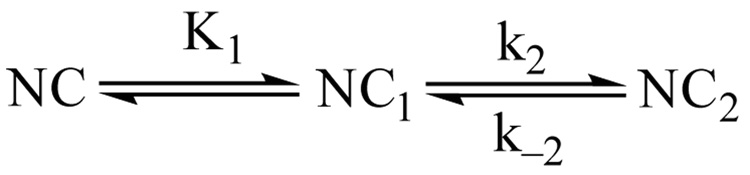Abstract
We have performed a kinetic analysis of Ca2+-dependent switching in the complex between calmodulin (CaM) and the IQ domain from neuromodulin, and have developed detailed kinetic models for this process. Our results indicate that the affinity of the C-ter Ca2+-binding sites in bound CaM is reduced due to a ~10-fold decrease in the Ca2+ association rate, while the affinity of the N-ter Ca2+-binding sites is increased due to a ~3-fold decrease in the Ca2+ dissociation rate. Although the Ca2+-free and Ca2+-saturated forms of the CaM-IQ domain complex have identical affinities, CaM dissociates ~100 times faster in the presence of Ca2+. Furthermore, under these conditions CaM can be transferred to the CaM-binding domain from CaM kinase II via a ternary complex. These properties are consistent with the hypothesis that CaM bound to neuromodulin comprises a localized store that can be efficiently delivered to neuronal proteins in its Ca2+-bound form in response to a Ca2+ signal.
At some level, all physiological responses involve coordinated biochemical changes triggered by intracellular Ca2+ signals. The Ca2+-binding protein calmodulin (CaM1), which engages in modulatory interactions with more than 100 known target proteins, is the primary mediator of Ca2+-dependent biochemical changes. Although many CaM targets, such as phosphodiesterase, myosin light chain kinase and the constitutive nitric oxide synthases do not interact significantly with the Ca2+-free protein (1–4), a large class bind Ca2+-free CaM at least as well as the Ca2+-bound forms of the protein. The majority of these, including neuromodulin, neurogranin, the unconventional myosins and some Ca2+, K+ and Na+ channels, interact with CaM via IQ domains, which have the consensus sequence: [I,L,V]QxxxR[G,x]xxx[R,K] (5–8). These regions bind apoCaM and/or Ca2+-bound CaM with dissociation constants ranging from subnanomolar to micromolar, depending upon their precise sequence and context (6). Complexes between CaM and IQ domains have been demonstrated to participate in a variety of regulatory processes, including positive and negative modulation of ion channels and unconventional myosin based motility (6). Some IQ domain proteins have been proposed to provide localized intracellular stores of CaM (9). The potential significance of such stores has been highlighted by recent investigations demonstrating that the intracellular CaM concentration can be limiting (10–12). Neuromodulin, a neuronal IQ domain protein found in axons and terminals, is perhaps the best studied example of such a protein (9). Neurogranin, a smaller protein with an essentially identical IQ domain, is thought to perform a similar function in dendritic spines (13, 14).
The results presented in this paper indicate that the properties of the neuromodulin CaM-IQ domain complex promote dissociation of CaM in its Ca2+-bound form, and allow it to be transferred to target proteins via a ternary complex. These features are conducive to the efficient delivery of Ca2+-bound CaM to target proteins. Our results also demonstrate that the association rate constant for the C-ter EF pair of Ca2+-binding sites in CaM is reduced by a factor of ~10 in the IQ domain complex. This somewhat novel finding is consistent with the accompanying reduction in the affinity of the complex (15). The detailed kinetic models that we present for Ca2+-dependent switching in this complex may be applicable to CaM-IQ domain complexes in proteins where Ca2+-dependent switching appears to directly modulate function, such as ion channels and unconventional myosins (5–8).
Materials and Methods
Proteins
The composition, expression and purification of the fluorescent reporter, BSCaMIQ, have been described in detail elsewhere (15), as have the methods used to express and purify native and mutant CaMs (16, 17). The two mutant CaMs used in these studies are NxCCaM, in which glutamic acid residues at positions 31 and 67 in the N-ter EF-hands have been replaced by alanines, and NCxCaM, in which the homologous glutamic acid residues at positions 104 and 140 have been replaced. As designated by the “x” subscript, these mutations eliminate Ca2+ binding to the N-ter or C-ter EF hand pair. Two synthetic peptides were used in these studies. One, termed ckPEP, is based on the CaM-binding domain in CaM-dependent protein kinase II and has the sequence: MHRQETVDCLKKFNARRKLKGAILTTMLA (Calbiochem, San Diego, CA). The other, termed ngPEP, is based on the IQ domain in neurogranin and has the sequence: AAKIQASFRGHMARKK (Promega , Madison, WI).
Stopped-flow fluorescence measurements
All dynamic fluorescence measurements were performed using an SX18-MV Stopped-Flow Reaction Analyzer with a nominal ~1.4 ms dead time (Applied Photophysics, Leatherhead, UK). Excitation light from a 75 watt xenon arc lamp is supplied via a fiber optic-coupled monochromator (5 nm slit widths). Fluorescence emission is monitored using a high voltage PMT fitted with an appropriate absorbance filter. To monitor changes in FRET between ECFP and EYFPC in BSCaMIQ, ECFP fluorescence was excited at 430 nm and EYFPC emission was monitored using an LG535 long-pass absorbance filter (Corion, Franklin, MA). Ca2+-dependent changes in quin-2 fluorescence excited at 330 nm were monitored using an LG505 long pass emission filter. In experiments where quin-2 or CaM-binding peptides were used to trap Ca2+ or CaM, a range of quin-2 or peptide concentrations were examined to ensure the absence of rebinding. All progress curves presented are the average of at least 6 individual determinations. Reactions were initiated by rapidly mixing equal volumes of reactant solutions to produce the stated final reactant concentrations. The base experimental buffer contained 25 mM Tris; pH 7.5, 0.1 M KCl and100 µg/ml BSA, with other components as specified in the text or captions. Where indicated, proteins and buffers were decalcified by successive treatments with Chelex™ and a BAPTA-polystyrene column (Molecular Probes, Inc.). Contaminating amounts of Ca2+ in decalcified protein solutions were 50 nM or less, based on the absence of any detectible effect on the A263 of BAPTA. Stopped-flow experiments were performed at 22 °C.
Analysis and modeling of kinetic data
Rate constants were derived from fits of individual fluorescence time courses to mono or double exponentials or from global fits of multiple time courses to explicit kinetic models performed using the Dynafit program (18). Standard errors given for the fitted parameters are the square roots of the diagonal elements in the variance-covariance matrices calculated for the non-linear least squares fits. In order to perform global fitting to explicit models it was necessary to assign relative molar fluorescence amplitudes to all kinetic species so as to account for their contributions to observed fluorescence. Values of 1.0 and 0.68 were assigned to free BSCaMIQ and the Ca2+-free CaM-BSCaMIQ complex, and values of 0.83 and 0.85 were assigned to the Ca2+-saturated complex and the intermediate complexes in which one CaM EF hand pair or the other is Ca2+-saturated. These values were previously determined under equilibrium conditions, and were validated in the stopped-flow fluorometer (15). Occupancy of an EF hand pair by only a single Ca2+ ion was assumed to have no effect on fluorescence, because fluorescence changes observed under equilibrium conditions appear to require occupancy of both Ca2+-binding sites (15).
Ca2+ association and dissociation
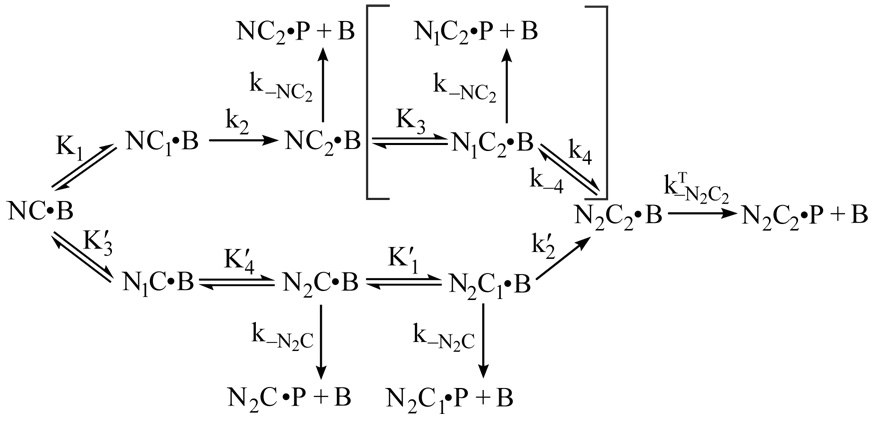
We have modeled Ca2+ binding to each EF hand pair in CaM according to a sequential model, as illustrated by Scheme 1. By convention, the two Ca2+-binding steps corresponding with occupancy of the C-ter EF hand pair are numbered 1 and 2 (as in Scheme 1), while the two N-ter Ca2+-binding steps are numbered 3 and 4. A sequential Ca2+ binding model is the simplest one that accounts for the fact that dissociation of both Ca2+ ions from an EF hand pair is always observed to occur with a single apparent rate (19, 20). The trivial explanation that Ca2+ dissociates independently from both sites at the same rate is inconsistent with the high degree of binding cooperativity (19). It is also inconsistent with 15N spin relaxation experiments, which indicate that the second Ca2+ release step is at least 100 times faster than the first (21). Since the second Ca2+ ion is released much faster than the first, we also treat the first Ca2+ binding step as rapidly equilibrating. This assumption becomes invalid at free Ca2+ concentrations where k2[Ca2+] ≈ k−1 or k4[Ca2+] ≈k−3, but it appears to be applicable over the range of Ca2+ concentrations used in these investigations (see Fig. 5).
