Abstract
There has been recent interest in the depletion of regulatory T cells (Tregs) as part of a multi-faceted approach to the immunotherapy of melanoma patients. This is in part due recent findings that convincingly show that Tregs are an integral part of regulating and even suppressing an immune response to growing tumor cells. We therefore compared three methods of Treg depletion and/or elimination, utilizing low dose cyclophosphamide (CY), a specific antibody directed against the IL-2 receptor found on Tregs (PC61) and the use of denileukin diftitox (DD), which is a fusion protein designed to have a direct cytocidal action on cells which express the IL-2 receptor. We show that CY administration resulted in the highest reduction in Tregs among the three reagents. However, the reduction in Tregs with CY was also associated with the concomitant reduction of CD8(+) T cells and a lack of tumor antigen priming. Utilization of DD resulted in a >50% Treg cell reduction without parallel cytocidal effects upon other T cell subsets but did not enhance anti-tumor immunity against B16 melanoma. Lastly, the PC61 showed a moderate reduction of Tregs that lasted longer than the other reagents, without a reduction in the total number of CD8(+) T cells. Furthermore, PC61 treatment did not abrogate tumor antigen-specific immunity elicited by dendritic cells (DC). We therefore conclude that PC61 administration was the most effective method of reducing Tregs in a murine melanoma model in addition to providing evidence of a synergistic effect when combined with DC-based immunotherapy.
Keywords: Regulatory T cell, Dendritic cell, Melanoma, Cyclophosphamide, Denileukin diftitox, Anti-CD25 antibody
Introduction
Early studies in the 1980’s described an immunological “obstacle” preventing the induction of anti-tumor immunity by immunotherapeutic approaches. In those studies, varying concentrations of cyclophosphamide (CY) were used to deplete a population of precursor L3T4+ suppressor T cells, and it was noted that the elimination of such cells allowed for the CY-mediated regression of established tumor in a CY-resistant murine tumor model (North, 1984a; North, 1984b; DiGiacomo and North, 1986; Awwad and North, 1989). Recent studies have shown that these depleted cells were probably regulatory T cells (Tregs), characterized by the expression of CD4, CD25 and Foxp3. These cells were subsequently described as a naturally arising population of CD4(+) regulatory T cells that were deemed critical in immune self-tolerance and as an inhibitory control mechanism of the host immune response (Sakaguchi, 2004).
Once the functional role of Tregs was firmly established, various methods of depletion were employed, such as the use of cyclophosphamide (CY), denileukin diftitox (DD; ONTAK) and anti-CD25 antibodies, such as the PC61. Cyclophosphamide is an alkylating agent that interferes with the growth of rapidly proliferating cells, most likely through the inhibition of DNA synthesis. Denileukin diftitox is a recombinant DNA-derived cytotoxic fusion protein composed of the amino acid sequences for the diphtheria toxin, fragments A and B, followed by the sequences for Interleukin-2 (IL-2). Denileukin diftitox is capable of binding to cells that express the CD25 cell surface receptor, subsequently internalized into the cell. This is followed by the enzymatic inhibition of protein synthesis and cell death (Kelley et al., 1988; Hu et al., 1998; Kreitman, 2003; Dannull et al., 2005). Lastly, the anti-CD25 antibody (PC61), derived from the PC61 hybridoma, is capable of specifically binding to the IL-2 receptor α-chain (CD25). Thus, the purpose of this study was to compare the various known methods of Treg depletion, with a particular focus on the subsequent effect upon an immunotherapeutic approach to treating B16 melanoma in a murine model. The inhibition of Tregs as part of a multi-modal approach to immunotherapy may have important implications in our design of novel treatment options. In particular, this may allow the host immune system to overcome such “immunoregulatory roadblocks”, thereby allowing for a complete and robust anti-tumor immune response that is paramount to eliminating the tumor burden associated with patients with advanced disease, in particular patients with metastatic melanoma.
Materials and Methods
Animals
Five to eight-week-old female C57BL/6 mice (denoted B6) were purchased from Harlan Laboratories (Indianapolis, IN) and Charles River Laboratories, Inc. (Wilmington, MA), and maintained at the Animal Maintenance Facility at the H. Lee Moffitt Cancer Center and Research Institute (Tampa, FL). All mice were housed at least 1 week prior to beginning the experimental design. Mice were humanely euthanized when tumors exceeded 1.5 cm in diameter, appeared necrotic or interfered with locomotion. All animal experiments were permitted and approved by the institutional guidelines established by the animal review board for animal care at H. Lee Moffitt Cancer Center and Research Institute.
Culture medium
Complete medium (CM) consists of RPMI1640 medium (Mediatech, Inc., Herndon, VA) supplemented by 10% heat-inactivated fetal bovine serum (Atlanta biologicals, Lawrenceville, GA), 1mM sodium pyruvate (Mediatech, Inc.), 0.1mM nonessential amino acids (Mediatech, Inc.), 100units/ml penicillin, 100µg/ml streptomycin (Mediatech, Inc.), 50µM 2-mercaptoethanol (Sigma, St. Louis, MO), 0.5µg/ml fungizone (Cambrex, Walkersville, MD), and 10µg/ml gentamicin (Cambrex).
