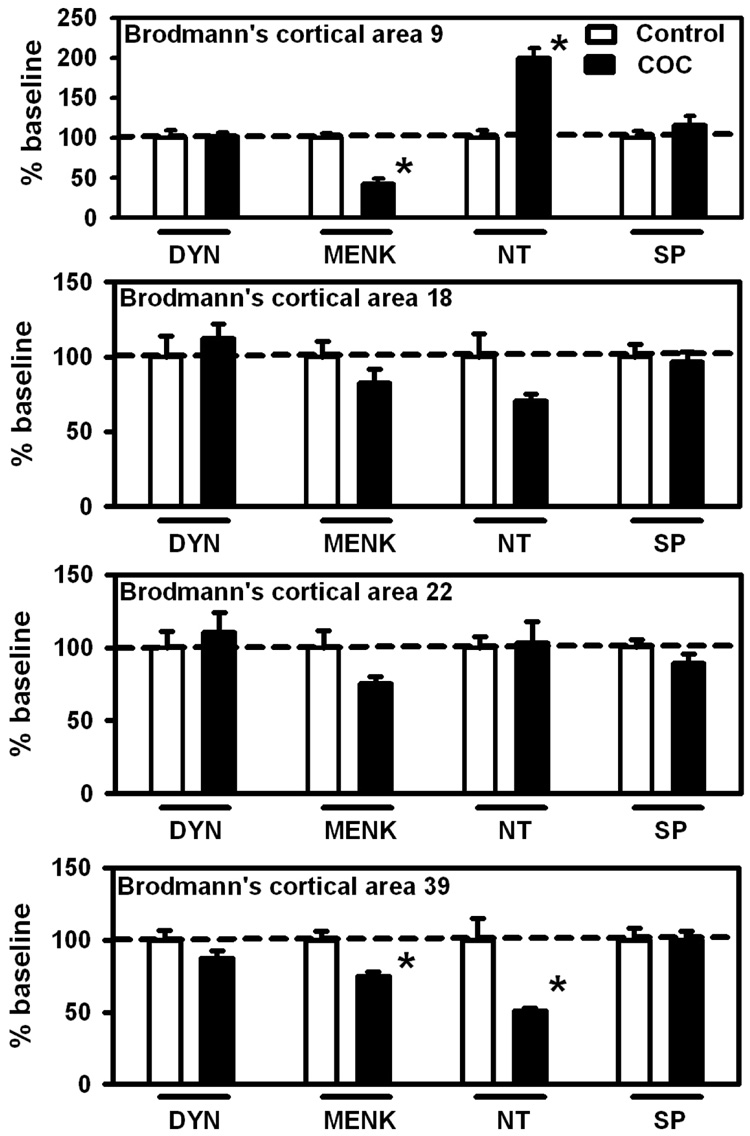
In the extra-striatal subcortical areas (Figure 2), changes were limited to markedly increased DYN (+346%) and NT (+104%) in the ventral pallidum. In cerebral cortex NT levels were significantly increased (+99%) in Brodmann’s cortical area 9 (Figure 3) while MENK levels were significantly decreased (−58%). Brodmann’s cortical area 39 (Figure 3) displayed significant decreases in both MENK (−25%) and NT levels (−49%) in the COC group.
Figure 2.
Neuropeptide levels in the human ventral pallidum (substantia innominata), medial pulvinar thalamus and lateral thalamus in control (no drug history) or COC abusers. Baseline levels of peptides (ng/mg ± SEM) for these regions where significant differences were found are (Ventral Pallidum: DYN: 2.45 ± 0.77; NT: 0.78 ± 0.16). Data (n = 12 – 17 per group) are expressed as percentage of baseline ± SEM *p < 0.05 versus control. Abbreviations are the same as Fig. 1.
Figure 3.