SCHEME 1.
Fig. 5. Dissociation of the initially Ca2+-free complex between BSCaMIQ and native CaM or NxCCaM after addition of Ca2+ and ckPEP.
Fluorescence data were measured after adding a solution containing 200 µM ckPEP, along with CaCl2 in amounts producing the specified final concentrations, to an equal volume of a decalcified solution containing 20 µM BSCaMIQ and either 4 µM native CaM or NxCCaM. (A) Dissociation of the NxCCaM-BSCaMIQ complex in the presence of ckPEP with Ca2+ at concentrations of 30, 45, 67.5, 125 or 250 µM (lowermost to uppermost trace). These data were globally fit according to an abbreviated version of the model presented in Scheme 3 containing only the steps associated with formation and dissociation of NC2-B. The fit shown was produced by iteratively varying the values for K1 and k2 using the DynaFit program (18). Relative molar fluorescence amplitudes were assigned to the various kinetic species as detailed under “Materials and Methods”. The dissociation rate constants assigned to the NC2-B species is given in Table 1. The NC-B species is effectively non-dissociating on this time scale. No attempt was made to include the standard errors associated with the fixed parameter value. The K1 and k2 values derived from the global fit shown are 4.6±0.9 µM and 3.1±0.1×106 M−1-s−1. (B) Dissociation of the native CaM-BSCaMIQ complex in the presence of ckPEP with Ca2+ at a concentrations of 30, 45, 67.5, 125 or 250 µM (lowermost to uppermost trace). Fluorescence time courses were globally fit according to the model presented in Scheme 3 by iteratively varying the values for K′1 and k′2 using the DynaFit program (18). Previously determined values of 122 and 9.4 µM were assigned to K′3 and K′4 (15). The dissociation rate constants assigned to the NC-B, N2C-B, NC2-B and N2C2-B species are given in Table 1. The values used for the N2C-B and NC2-B species were determined using NxCCaM and NCxCaM. Values of 4.6±0.9 µM and 3.1±0.1×106 M−1-s−1, which were determined using NxCCaM, were assigned to K1 and k2 (Table 2). Relative molar fluorescence amplitudes were assigned to the various kinetic species as detailed under “Materials and Methods”. The K′1 and k′2 values derived from the global fit shown are 4.1±0.6 µM and 3.2±0.1×106 M−1-s−1.
The transition from Ca2+-saturated to Ca2+-free complex
The data presented in Fig. 4 were globally fit to the kinetic model given in scheme 2. In this scheme, B corresponds with BSCaMIQ, NC corresponds with Ca2+-free CaM, and N2C2 corresponds with CaM in which both the N-ter (N) and C-ter (C) EF hand pairs are replete with Ca2+. Intermediate Ca2+- bound states are designated appropriately. Since Ca2+ dissociates much more rapidly from the N-ter EF hand pair than it does from the C-ter pair, dissociation of Ca2+ is treated as a sequential process, with the N-ter pair of sites going first. The vertical dashed arrows denote steps that were omitted from fitting calculations because dissociation of the second Ca2+ ion from an EF hand pair is assumed to be much faster than dissociates of BSCaMIQ. “Fast” rate constants were arbitrarily assigned values 10-fold larger than the rate constants preceding them.
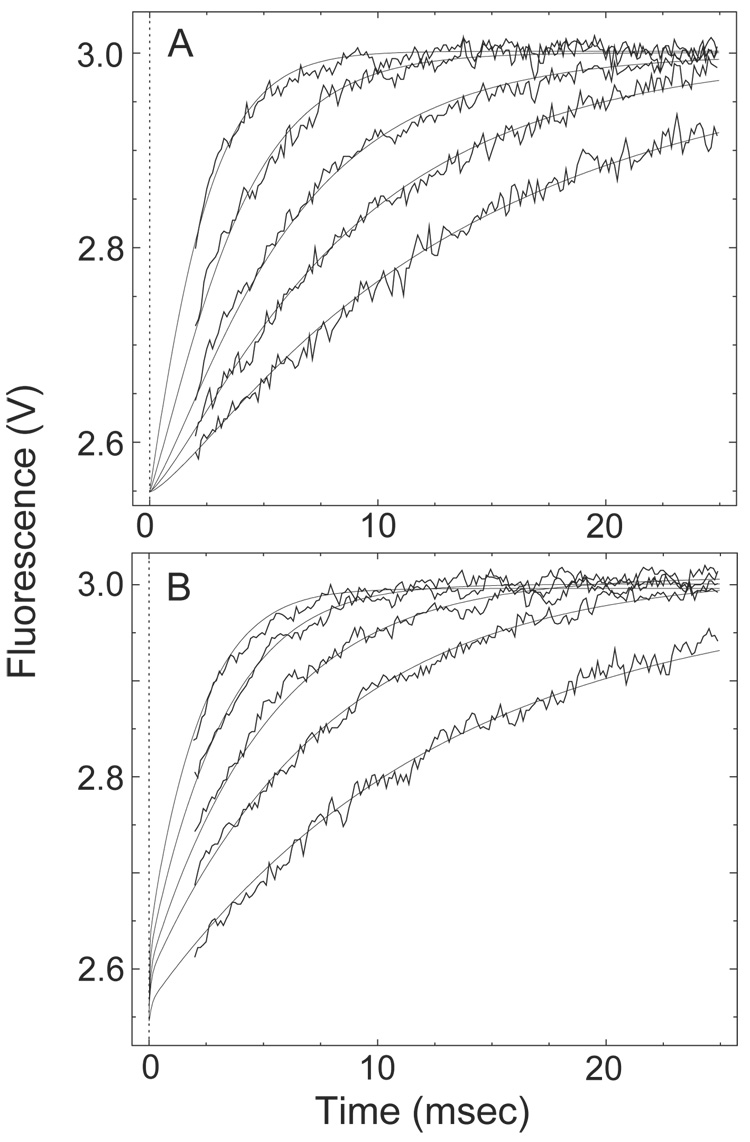
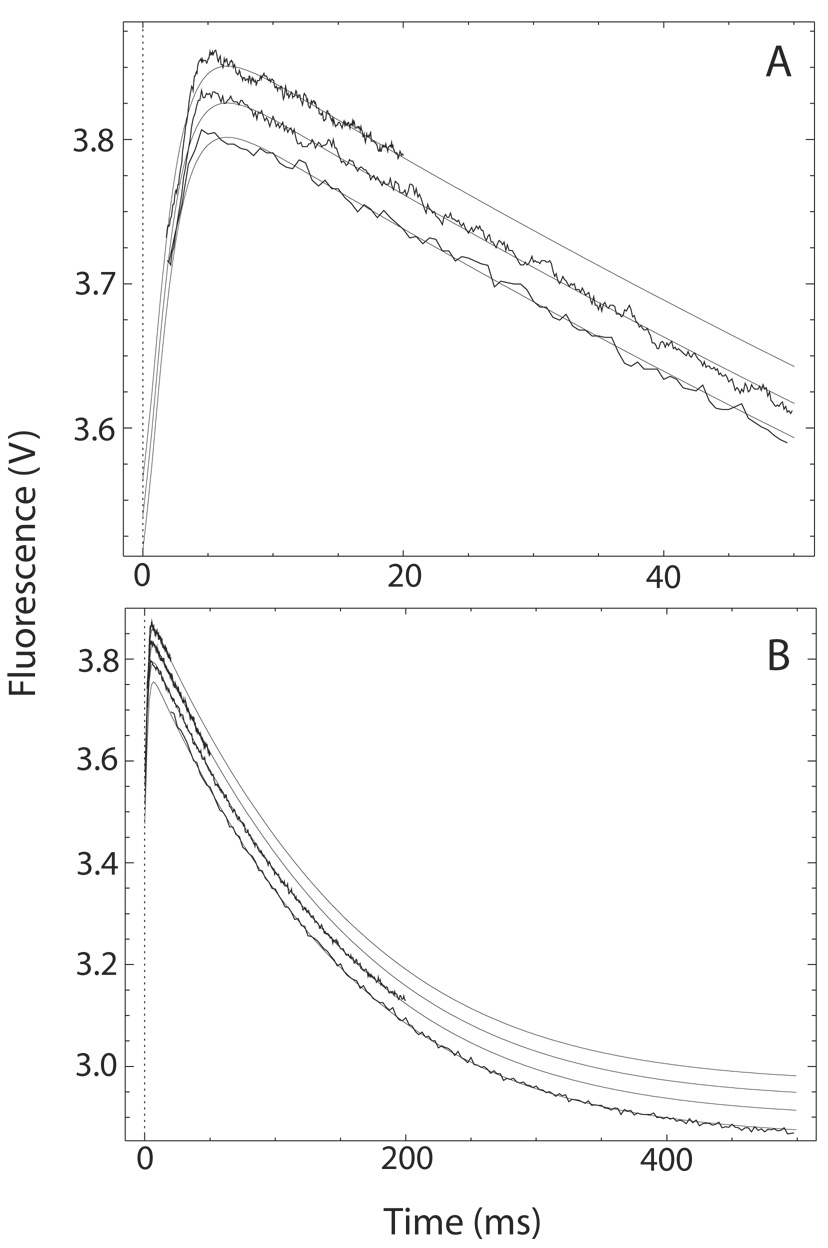
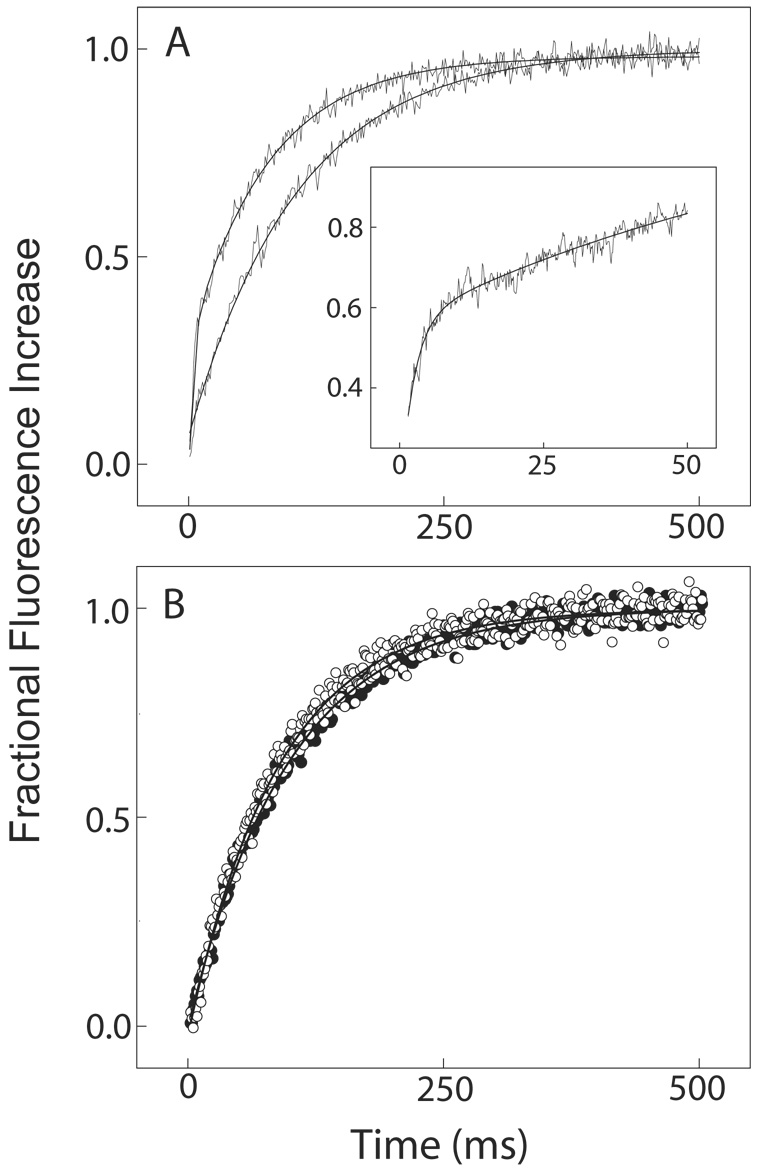
Fig. 4. The transition from Ca2+-saturated to Ca2+-free CaM-BSCaMIQ complex.
(A, B) Buffer containing 48 µM CaM and 4 µM BSCaMIQ in 500 µM CaCl2 was rapidly mixed with an equal volume of buffer containing 10 mM BAPTA. Data measured over 20, 50 and 200 ms time intervals are presented in panels A and B. Data measured over a 500 ms time interval are included in panel B. The individual data sets are offset on the y axis for presentation purposes. These data were globally fit to the model presented in Scheme 2 by iteratively varying the values for k′−2 and k′−4, the Ca2+ dissociation rate constants for bound CaM, using the DynaFit program (18). Relative molar fluorescence amplitudes were assigned to the various kinetic species as detailed under “Materials and Methods”. Association and dissociation rate constants applied to the various Ca2+-bound forms of the CaM-BSCaMIQ complex are given in Table 1. Ca2+ dissociation rate constants applied to free CaM are given in Table 2. Parameters assigned to the NC2-B species were determined using NxCCaM. No attempt was made to include the standard errors associated with fixed parameter values. Steps marked as fast were arbitrarily assigned rates 10-fold faster than the steps preceding them. The fitted values obtained for k′−4 and k′−2 are 528.3±3.3 and 13.5±0.1 s−1.
SCHEME 2.
Release of BSCaMIQ after addition of Ca2+and ckPEP
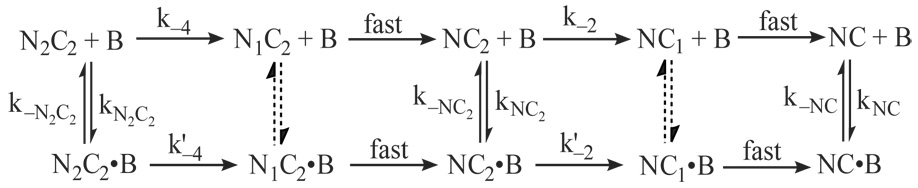
The data presented in Fig. 5 were globally fit according to the model given in Scheme 3. In this scheme, P corresponds with a peptide (ckPEP) based on the CaM-binding domain in CaM kinase II. Since the peptide prevents reassociation of CaM, rate constants for reversal of the second C-ter Ca2+-binding step (k−2 and k′−2) can be omitted because the rate constants for dissociation of the CaM complex are much larger (see Table 1 and Table 2). Dissociation of the Ca2+-free complex can also be ignored, as it occurs at a rate of ~1 s−1, which is far slower than the Ca2+ binding steps. Dissociation of CaM from other intermediates is effectively irreversible due the presence of a large molar excess of ckPEP. Since the dissociation rate constant for the N2C-B complex (kN2C) is much smaller than k′−4 (see Table 1 and Table 2), we can treat both N-ter Ca2+-binding steps on the lower pathway in the scheme as rapidly equilibrating, with dissociation constants of K′3, K′4. This approach is not applicable to the N-ter Ca2+-binding steps on the upper pathway because the apparent Ca2+ dissociation rate constant for the N-ter sites (k−4) is similar to the rate for dissociation of a presumptive peptide ternary complex ( see Table 1 and Table 2). However, this is not a significant issue because the bracketed steps can be omitted from fitting calculations without significantly affecting the fit or the derived parameter values. The probable basis for this is explained under “Results”.
SCHEME 3.
Table 1. Association and dissociation rate constants for native and mutant CaM-BSCaMIQ complexes.
The first two columns contain kon and koff values derived from linear least-squares fits to replots of kobs vs [CaM] (see Fig. 1). Kd values calculated using these rate constants are consistent with previously determined equilibrium Kd values (15). The last column contains koff values derived from dissociation time courses measured in the presence of larger molar excesses of ngPEP or ckPEP (see Fig. 2).
| kobsvs [CaM] plot | koff/kon | Equil | Peptide trap | ||
|---|---|---|---|---|---|
| CaM | kon (M−1s−1) | koff (s−1) | Kd (µM) | Kd (µM) | koff (s−1) |
| NC | 3.6±0.2×105 | 0.9±0.2 | 2.5±0.6 | 2.3±0.1 | 0.8±0.2 |
| NxC | 3.9±0.6×105 | 1.1±0.3 | 2.8±0.7 | 2.1±0.2 | 0.9±0.2 |
| NCx | 2.9±0.5×105 | 0.9±0.2 | 3.1±0.9 | 2.5±0.3 | 0.7±0.3 |
| NxC2 | 4.1±0.2×107 | 558.8±10.1 | 13.6±1.1 | 14.4±1.3 | 630.7±16.4 |
| N2Cx | 1.1±0.2×106 | 30.9±1.6 | 28.1±5.3 | 36.7±3.9 | 30.2±4.2 |
| N2C2 | 3.1±0.2×107 | 69.4±9.6 | 2.2±0.3 | 2.5±0.1 | 565.1±13.1 |
Table 2. Ca2+association and dissociation rate constants for native and mutant CaMs free in solution or bound to BSCaMIQ.