Neuropeptide levels in Brodmann’s cortical areas 9, 18, 22 and 39 in control (no drug history) or COC abusers. Baseline levels of peptides (ng/mg ± SEM) for these regions where significant differences were found are (Brodmaan’s cortical area 9: MENK: 1.54 ± 0.08; NT: 0.07 ± 0.01; Brodmann’s cortical area 39: MENK: 7.12 ± 0.42; NT: 0.081 ± 0.012). Data (n = 12 – 17 per group) are expressed as percentage of baseline ± SEM *p < 0.05 versus control. Abbreviations are the same as Fig. 1.
Neuropeptide levels vs. DA and VMAT2 binding
Pearson correlations, using data obtained in Wilson et al (1996) were conducted to establish whether there might be any relationship between levels of neuropeptides in striatum and nucleus accumbens vs. concentrations of the neurotransmitter dopamine and those of the vesicular monoamine transporter (VMAT2, assessed by measurement of 3H-dihydrotetrabenazine binding Bmax), considered to be a marker of the integrity of dopamine neurones in striatum (see Wilson et al., 1996). Statistically significant correlations were limited to DA and NT in the caudate (r=0.58) and nucleus accumbens (r=0.68) and MENK and VMAT2 binding in the nucleus accumbens (r=−0.84).
4. Discussion
Our major finding is that, as predicted by animal data, levels of the neuropeptide DYN were increased in the caudate of human COC users whereas striatal concentrations of the other peptides were either normal or, in the case of NT, decreased. In addition, we found that DYN concentration was strikingly increased in an anatomically adjacent area, the ventral pallidum.
In our investigation we attempted as much as possible to address the potential confounding variables inherent in all autopsied brain investigations by matching the control and COC user groups with age, postmortem time, and by confirming by forensic drug analyses that the COC users used COC and the controls did not. However, it needs to be acknowledged that both drug and control groups might have previously used other drugs or have other characteristics that could have affected brain neuropeptide levels.
In animal models, high doses of COC (e.g., ~30 mg/kg, 4 i.p. injections, 2-hr intervals) over a relatively short period of time (e.g. 6–8 hours) increase DYN, NT and SP levels both in the striatum and nucleus accumbens (Alburges et al., 2000; Hanson et al., 1989; Smiley et al., 1990). The likely explanation for these changes is that COC, via dopamine transporter inhibition stimulate the DA D-1 and/or D-2 receptors and increases synthesis of NT, DYN (Adams et al., 2003) and SP (Adams et al., 2001). This is supported by findings that D-1 antagonists block, and a selective D-1 agonist mimics, these COC effects (Hanson et al., 1989) while either D-1 or D-2 selective antagonists block increased levels of DYN (Smiley et al., 1990) or SP (Alburges et al., 2000). Our finding of increased caudate DYN in human COC users (who all had used the drug chronically and recently) is somewhat supported by the early report of modestly increased expression of preprodynorphin mRNA in the putamen (with a trend in caudate) in human COC users (Hurd and Herkenham, 1993) and suggests that COC is able to stimulate production of striatal DYN even after long-term repeated exposure. In the normal human we found, as expected, that the concentration of DYN is markedly enriched in the nucleus accumbens (7.8 ng/mg) as compared with that in the caudate (0.4 ng/mg; see Table 1). Although the results are not entirely consistent across species, animal studies (Fagergren et al., 2003) generally show that activation of the dynorphin system is more prominent in the striatum (caudate and putamen) rather than in the nucleus accumbens—experimental findings in agreement with our postmortem human data. Although only one portion of the nucleus accumbens (see methods), was sampled in our study, and inferences based on peptide changes or lack of changes must be tentative, our data do suggest that the dorsal, rather than the ventral, striatal DYN system might be more responsive to chronic COC exposure in the human.
We also observed a striking (almost fourfold) increase in DYN concentration in a brain area previously described as part of the “substantia innominata” and now known as the ventral pallidum. This area in human brain, ventral to the external globus pallidus and the anterior commissure (Heimer, 2003; Riley, 1943), is rich in DYN possibly localized to neuronal projections from the striatum (Reiner et al., 1999) or the nucleus accumbens (Zhou et al., 2003). In this regard, our human data suggest that COC activates DYN neurons that innervate the ventral pallidum, a brain system which does not yet appear to have been examined in parallel experimental animal studies. In our previous postmortem brain study of chronic human users of the dopaminergic stimulant methamphetamine, we observed a statistically nonsignificant trend for increased DYN in both the caudate and the ventral pallidum/substantia nigra (Frankel et al., 2007). Taken together these findings add more support to the notion that activation of the striatal/ventral pallidum DYN system is a consequence of exposure to psychostimulant drugs in the human.
In contradistinction to animal data showing that COC increases levels of DYN (Alburges et al., 1999; Smiley et al., 1990), MENK (Alburges et al., 2001), NT (Hanson et al., 1989) and SP (Alburges et al., 2000) and mRNA for DYN (Fagergren et al., 2001; Schlussman et al., 2003), NT and SP (Adams et al., 2001) we only found an increase in the levels of DYN in caudate of the COC users. In this regard, many of the drug users included in the current study regularly abused COC for 3 to > 10 years (Kalasinsky et al., 2000) contrasting with the typical short-term COC treatments administered in the animal studies. The importance of such differences between the conditions associated with the animal experiments and this study are underscored by the observation that the pattern of neuropeptide responses in COC users is different from the reported findings in non-contingent animal models employing high doses of this stimulant (Hanson et al, 1989). Thus, with the exception of a significant increase in caudate DYN content in COC users (Fig. 1), the other peptide responses in the basal ganglia and nucleus accumbens were quite different from that observed in animal studies. Assuming that the basal ganglia and accumbal DA-linked neuropeptides are comparably regulated, possible explanations for the neuropeptide differences in COC-dependent humans and laboratory animal models include the patterns of drug administration such as doses, contingency (i.e., the ability to manage drug exposure through self administration) and duration of drug exposure [i.e., days (animal model) vs. years (human users)]. Alternatively, it is possible that in the human COC exposure does not activate the forebrain NT, SP or MENK systems, or that it does but only acutely, with tolerance to this effect occurring after chronic drug exposure. Another consideration is that stimulation of D-2 (vs. D1) dopamine receptors with a selective agonist decreases striatal levels of NT (Merchant et al., 1989), suggesting that the decreased caudate level of NT (and trend for decreased SP) observed in human COC users could be mediated by D-2 receptor activation, while the caudate DYN increase is a D-1-mediated effect.
In this exploratory study we also examined the effects of long-term COC use on neuropeptide levels in extra-striatal brain areas and observed variable changes in MENK and NT levels in frontal and parietal cerebral cortices. It is not possible to relate the changes in these extra-caudate neuropeptide levels found in COC abusers to preclinical studies as corresponding neuropeptide systems have not been examined in laboratory animals. In principle, it is possible that, overall, they reflect altered cortical functions associated with the addiction process (Kalivas and Volkow, 2005; Winder et al., 2002). In this regard, we observed that the neuropeptide levels in cortical regions particularly altered in the COC users were Brodmann’s areas 9 (frontal cortex) and 39. These brain areas are especially important for functions such as decision making, cognitive processing, initiative states and planning, functions reported to be altered in drug abusers (Chen et al., 2003; Gouzoulis-Mayfrank et al., 1999; Iyo et al., 1997; Srisurapanont et al., 2003).
Most efforts to develop pharmacotherapies for treatment of COC addiction have focused on drugs acting on the brain DA system. However, this approach has not yet resulted in success in the clinic and an additional focus has involved development of drugs acting on other target sites (e.g., neuropeptides) that are considered to interact with the brain DA system, such as DYN, the endogenous ligand for the κ-opioid receptor (Shippenberg et al., 2007). The physiological role of DYN in human brain is unresolved, but a variety of possible functions have been ascribed, including involvement in a feedback system to suppress dopaminergic activity (Shippenberg et al., 2007) and as a mediator of the dysphoric component of stress (reviewed in Land et al., 2008). In human clinical studies κ-opioid agonist administration produces a variety of subjective effects including dysphoria and perceptual disturbances (Walsh et al., 2001). Dynorphin has been selected as a drug target for COC relapse in large part because of animal data showing that dynorphin levels are increased in basal forebrain regions considered to be relevant to mechanisms of drug addiction following cocaine exposure. Our postmortem data provide the first evidence that a change in DYN tissue levels in brain regions considered to be important for drug dependence is associated with long-term cocaine exposure in the human. Although this finding by itself does not identify the preferred direction of therapeutic intervention in cocaine addiction (i.e., do increased peptide levels suggest κ-opioid agonist vs. antagonist therapy is to be preferred?) it should provide further stimulus to the clinical testing of therapeutics targeting the κ-opioid receptor system in human psychostimulant users.