Ca2+ binding was modeled according to a sequential mechanism with a rapidly equilibrating first step (Scheme 1), so these rate constants correspond with the second Ca2+-binding step. Values indicated by an “NA” are not applicable; those indicated by an “ND” are too fast to measure in the stopped-flow fluorometer, which has a dead-time of ~1.4 ms. Association rate constants (kon) designated by a superscript “A” were calculated using the dissociation rate constants (koff ) given in this table and previously determined equilibrium constants for the second Ca2+ binding step (15). Association rate constants for the C-ter EF hand pair in the CaM-BSCaMIQ complex were derived from a global analysis of the fluorescence time courses presented in Fig. 5. koff values designated by a superscript “B” were determined using a 5 µl observation cell, which reduces the stopped-flow dead time to below 1 ms, but unfortunately cannot be used with solutions containing BSCaMIQ due to their higher viscosity. koff values for the CaM-BSCaMIQ complex given in parenthesis were derived from a global analysis the fluorescence time courses presented in Fig. 4. All other koff values were derived from quin-2 fluorescence data (Fig. 3).
| N-ter EF hand pair | C-ter EF hand pair | |||
|---|---|---|---|---|
| CaM | kon (M−1s−1) | koff (s−1) | kon (M−1s−1) | koff (s−1) |
| NC | 3.1±1.2×108 A | 1152±117B | 3.4±2.4×107 A | 10.3±0.7 |
| NxC | NA | NA | 5.8±3.2×107 A | 10.8±0.6 |
| NCx | 3.4±1.5×108 A | 1298±135B | NA | NA |
| CaM-BSCaMIQ | kon (M−1s−1) | koff (s−1) | kon (M−1s−1) | koff(s−1) |
| NC | 3.0±1.2×108 A | 473.4±37.9 (528.3±3.3) |
3.2±0.1×106 | 11.7±0.5 (13.5±0.1) |
| NxC | NA | NA | 3.1±0.1×106 | 11.8±0.5 |
| NCx | ND | ND | NA | NA |
Results
Association of CaM and BSCaMIQ
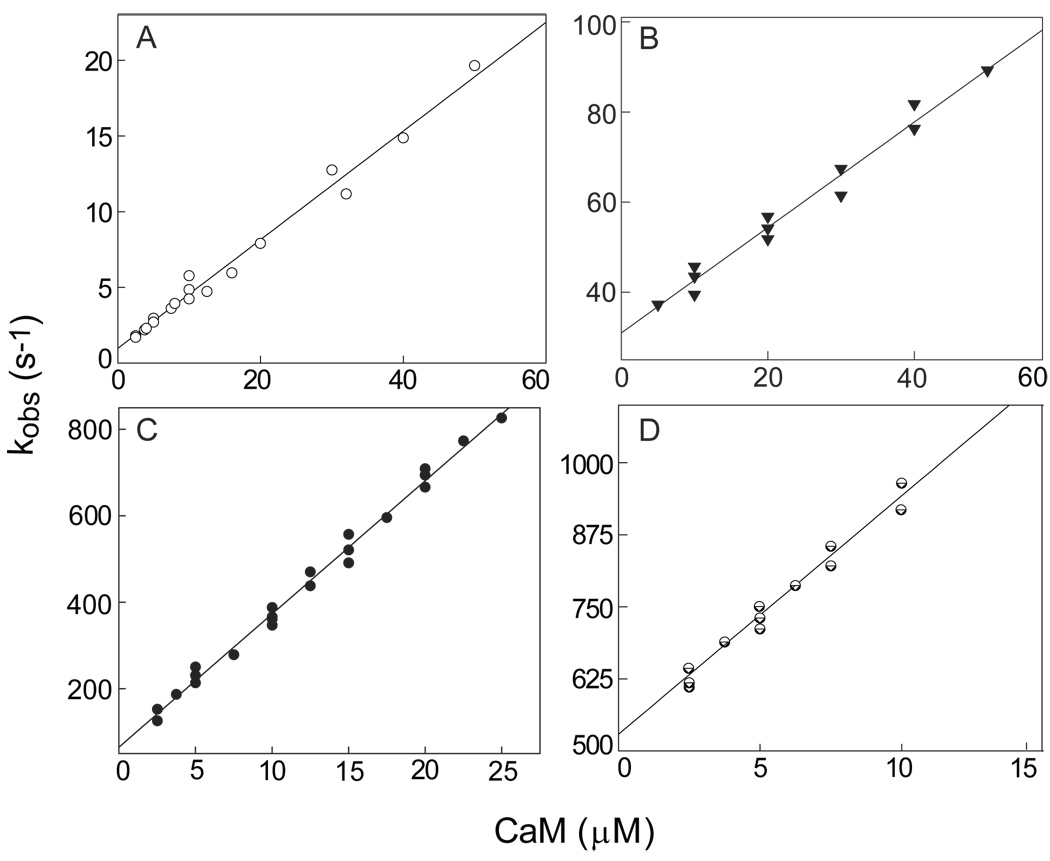
The first step in these investigations was to derive apparent association and dissociation rate constants (kon and koff ) for the various complexes between native or mutant CaM and BSCaMIQ, a fluorescent reporter containing the IQ domain from neuromodulin (15). This was accomplished by determining observed association rates (kobs) under pseudo-first order conditions at a series of different CaM concentrations. Values for kon and koff were then derived from linear least-squares fits to replots of kobs vs [CaM]. Values for kon and koff of 3.6±0.2×105 M−1s−1 and 0.9±0.2 s−1 were derived for the Ca2+-free native CaM complex from the replot presented in Fig. 1A. The koff /kon ratio of 2.5±0.6 µM calculated for this complex is essentially identical to the equilibrium Kd value of 2.3±0.1 µM (Table 1). Similar rate constants were derived for the Ca2+-free NxCCaM and NCxCaM complexes (Table 1; data not shown). Values for kon and koff of 1.1±0.2×106 M−1-s−1 and 30.9±1.6 s−1 were derived for the Ca2+-saturated NCxCaM complex from the replot in Fig. 1B, and the koff /kon ratio of 28.1±5.3 µM calculated for this complex agrees well with the equilibrium Kd value of 36.7±3.9 µM (Table 1). Values for kon and koff of 3.1±0.2×107 M−1-s−1 and 69.4±9.6 s−1 were derived for the Ca2+-saturated complex with native CaM from the replot in Fig. 1C; and respective values of 4.1±0.2×107 M−1-s−1 and 558.8±10.1 s−1 were derived for the Ca2+-saturated NxCCaM complex from the replot in Fig. 1D. The koff /kon ratios of 2.2±0.3 and 13.6±1.1 µM calculated for these complexes are essentially identical to their equilibrium Kd values of 2.5±0.1 and 14.4±1.3 µM (Table 1). The overall agreement between koff /kon ratios and equilibrium Kd values confirms that CaM binding to the neuromodulin IQ domain is a simple bimolecular process. Occupancy of one or both EF hand pairs in CaM significantly accelerates binding kinetics, although the greatest effect is seen when the C-ter EF hand pair is occupied. The accelerated rates observed in the presence of Ca2+ may serve to mobilize neuromodulin-bound CaM in response to a Ca2+ signal. The association rate constants for the CaM-IQ domain complexes in which the C-ter EF hand pair is Ca2+ bound are similar to those for the complexes between CaM and canonical target proteins, such as myosin light chain kinase or nitric oxide synthase (22, 23). In contrast, the association rates derived for complexes in which the C-ter EF hand pair is Ca2+-free are 30–100 times slower.
Fig. 1. Association of native and mutant CaMs with BSCaMIQ.
Values for kobs were derived from mono-exponential fits to time courses for BSCaMIQ fluorescence measured after addition of CaM. The lowest final CaM concentration produced was in all cases at least 5-fold greater than final BSCaMIQ concentration. Pseudo-first order conditions were thus maintained so that kobs = kon[CaM] + koff. (A) Replot of kobs values determined in the absence of Ca2+ vs the concentration of CaM. Nominally Ca2+-free conditions were produced by including 3 mM BAPTA in all experimental buffers. The kon and koff values derived from the linear fit shown are 3.6±0.2×105 M−1-s−1 and 0.9±0.2 s−1. kon and koff values derived for Ca2+-free NxCCaM are 3.2±0.1×105 M−1-s−1 and 1.1±0.1 s−1, and values of 2.9±0.1×105 M−1-s−1 and 0.7±0.1 s−1 were derived for Ca2+-free NCxCaM (data not shown). (B) Replot of kobs values determined in the presence of 250 µM CaCl2 vs the concentration of NCxCaM. The kon and koff values derived from the linear fit shown are 1.1±0.2×106 M−1-s−1 and 30.9±1.6 s−1. (C) Replot of kobs values determined in the presence of 250 µM CaCl2 vs the concentration of native CaM. The kon and koff values derived from the linear fit shown are 3.1±0.2×107 M−1-s−1 and 69.4±9.6 s−1. (D) Replot of kobs values determined in the presence of 250 µM CaCl2 vs the NxCCaM concentration. kon and koff values of 4.1±0.2×107 M−1-s−1 and 558.8±10.1 s−1 were derived from linear fit shown.
Dissociation of CaM in the presence of CaM-binding peptides
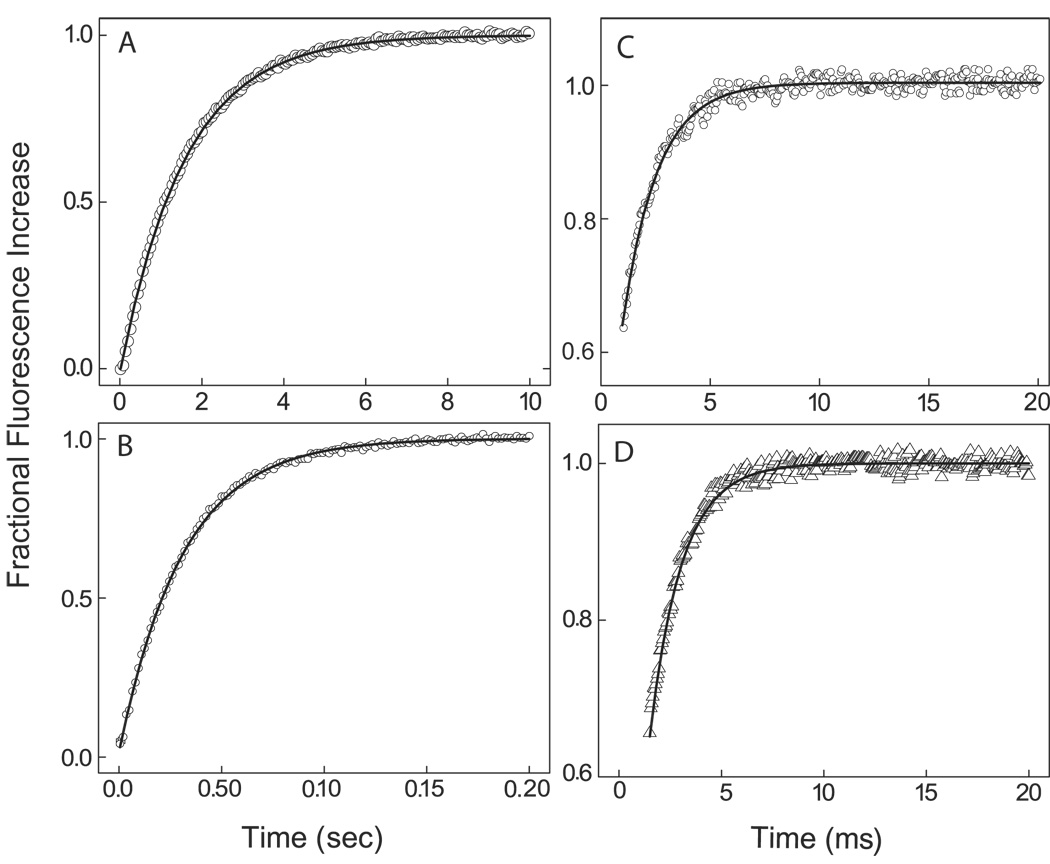
We extended our analysis of the kinetics of native and mutant CaM-BSCaMIQ complexes by examining dissociation in the presence of a large molar excess of peptides based on the IQ domain in neurogranin (ngPEP) and the CaM-binding domain in CaM kinase II (ckPEP), which does not bind CaM with significant affinity in the absence of Ca2+. In the presence of ngPEP, Ca2+-free native CaM dissociates with an apparent rate constant of 0.8±0.2 s−1 (Fig. 2A), and essentially identical rate constants were derived for the Ca2+-free complexes with the two mutant CaMs (data not shown). In the presence of ckPEP, Ca2+-saturated NCxCaM and NxCCaM dissociate with rate constants of 30.2±4.2 and 630.7±16.4 s−1 (Figs. 2B and D). These values agree with those derived from replots of kobs vs [CaM] (Table 1). In contrast, Ca2+-saturated native CaM dissociates with an apparent rate constant of 565.1±13.1 s−1 in the presence of ckPEP (Fig. 2C), which is ~10-fold faster than the rate derived from a replot of kobs vs [CaM] (Table 1). The accelerated dissociation rate observed in the presence of ckPEP indicates that dissociation occurs via a ternary complex between ckPEP, CaM and BSCaMIQ. Since the measured fluorescence time course is monophasic and has an amplitude consistent with formation of free BSCaMIQ, formation of the ternary complex itself does not appear to be associated with a significant change in fluorescence. However, it is conceivable that the fluorescence time course corresponds with formation of the ternary complex itself, rather than dissociation of BSCaMIQ. This is unlikely because the apparent dissociation rate does not exhibit a second-order dependence on the ckPEP concentration (data not shown).
Fig. 2. Dissociation of complexes between native and mutant CaMs and BSCaMIQ in the presence of CaM-binding peptides.
In all cases a range of peptide concentrations was investigated to ensure that a concentration sufficient to prevent rebinding of CaM was used. (A) Dissociation of the Ca2+-free CaM-BSCaMIQ complex after addition of a peptide (ngPEP) based on the CaM-binding domain in neurogranin. Nominally Ca2+-free conditions were produced by including 3 mM BAPTA in all experimental buffers. The final BSCaMIQ and CaM concentrations were 10 and 1 µM; the final peptide concentration was 100 µM. The koff value derived from mono-exponential fits to these and similar data is 0.8±0.2 s −1. (B) Dissociation of the NCxCaM-BSCaMIQ complex in the presence of 250 µM CaCl2 after addition of a peptide (ckPEP) based on the CaM-binding domain in CaM kinase II. The final BSCaMIQ and NxCCaM concentrations were 50 and 10 µM; the final ckPEP concentration was 100 µM. The dissociation rate constant derived from fits of these and similar data to single exponentials is 30.2±4.2 s−1. (C) Dissociation of CaM-BSCaMIQ complex in the presence of 250 µM CaCl2 after addition of ckPEP at a final concentration of 100 µM. The final BSCaMIQ and CaM concentrations were 12.5 and 2.5 µM. The apparent dissociation rate constant derived from fits to these and similar data to single exponentials is 565.1±13.1 s−1. (D) Dissociation of the Ca2+-saturated NxCCaM-BSCaMIQ complex in the presence of 250 µM CaCl2 after addition of ckPEP at a final concentration of 100 µM. The final BSCaMIQ and NxCCaM concentrations were 25 and 5 µM. The apparent dissociation rate constant derived from fits of these and similar data to single exponentials is 630.7±16.4 s−1.
Dissociation of Ca2+
We have previously reported that under equilibrium conditions Ca2+ binding to the C-ter EF hand pair in the CaM-BSCaMIQ complex reduces the affinity of the complex, and subsequent binding to the N-ter EF hand pair raises it (15). The Ca2+-binding affinity of the C-ter EF hand pair is thus reduced due to negative energy coupling, while the affinity of the N-ter pair is increased due to positive energy coupling (15). To investigate the mechanistic basis for these changes in Ca2+-binding affinity, we determined Ca2+ dissociation rates for free and bound native and mutant CaMs. In general agreement with others (19, 24–27), Ca2+ was found to dissociate from the C-ter and N-ter EF hand pairs in free CaM with rate constants of 10.3±0.7 and 1152±117 s−1. It is important to note that dissociation rate constants were determined for free CaM using a 5 µl observation cell, rather than the standard 20 µl cell. This reduces the dead time of the stopped-flow fluorometer to below 1 ms, allowing this fast rate to be measured. Unfortunately, efficient rapid mixing was not obtained when we attempted to use the smaller cell with solutions containing BSCaMIQ, presumably due to their higher viscosities. Dissociation rate constants of 11.7±0.5 s−1 and 473.4±37.9 s−1 were derived when CaM is bound to BSCaMIQ (Fig. 3A). To confirm that the slower rate constant corresponds with the C-ter EF hand pair, dissociation rate constants were derived for NxCCaM in the presence and absence of BSCaMIQ (Fig. 3B), and respective values of 11.8±0.5 and 10.8±0.6 s−1 were obtained. The dissociation rate constant of 473.4±37.9 s−1 derived for the native CaM complex therefore corresponds with the N-ter EF hand pair and the slower rate corresponds with the C-ter pair. The increased Ca2+-binding affinity of the N-ter sites in the CaM-BSCaMIQ complex is thus correlated with a decrease in the dissociation rate for Ca2+. The reduced affinity of the C-ter pair of sites does not appear to involve a change in the Ca2+ dissociation rate. Determination of a Ca2+ dissociation rate for the NCxCaM-BSCaMIQ complex, which exhibits negative energy coupling (15), was not possible because maintenance of the complex requires BSCaMIQ solutions with viscosities that preclude efficient mixing.
Fig. 3. Dissociation of Ca2+ from the complex between BSCaMIQ and CaM or NxCCaM.
The fluorescent Ca2+ chelator quin-2 was used as a Ca2+ trap. Dissociation of Ca2+ from native or mutant CaM therefore corresponds directly with a Ca2+-dependent increase in quin-2 fluorescence. A range of quin-2 concentrations was investigated to ensure that a concentration sufficient to prevent rebinding of Ca2+ was used. (A) Dissociation of Ca2+ from free CaM (lower trace) and from the CaM-BSCaMIQ complex (upper trace). The quin-2 fluorescence time course for Ca2+ dissociation from free CaM was generated by mixing a solution containing 4 µM CaM and 50 µM CaCl2 with an equal volume of 400 µM quin-2. These data are fit by a single exponential with a rate constant of 10.3±0.7 s−1 and an amplitude corresponding with dissociation of ~2 Ca2+ ions. Dissociation of two additional Ca2+ ions occurred within the instrument dead time. The quin-2 fluorescence time course for Ca2+ dissociation from the CaM-BSCaMIQ complex was generated by mixing a solution containing 25 µM BSCaMIQ, 4 µM CaM and 50 µM CaCl2 with an equal volume of 400 µM quin-2. The data presented are fit by a double exponential. The slower process has an apparent rate constant of 14.7±0.5 s−1 and an amplitude corresponding with dissociation of ~2 Ca2+ ions. The faster rate was derived from data measured over a shorter time interval (inset). These data are fit by a double exponential with rate constants of 427.4±35 and 11.7±0.5 s−1, and respective amplitudes corresponding with dissociation of ~1.5 and ~2 Ca2+ ions. If the faster process is forced to have an amplitude corresponding with dissociation of 2 Ca2+ ions, then apparent rate constants of 473.4±37.9 s−1 and 12.9±0.7 s−1 are derived for the faster and slower rates. (B) Dissociation of Ca2+ from the NxCCaM-BSCaMIQ complex (●) and from free NxCCaM (○). For these experiments a solution containing 4 µM NxCCaM and 50 µM CaCl2 in the presence or absence of 25 µM BSCaMIQ was mixed with an equal volume of 400 µM quin-2. The two resulting quin-2 fluorescence time courses are essentially superimposable. Ca2+-dissociation rate constants of 10.8±0.6 s−1 and 11.8±0.5 s−1 were derived for free and bound NxCCaM from fits of these data to single exponentials with amplitudes corresponding to dissociation of ~2 Ca2+ ions.