Acknowledgements
This work was supported by PHS grants from NIDA, DA09407, DA00378, and DA07182 (to SK).
Abbreviations used
- ChAT
choline acetyltransferase
- DA
dopamine
- DYN
dynorphin
- MENK
metenkephalin
- METH
methamphetamine
- NT
neurotensin
- SP
substance P
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DH, Hanson GR, Keefe KA. Differential effect of cocaine and methamphetamine on neurotensin/neuromedin N and preprotachykinin messenger RNA expression in unique regions of the striatum. Neuroscience. 2001;102:843–851. doi: 10.1016/s0306-4522(00)00530-3. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hanson GR, Keefe KS. Distinct effects of methamphetamine and cocaine on preprodynorphin messenger RNA in rat striatal patch and matrix. Journal of Neurochemistry. 2003;84:87–93. doi: 10.1046/j.1471-4159.2003.01507.x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hanson GR. Ibogaine pretreatment dramatically enhances the dynorphin response to cocaine. Brain Research. 1999;847:139–142. doi: 10.1016/s0006-8993(99)02017-x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Ramos BP, Bush L, Hanson GR. Responses of the extrapyramidal and limbic substance P systems to ibogaine and cocaine treatments. European Journal of Pharmacology. 2000;390:119–126. doi: 10.1016/s0014-2999(99)00919-x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Keefe KA, Hanson GR. Contrasting responses by basal ganglia metenkephalin systems to low and high doses of methamphetamine in a rat model. Journal of Neurochemistry. 2001;76:721–729. doi: 10.1046/j.1471-4159.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, Chiang YL, Ree SC, Lee CH, Murray RM. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine. 2003;33:1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. European Journal of Neuroscience. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Brain levels of neuropeptides in human chronic methamphetamine users. Neuropharmacology. 2007;53:447–454. doi: 10.1016/j.neuropharm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Büll U, Sass H. Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDA) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology. 1999;20:565–581. doi: 10.1016/S0893-133X(98)00089-X. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Characterization of methamphetamine effects on the striatal-nigral dynorphin system. European Journal of Pharmacology. 1988;155:11–18. doi: 10.1016/0014-2999(88)90397-4. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Smiley P, Johnson M, Letter A, Bush L, Gibb JW. Response by the neurotensin systems of the basal ganglia to cocaine treatment. European Journal of Pharmacology. 1989;160:23–30. doi: 10.1016/0014-2999(89)90650-x. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Bush LG, Taylor VL, Gibb JW, Davis K, Schmidt CJ. Comparison of neurotensin responses to MDL 100,907, a selective 5-HT2A antagonist, with clozapine and haloperidol. Brain Research Bulletin. 1997;42:211–219. doi: 10.1016/s0361-9230(96)00258-4. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. American Journal of Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Research Molecular Brain Research. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Iyo M, Namba H, Yanagisawa M, Hirai S, Yui N, Fukui S. Abnormal cerebral perfusion in chronic methamphetamine abusers: a study using 99MTc-HMPAO and SPECT. Progress in Neuropsychopharmacology and Biological Psychiatry. 1997;5:789–796. doi: 10.1016/s0278-5846(97)00079-1. [DOI] [PubMed] [Google Scholar]
- Kalasinsky KS, Bosy TZ, Schmunk GA, Ang L, Adams V, Gore SB, Smialek J, Furukawa Y, Guttman M, Kish SJ. Regional distribution of cocaine in postmoretem brain of chronic human cocaine users. Journal of Forensic Science. 2000;45:1041–1048. [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Naloxone depresses cocaine self administration and delays its initiation on the following day. Neuroreport. 2003;14:251–255. doi: 10.1097/00001756-200302100-00019. [DOI] [PubMed] [Google Scholar]
- Kosten T, Silverman DG, Fleming J, Kosten TA, Gawin FH, Compton M, Jatlow P, Byck R. Intravenous cocaine challenges during naltrexone maintenance: a preliminary study. Biological Psychiatry. 1992;32:543–548. doi: 10.1016/0006-3223(92)90223-m. [DOI] [PubMed] [Google Scholar]
- Kuzmin AV, Gerrits MA, van Ree JM. Naloxone inhibits the reinforcing and motivational aspects of cocaine addiction in mice. Life Science. 1997;60:257–264. doi: 10.1016/s0024-3205(97)00130-6. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. Journal of Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Progress in Neuropsychopharmacology and Biological Psychiatry. 2007;31:1061–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Gibb JW, Hanson GR. Role of dopamine D-1 and D-2 receptors in the regulation of neurotensin systems of the neostriatum and the nucleus accumbens. European Journal of Pharmacology. 1989;160:409–412. doi: 10.1016/0014-2999(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Maidment NR, Siddall BJ, Rudolph VR, Erdelyi E, Evans CJ. Dual determination of extracellular cholecystokinin and neurotensin fragments in rat forebrain: microdialysis combined with sequential multiple antigen radioimmunoassay. Neuroscience. 1991;45:81–93. doi: 10.1016/0306-4522(91)90105-w. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Pettinati HM, Volpicelli JR, Wolf AL, Kampman KM, O’Brien CP. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. Journal of Substance Abuse and Treatment. 1999;2:163–167. doi: 10.1016/s0740-5472(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Posnett DN, McGrath H, Tamm JP. A novel method for producing anitpeptide antibodies. Journal of Biological Chemistry. 1988;261:1719–1725. [PubMed] [Google Scholar]
- Reiner A, Medina L, Haber SN. The distribution of dynorphinergic terminals in striatal target regions in comparison to the distribution of substance P-containing and enkephalinergic terminals in monkeys and humans. Neuroscience. 1999;88:775–793. doi: 10.1016/s0306-4522(98)00254-1. [DOI] [PubMed] [Google Scholar]
- Riley HA. An Atlas of the Basal Ganglia, Brain Stem, and Spinal Cord. Baltimore, MD: Williams and Wilkins; 1943. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Yuferov V, LaForge KS, Ho A, Kreek MJ. Acute ‘binge’ cocaine administration elevates dynorphin mRNA in the caudate putmen of C57BL/6J but not 129/J mice. Brain Research. 2003;974:249–253. doi: 10.1016/s0006-8993(03)02561-7. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addictive Behaviors. 2001;2:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacology and Therapeutics. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramindal and limbic dynorphin systems. Journal of Pharmacology and Experimental Therapeutics. 1990;253:938–943. [PubMed] [Google Scholar]
- Srisurapanont M, Ali R, Marsden J, Sunga A, Wada K, Monteiro M. Psychotic symptoms in methamphetamine psychotic in-patients. International Journal of Neuropsychopharmacology. 2003;6:347–352. doi: 10.1017/S1461145703003675. [DOI] [PubMed] [Google Scholar]
- Tong J, Horkiewicz O, Kish SJ. Identification of a noradrenaline-rich subdivision of the human nucleus accumbens. Journal of Neurochemistry. 2006;96:349–354. doi: 10.1111/j.1471-4159.2005.03546.x. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu M/E/, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphine in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams VI, Smialek J, Anderson WR, Shannak K, Deck J, Niznik HB, Kish SJ. Striatal dopamine, dopamine transporter and vesicular monoamine transporter in chronic cocaine users. Annals of Neurology. 1996;40:428–439. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- Winder DG, Egli RE, Schramm NL, Matthews RT. Synaptic plasticity in drug reward circuitry. Current Molecular Medicine. 2002;7:667–676. doi: 10.2174/1566524023361961. [DOI] [PubMed] [Google Scholar]
- Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120:783–798. doi: 10.1016/s0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]