The transition from Ca2+-saturated to Ca2+-free complex
Having determined binding kinetics and Ca2+ dissociation rates for mutant and native CaM-BSCaMIQ complexes, we next analyzed the fluorescence changes associated with the transition from the Ca2+-saturated to the Ca2+-free complex. Fluorescence time courses were measured after addition of a large molar excess of BAPTA to the Ca2+-saturated CaM-BSCaMIQ complex (Fig. 4A and B). Individual time courses measured on different time scales are offset in the figure for presentation purposes. These data were globally fit to the kinetic model presented in Scheme 2. In this model Ca2+-dissociates sequentially from the N-ter and C-ter EF hand pairs because the N-ter sites have much faster kinetics. Relative molar fluorescence amplitudes were assigned to all kinetic species as detailed under “Materials and Methods”. The fitted curves presented in the figure were generated by varying only k′−2 and k′−4, the Ca2+ dissociation rate constants for N-ter and C-ter EF hand pairs in bound CaM. All other necessary parameters were fixed to the values given in Table 1 & Table 2. Parameter values derived for NxCCaM were applied to the intermediate species in which Ca2+ remains bound to the C-ter EF hand pair (NC2-B). Rate constants for “fast” steps were arbitrarily assigned values 10-fold higher than the rate constants for the steps preceding them. The rising phase in fluorescence corresponds largely with formation and dissociation of the NC2-B intermediate, although dissociation of the initial Ca2+-saturated complex due to dilution also contributes. The falling phase corresponds with formation of the final Ca2+-free complex, which is not as fluorescent as the initial complex. The slight discrepancy between the fitted curves and the data measured during the first few milliseconds is probably due to the simplifying assumption that short-lived intermediates with Ca2+ bound to only a single site in an EF hand pair are effectively non-dissociating (dashed arrows in Scheme 2). The overall quality of the fit suggests that this scheme nevertheless represents a reasonable minimal model. The validity of the model is lent further support by the fact that fitted values of 13.5±0.1 and 528.3±3.3 s−1 were derived for k′−2 and k′−4, which are essentially identical to the Ca2+-dissociation rate constants derived from Ca2+ trapping experiments (Table 2).
Ca2+-dependent release of CaM in the presence of a CaM-binding peptide
Given the hypothesis that neuromodulin participates in CaM storage and delivery (9, 28, 29), we were particularly interested in investigating release of CaM from the neuromodulin IQ domain in the presence of Ca2+ and a peptide (ckPEP) based on the CaM-binding domain in CaM kinase II, an abundant enzyme in neurons (30). From an experimental standpoint, analysis of this process is complicated by the fact that two pathways for sequential occupancy of the N-ter and C-ter EF hand pairs must be considered (see Scheme 3). We therefore initially analyzed the behavior of the NxCCaM-BSCaMIQ complex to eliminate the contribution of the N-ter pair of Ca2+ binding sites.
Fluorescence time courses measured after adding excess ckPEP and different amounts of Ca2+ to the Ca2+-free mutant complex are presented in Fig. 5A. These data were globally fit according the steps in Scheme 3 that correspond with formation and dissociation of the intermediate NC2-B complex. The second Ca2+-binding step is effectively irreversible because Ca2+ dissociates from the NC2-B complex at a rate of ~10 s−1, while the complex itself dissociates at a rate of ~500 s−1 (Table 1 & Table 2). The fit presented in Panel A was produced by varying the dissociation constant (K1) for the first C-ter Ca2+-binding step and the association rate constant (k2) for the second Ca2+-binding step. The other necessary parameter was the dissociation rate constant for the NC2-B intermediate (k−NC2), which was fixed to the value given in Table 2. Dissociation of this intermediate is effectively irreversible, as there is a large molar excess of ckPEP. Relative molar fluorescence amplitudes were assigned to all kinetic species as detailed under “Materials and Methods”. The observed increases in fluorescence reflect the increase that occurs when the NC2-B intermediate forms, and the further increase that occurs when it dissociates. The quality of the fit suggests that a three step kinetic model we have used is a reasonable minimal description of this process. The fitted values obtained for K1 and k2 are 4.8±0.9 µM and 3.1±0.1×106 M−1-s−1. The latter value is ~10-fold less than the Ca2+ association rate constant estimated for free CaM (Table 2).
We next measured a series of fluorescence time courses for the native CaM-BSCaMIQ complex using the same experimental approach (Fig. 5B). These data were globally fit according to the model depicted in Scheme 3. The rate constant for dissociation of the Ca2+ saturated CaM-BSCaMIQ complex in the presence of ckPEP is designated as since this step appears to involve formation of a ternary complex with the peptide. The bracketed portion of the scheme can be omitted from fitting calculation without significant effect, apparently because in the presence of ckPEP the NC2-B and N2C2-B intermediates dissociate at essentially the same rate (Table 2), and there is also little difference in their fluorescence (15). Binding of Ca2+ to the N-ter EF hand pair prior to the C-ter pair is modeled as rapidly equilibrating because the rates at which N2C-B dissociates or is converted to the N2C2-B intermediate are relatively slow (see Table 1 and Table 2). The fit shown in Fig. 5B was produced by varying only K′1 and k′2. Previously-determined dissociation constants of 122 and 9.4 µM were assigned to K′3 and K′4 (15). Other required kinetic parameters, including K1 and k2, were fixed to the values given in Table 1 & Table 2. The parameters for reactions involving NC2-B and N2C-B were derived using NxCCaM and NCxCaM. Relative molar fluorescence amplitudes were assigned to all kinetic species as detailed under “Materials and Methods”. The observed fluorescence time courses reflect increases due to formation of the NC2-B and N2C-B intermediates, and to release of BSCaMIQ (B). Formation of the N2C-B intermediate produces a fluorescence increase that occurs within the ~1.4 ms dead time of the stopped-flow (Fig. 5B). This increase thus corresponds with rapidly-equilibrating binding of Ca2+ to the N-ter EF hand pair, and its amplitude increases as a function of the free Ca2+ concentration in accordance with the values used for K′3 and K′4 . The amplitude of this phase is also linked to the amount of BSCaMIQ dissociation occurring via the lower pathway in Scheme 3. Thus, at a free Ca2+ concentration of ~10 µM, dissociation via this pathway is negligible, while at a free Ca2+ concentration of ~70 µM dissociation via the upper and lower pathways is equivalent (data not shown). At a free Ca2+ concentration of 250 µM the proportion of dissociation via the lower pathway saturates at a value of ~65%. This occurs in spite of the much faster Ca2+-binding kinetics of the N-ter EF hand pair because the N2C-B intermediate dissociates relatively slowly. The overall quality of the fit indicates that Scheme 3 represents an acceptable minimal kinetic model. Fitted values of 4.1±0.6 µM and 3.2±0.1×106 M−1-s−1 were derived for K′1 and k′2, which are not significantly different from the values derived for K1 and k2. The association rate constant for the C-ter Ca2+-binding sites in CaM therefore appears to be reduced to the same extent, regardless of whether Ca2+ is bound to the N-ter pair of sites.
Discussion
In this paper we present the results of a kinetic analysis of the complex between CaM and BSCaMIQ, a fluorescent reporter containing the IQ domain from neuromodulin (15). Association and dissociation rate constants for various Ca2+-bound forms of the complex were determined, as well as many of the rate constants governing Ca2+ binding to the complex. Kinetic models incorporating these rate constants were developed that account for the BSCaMIQ fluorescence responses observed when Ca2+ is removed from the Ca2+-saturated complex or when Ca2+ is added to the Ca2+-free complex in the presence of a peptide based on the CaM-binding domain in CaM kinase II. Three key points are evident: (1) Association and dissociation of the complex is accelerated in the presence of Ca2+. (2) When CaM is bound to the IQ domain, the Ca2+-association rate constant for the C-ter EF hand pair is reduced ~10-fold and the Ca2+-dissociation rate constant for the N-ter EF hand pair is reduced ~3-fold. (3) Ca2+-saturated CaM can be transferred from the neuromodulin IQ domain to canonical CaM-binding domains, such as the one in CaM kinase II, via an intermediate ternary complex.
CaM-binding kinetics
As seen in Table 1, there is complete agreement between the association and dissociation rate constants for various native and mutant CaM-BSCaMIQ complexes derived from replots of kobs vs [CaM] and the corresponding equilibrium Kd values. The association and dissociation rates for the Ca2+-saturated CaM-IQ domain complex and the C-ter Ca2+-bound intermediate are 30 to 100-fold faster than for the Ca2+-free complex (Table 1). The association rate constants derived for the Ca2+-saturated complex and the C-ter Ca2+-bound intermediate are similar to those derived for the Ca2+-saturated complexes between CaM and other types of CaM-binding sequences (22, 23, 31). The kinetics of the N-ter Ca2+-bound intermediate are comparatively slow, as its association rate constant is only 3-fold faster than the rate constant for the Ca2+-free complex (Table 1). This indicates that slow binding kinetics are a feature of interactions between the Ca2+-free C-ter CaM lobe and the IQ domain.
Dissociation rate constants were also determined for native and mutant CaM-BSCaMIQ complexes by using ckPEP or ngPEP to trap CaM. With only one exception, these values agree with those derived from replots of kobs vs [CaM] (Table 1). The exception is the Ca2+-saturated native CaM-BSCaMIQ complex, which dissociates at a rate of 565.1±13.1 in the presence of ckPEP, compared with a rate of 69.4±9.6 derived from an analysis of binding kinetics (Table 1). Dissociation is accelerated in the presence of the peptide, since the slower rate is consistent with the Kd value for this complex (Table 1). The only reasonable explanation for this behavior is that in the presence of ckPEP dissociation occurs via a ternary complex between the peptide, CaM and BSCaMIQ. The absence of an effect of ckPEP on the dissociation rates for the intermediate CaM-BSCaMIQ complexes in which Ca2+ is bound only to the N-ter or C-ter EF hand pair suggests that neither can participate in a ternary complex, or that they do so with no apparent effect on their kinetic properties. Gaertner and co-workers have previously presented evidence consistent with formation of a ternary complex between intact CaM kinase II, CaM and the IQ domain in neurogranin (32). A ternary complex intermediate provides a route for efficient delivery of CaM from neuromodulin to other CaM-binding proteins, consistent with its proposed role in CaM storage and delivery.
Ca2+-binding kinetics
Ca2+ dissociation rates for the N-ter and C-ter EF hand pairs in bound native and mutant CaMs were directly evaluated in Ca2+ trapping experiments (Table 2). These rates agree with those derived from a global analysis BSCaMIQ fluorescence data measured during the transition from the Ca2+-saturated to the Ca2+-free complex (Fig. 4A and B; Table 2). The dissociation rate derived for the N-ter EF hand pair in bound CaM (k′−4) is 473.4±37.9 s−1, while the corresponding value for free CaM (k−4) is 1298±135 s−1. In principle, the k−4/k′−4 ratio of 2.7±0.4 should be comparable to the K4/K′4 ratio, but in practice the individual dissociation constants for an EF hand pair are not as well determined as the product of the constants (15). It is therefore preferable to compare the K3K4/K′3K′4 ratio with the square of the k−4/k′−4 ratio. The latter ratio is squared because k−4 and k′−4 both correspond with dissociation of two Ca2+ ions (see Scheme 2). The value of 10.2±1.8 derived for K3K4/K′3K′4 from data published previously is in reasonable agreement with the square of k−4/k′−4, which is 7.3±1.1 (15). This suggests that the increased Ca2+-binding affinity of the N-ter EF hand pair in the CaM-IQ domain complex is due to a decreased Ca2+ dissociation rate. The Ca2+ dissociation rates determined for the C-ter EF hand pair in bound and free CaM are essentially identical (Table 2), so the reduced affinity of these sites in the CaM-IQ domain complex is not due to an accelerated dissociation rate.
Association rates for Ca2+ were derived for the C-ter EF hand pair in bound CaM from a global analysis of fluorescence data measured after addition of Ca2+ and ckPEP to the Ca2+-free native or NxCCaM complex with BSCaMIQ (Fig. 5A and B; Table 2). Association rates could not be determined for the N-ter EF hand pair. Since essentially the same kinetic parameters were derived for the C-ter EF hand pairs in the native and NxCCaM complexes with BSCaMIQ, we shall only discuss our analysis of the latter in detail. We have modeled Ca2+ binding to an EF hand pair as a sequential process, with a rapidly equilibrating first step and a slower second step. Fitted values of 4.6±0.9 µM and 3.1±0.1×106 M−1-s−1 were derived for K1, the dissociation constant for the first step and k2, the association rate constant for the second step. In the presence of ckPEP, we can ignore k−2, the rate constant for reversal of the second step, because Ca2+ dissociates ~50-fold slower from the complex than does Ca2+-saturated NxCCaM, which is then trapped by ckPEP (Table 1). The value derived for k2 is ~10-fold less than the corresponding value for free NxCCaM (Table 2). By combining the values we have derived for K1 and k2 with a k−2 value of 11.8±0.5 (Table 2), we can calculate a value of 18.2±5.1 µM2 for the product of the two Ca2+ dissociation constants for the NxCCaM-BSCaMIQ complex. This is in reasonable agreement with the value of 24.6±2.7 µM2 derived from equilibrium Ca2+-binding data (15). Thus, the reduced affinity of the C-ter EF hand pair in bound CaM appears to be due to a reduced Ca2+ association rate, as expected given the absence of any effect on the dissociation rate.
A surprising aspect of our results is that the same association rate constant is derived for Ca2+ binding to the C-ter EF hand pair, regardless of whether the N-ter EF hand pair is occupied by Ca2+. Since the affinity of C-ter EF hand pair for Ca2+ is higher when the N-ter sites are occupied, it appears that the dissociation rate constant must be reduced to account for this (15). This is difficult to prove because Ca2+ dissociates from the C-ter sites much more slowly than it does from the N-ter sites. Thus, dissociation from the C-ter sites while the N-ter sites remain occupied cannot be observed in Ca2+ trapping experiments.
Implications for neuromodulin function
We have shown previously that under equilibrium conditions the apparent dissociation constant for the neuromodulin CaM-IQ domain complex has a bell-shaped dependence on the free Ca2+ concentration, peaking at a concentration of ~ 6 µM (15). Thus, release of CaM is promoted over a relatively narrow range of free Ca2+ concentrations. During a Ca2+ transient in a neuron the free Ca2+ concentration is likely to pass rapidly through this range, so we have proposed that release of CaM is only promoted briefly (15). Although the bell-shaped response curve has important implications with regard to regulation via CaM-IQ domain switches under equilibrium or quasi-equilibrium conditions, the analysis presented in this paper suggests some modifications to our understanding of what occurs during a rapid Ca2+ transient. The overall picture that emerges is one in which neuromodulin continuously maintains a local store of CaM that is efficiently delivered to target proteins in its Ca2+-bound form during a Ca2+ transient.
The principal difference between the equilibrium and transient behaviors of the CaM-IQ domain complex is that the N-ter Ca2+-bound intermediate plays a significant role when Ca2+ is rapidly increased to levels above 10 µM, as can occur locally during a neuronal Ca2+ transient (33–38). This intermediate does not form to a significant extent under equilibrium conditions, primarily because the C-ter Ca2+-binding sites, although slower kinetically, have an EC50(Ca2+) of ~5 µM, while the value for the N-ter sites is ~ 35 µM (15). The N-ter Ca2+ bound intermediate dissociates at a relatively slow rate of ~30 s−1, compared with a rate of ~500 s−1 for the C-ter intermediate. The kinetic properties of the CaM-IQ domain complex therefore appear to inhibit release of CaM in its Ca2+ free and N-ter Ca2+ bound intermediate forms (Table 1). When the C-ter Ca2+-bound CaM intermediate dissociates it is presumably captured by target proteins and rapidly converted to Ca2+-saturated CaM, given the essentially diffusion-limited Ca2+ association rate constants for the N-ter EF hand pair (Table 2). If the N-ter Ca2+-bound intermediate were to dissociate to a significant extent it could have negative consequences. First, since at least some targets appear to capture CaM via its C-ter lobe, the N-ter Ca2+-bound intermediate may simply not be bound in some cases (39). Second, if a target were to capture this intermediate, the relatively slow Ca2+ association rate constants for the C-ter EF hand pair could impose a significant delay on target activation (Table 2). An implication of the latter point is that an abundant localized store accelerates production of CaM in its Ca2+-saturated and C-ter Ca2+-bound intermediate forms sufficiently to meet the speed requirements of neuronal signaling. Given a high enough local CaM concentration, this could be effected through simple mass action. The similar affinities of neuromodulin for Ca2+-free and Ca2+-saturated CaM may serve not only to minimize diffusional losses of CaM, as we have proposed previously, but also to continuously replenish the CaM store through diffusional recruitment from adjacent regions in the cell. The degree to which ternary complexes play a role in CaM delivery in neurons remains to be established, although they clearly have the potential to facilitate this process.
We are currently extending these investigations by determining how the CaM-IQ domain complex responds to synthetic Ca2+ transients. In conjunction with extensive modeling, this approach should allow us to develop a more quantitative understanding of Ca2+ switching among various states. The kinetic aspects of the CaM-IQ domain complex that we have described are likely to have counterparts in systems that are functionally modulated by CaM-IQ domain switches, such as ion channels and unconventional myosins. A particular intriguing possibility is that ratio of the N-ter and C-ter Ca2+-bound intermediates that is produced during a Ca2+ transient provides a mechanism for producing functional responses that depend upon the amplitude and speed of the transient.
Footnotes
This work was supported by NIH Grant GM074887 to A.P.
Abbreviations
CaM, calmodulin; BSCaMIQ, fluorescent biosensor containing a CaM-binding sequence based on the IQ domain in neuromodulin; ECFP, cyan emitting variant of green fluorescent protein, EYFPC, yellow emitting variant of green fluorescent protein; NxCCaM, mutant CaM with E31A and E67A substitutions; NCxCaM, mutant CaM with E104A and E140A substitutions; N-ter, the NH2-terminal end of a polypeptide; C-ter, the COOH-terminal end of a polypeptide; NC, Ca2+-free CaM; N2C, CaM with Ca2+ bound to both N-ter EF hands; NC2, CaM with Ca2+ bound to both C-ter EF hands; N2C2, CaM with Ca2+ bound to all four EF hands; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid; dibromo-BAPTA, 1,2-bis(2-amino-5,5'-dibromophenoxy)ethane-N,N,N',N'-tetraacetic acid; FRET, fluorescence resonance energy transfer; ckPEP, a synthetic peptide based on the CaM-binding domain in CaM-dependent protein kinase II; ngPEP, a synthetic peptide based on the CaM-binding domain in neurogranin.
References
- 1.Yagi K, Yazawa M, Kakiuchi S, Ohshima M, Uenishi K. Identification of an activator protein for myosin light chain kinase as the Ca2+-dependent modulator protein. J. Biol. Chem. 1978;253:1338–1340. [PubMed] [Google Scholar]
- 2.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Nat. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venema RC, Sayegh HS, Arnal JF, Harrison DG. Role of the enzyme calmodulin-binding domain in membrane association and phospholipid inhibition of endothelial nitric oxide synthase. J. Biol. Chem. 1995;270:14705–14711. doi: 10.1074/jbc.270.24.14705. [DOI] [PubMed] [Google Scholar]
- 4.Dabrowska R, Sherry JM, Aromatorio DK, Hartshorne DJ. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978;17:253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- 5.Jurado LA, Chockalingam PS, Jarrett HW. Apocalmodulin. Physiol. Rev. 1999;79:661–682. doi: 10.1152/physrev.1999.79.3.661. [DOI] [PubMed] [Google Scholar]
- 6.Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- 7.Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annu. Rev. Physiol. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- 8.Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin.[republished from Sci STKE. 2005 Dec 20;2005(315):re15; PMID: 16369047] Science's Stke [Electronic Resource]: Signal Transduction Knowledge Environment. 2006;2006:er1. doi: 10.1126/stke.3182006er1. [DOI] [PubMed] [Google Scholar]
- 9.Estep RP, Alexander KA, Storm DR. Regulation of free calmodulin levels in neurons by neuromodulin: relationship to neuronal growth and regeneration. Curr. Top. Cell. Regul. 1990;31:161–180. doi: 10.1016/b978-0-12-152831-7.50006-8. [DOI] [PubMed] [Google Scholar]
- 10.Tran QK, Black DJ, Persechini A. Intracellular coupling via limiting calmodulin. J. Biol. Chem. 2003;278:24247–24250. doi: 10.1074/jbc.C300165200. [DOI] [PubMed] [Google Scholar]
- 11.Rakhilin SV, Olson PA, Nishi A, Starkova NN, Fienberg AA, Nairn AC, Surmeier DJ, Greengard P. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science. 2004;306:698–701. doi: 10.1126/science.1099961. [DOI] [PubMed] [Google Scholar]
- 12.Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc. Natl. Acad. Sci. USA. 2004;101:6279–6284. doi: 10.1073/pnas.0308742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnegy ME. Calmodulin: effects of cell stimuli and drugs on cellular activation. Prog. Drug Res. 1995;45:33–65. doi: 10.1007/978-3-0348-7164-8_2. [DOI] [PubMed] [Google Scholar]
- 14.Gerendasy DD, Herron SR, Watson JB, Sutcliffe JG. Mutational and biophysical studies suggest RC3/neurogranin regulates calmodulin availability. J. Biol. Chem. 1994;269:22420–22426. [PubMed] [Google Scholar]
- 15.Black DJ, Leonard J, Persechini A. Biphasic Ca2+-dependent switching in a calmodulin-IQ domain complex. Biochemistry. 2006;45:6987–6995. doi: 10.1021/bi052533w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fruen BR, Black DJ, Bloomquist RA, Bardy JM, Johnson JD, Louis CF, Balog EM. Regulation of the RYR1 and RYR2 Ca2+ release channel isoforms by Ca2+-insensitive mutants of calmodulin. Biochemistry. 2003;42:2740–2747. doi: 10.1021/bi0267689. [DOI] [PubMed] [Google Scholar]
- 17.Tang W, Halling DB, Black DJ, Pate P, Zhang JZ, Pedersen S, Altschuld RA, Hamilton SL. Apocalmodulin and Ca2+ calmodulin-binding sites on the Ca(V)1.2 channel. Biophys. J. 2003;85:1538–1547. doi: 10.1016/s0006-3495(03)74586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 19.Forsén S, Vogel HJ, Drakenberg T. Biophysical studies of calmodulin. In: Cheung WY, editor. Calcium and Cell Function. New York: Academic Press; 1986. pp. 113–157. [Google Scholar]
- 20.Persechini A, White HD, Gansz KJ. Different Mechanisms For Ca2+ Dissociation From Complexes of Calmodulin With Nitric Oxide Synthase or Myosin Light Chain Kinase. J. Biol. Chem. 1996;271:62–67. doi: 10.1074/jbc.271.1.62. [DOI] [PubMed] [Google Scholar]
- 21.Malmendal A, Evenas J, Forsen S, Akke M. Structural dynamics in the C-terminal domain of calmodulin at low calcium levels. J. Mol. Biol. 1999;293:883–899. doi: 10.1006/jmbi.1999.3188. [DOI] [PubMed] [Google Scholar]
- 22.Bowman BF, Peterson JA, Stull JT. Pre-steady-state Kinetics of Activation of Rabbit Skeletal Muscle Myosin Light Chain Kinase by Ca2+/Calmodulin. J. Biol. Chem. 1992;267:5346–5345. [PubMed] [Google Scholar]
- 23.Kasturi R, Vasulka C, Johnson JD. Ca2+, caldesmon, and myosin light chain kinase exchange with calmodulin. J. Biol. Chem. 1993;268:7958–7964. [PubMed] [Google Scholar]
- 24.Bayley P, Ahlstrom P, Martin SR, Forsén S. The kinetics of calcium binding to calmodulin: Quin 2 and Ans stopped-flow fluorescence studies. Biochem. Biophys. Res. Comm. 1984;120:185–191. doi: 10.1016/0006-291x(84)91431-1. [DOI] [PubMed] [Google Scholar]
- 25.Martin SR, Teleman AA, Bayley PM, Drakenberg T, Forsén S. Kinetics of calcium dissociation from calmodulin and its tryptic fragments. A stopped-flow fluorescence study using Quin 2 reveals a two-domain structure. Eur. J. Biochem. 1985;151:543–550. doi: 10.1111/j.1432-1033.1985.tb09137.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin SR, Linse S, Bayley PM, Forsen S. Kinetics of cadmium and terbium dissociation from calmodulin and its tryptic fragments. Eur. J. Biochem. 1986;161:595–601. doi: 10.1111/j.1432-1033.1986.tb10483.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JD, Snyder C, Walsh M, Flynn M. Effects of myosin light chain kinase and peptides on Ca2+ exchange with the N- and C-terminal Ca2+ binding sites of calmodulin. J. Biol. Chem. 1996;271:761–767. doi: 10.1074/jbc.271.2.761. [DOI] [PubMed] [Google Scholar]
- 28.Chapman ER, Au D, Alexander KA, Nicolson TA, Storm DR. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J. Biol. Chem. 1991;266:207–213. [PubMed] [Google Scholar]
- 29.Liu YC, Storm DR. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol. Sci. 1990;11:107–111. doi: 10.1016/0165-6147(90)90195-e. [DOI] [PubMed] [Google Scholar]
- 30.Nairn AC, Picciotto MR. Calcium/calmodulin-dependent protein kinases. Semin. Cancer Biol. 1994;5:295–303. [PubMed] [Google Scholar]
- 31.Johnson JD, Holroyde MJ, Crouch TH, Solaro RJ, Potter JD. Fluorescence studies of the interaction of calmodulin with myosin light chain kinase. J. Biol. Chem. 1981;256:12194–12198. [PubMed] [Google Scholar]
- 32.Gaertner TR, Putkey JA, Waxham MN. RC3/neurogranin and Ca2+/calmodulin-dependent protein kinase II produce opposing effects on the affinity of calmodulin for calcium. J. Biol. Chem. 2004;279:39374–39382. doi: 10.1074/jbc.M405352200. [DOI] [PubMed] [Google Scholar]
- 33.Etter EF, Minta A, Poenie M, Fay FS. Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18. Proc. Natl. Acad. Sci. USA. 1996;93:5368–5373. doi: 10.1073/pnas.93.11.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsault R, Murgia M, Pozzan T, Rizzuto R. Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 1997;16:1575–1581. doi: 10.1093/emboj/16.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies EV, Hallett MB. High micromolar Ca2+ beneath the plasma membrane in stimulated neutrophils. Biochem. Biophys. Res. Commun. 1998;248:679–683. doi: 10.1006/bbrc.1998.9031. [DOI] [PubMed] [Google Scholar]
- 36.Klingauf J, Neher E. Modeling Buffered Ca2+ Diffusion Near the Membrane - Implications For Secretion In Neuroendocrine Cells. Biophys. J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon SM, Llinas RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys. J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llinas R, Moreno H. Local Ca2+ signaling in neurons. Cell Calcium. 1998;24:359–366. doi: 10.1016/s0143-4160(98)90059-8. [DOI] [PubMed] [Google Scholar]
- 39.Persechini A, McMillan K, Leakey P. Activation of myosin light chain kinase and nitric oxide synthase activities by calmodulin fragments. J. Biol. Chem. 1994;269:16148–16154. [PubMed] [Google Scholar]