Tumor lines
The B16-BL6 (denoted B16) melanoma cell line was derived from a spontaneous melanoma in a B6 mouse and is considered to be poorly immunogenic (Fidler, 1975). B16 was cultured in CM and maintained by serial in vitro passage. MO5 is OVA gene-transfected B16 tumor line (Falo et al., 1995). The MO5 cell line is maintained in CM containing 200 mg/ml hygromycin B (Invitrogen Corp., Carlsbad, CA). For tumor growth experiments, 1 × 105 B16 or 1 × 106 MO5 tumor cells were injected into the right flank of the mouse. Data are reported as the average tumor area ± SEM for each group.
Generation of dendritic cells (DC)
Bone marrow cells were collected from the femurs and tibias of B6 mice under sterile conditions. Erythrocytes were lysed with ACK lysing buffer (0.15M NH4Cl, 1mM KHCO3, and 0.1mM EDTA in sterile water). Erythrocyte-depleted bone marrow cells were then washed twice with Dulbecco’s phosphate-buffered saline (PBS) (Mediatech, Inc.) and re-suspended in CM containing 20ng/ml of recombinant mouse granulocyte/macrophage colony-stimulating factor (GM-CSF) and recombinant mouse interleukin-4 (IL-4) (both from R&D Systems, Minneapolis, MN) at the concentration 1 × 106cells/ml, and then incubated at 37°C 5% CO2. At day 5, non-adherent cells were collected and DCs were enriched by density centrifugation over OptiPrep (Axis-Shield PoC AS, Oslo, Norway). Analysis of the collected cells by flow cytometry revealed that the DC population was >80% positive for MHC class-II, CD80, and CD86, and >70% positive for CD11c (data not shown). For DC vaccination, 1 × 106 DC were inoculated into the left flank of mice (opposite side from tumor injection) subcutaneously (s.c.). For the negative control, PBS or unpulsed DC were injected s.c. into the mice.
Peptide pulsing of DC
For some experiments, DCs were pulsed with either the H-2Kb-restricted peptide TRP2180–188 (SVYDFFVWL) or OVA257–264 (SIINFEKL) (Invitrogen Corp.). These peptides were dissolved in dimethyl sulfoxide (DMSO; Sigma) and diluted with CM. After OptiPrep enrichment, DCs were re-suspended in CM at a concentration of 1 × 106cells/ml, and then 10ìg/ml peptide was added to the culture media. After a 4-hour co-culture, cells were washed three times with PBS and re-suspended in PBS prior to vaccination.
Reagents
CY was purchased from Bristol-Myers Squibb Oncology (New York, NY). It was diluted with PBS at a concentration of 20mg/ml. Denileukin diftitox (DD) was provided by Ligand Pharmaceuticals, Inc., San Diego, CA, (now Eisai Corporation). Aliquots for DD were prepared and stored at −20°C until the experiments were performed. For the anti-CD25 antibody, ascites from the hybridoma clone PC61 was injected at 100µl/mouse. All reagents were diluted in 500µl PBS and injected intraperitoneally (i.p.), with controls consisting of 500µl PBS injected i.p.
Preparation of lymphocytes from spleen or tumor
For some experiments, lymphocytes were collected from the spleens of select mice for each group. Briefly, spleens were removed from mice under sterile conditions and minced and filtered with a 40µm cell strainer (BD Biosciences, San Diego, CA). Erythrocytes were then lysed with ACK lysing buffer and cells were washed twice with PBS. After washing, cells were re-suspended in CM or PBS and used for further experiments. For analysis of tumor infiltrating lymphocytes (TIL), tumors were excised 15 days after treatment with PBS or PC61. All extraneous non-tumor tissue was removed and the tumors were minced and digested for 2 h at room temperature in 1 mg/ml type IV collagenase (Sigma). Digested tumors were then passed over a 70 µm nylon mesh, washed once with HBSS, and resuspended in PBS+ 3% BSA to a concentration of 1 × 106 cells/ml for flow cytometric analysis.
Flow cytometry
For flow cytometric experiments, the following anti-mouse antibodies were used: Fluorescein isothiocyanate (FITC)-conjugated anti-CD3e, anti-CD4, anti-CD11c, anti-CD19, anti-CD25 (clone 7D4), anti-CD62L (all from BD Biosciences), phycoerythrin (PE)-conjugated anti-CD4, anti-CD8a, anti-CD25 (clone PC61), anti-CD44, anti-Gr-1, anti-NK1.1 (all from BD Biosciences), anti-Foxp3 (eBiosciences), phycoerythrin-carbocyanin5 (PE-Cy5)-conjugated anti-CD4, anti-CD8a (all from BD Biosciences), APC-conjugated anti-CD25 (clone PC61) (BD Biosciences) and appropriate isotype-matched controls.
For cell surface marker staining, cells were washed with flow buffer (0.01% NaN3, 2% fetal bovine serum in PBS) and Fcγ III/II receptor blocking was performed by purified anti-mouse CD16/32 antibody (BD Biosciences). The blocking antibody (1µg/1 × 106cells) was added and cells were placed on ice for 10 minutes. After the blocking procedure, antibodies (1 µg/1 × 106cells) for cell surface staining were added into each sample and placed on ice for 30 minutes protected from light. After two additional washes with buffer solution, all cells were fixed with 1% paraformaldehyde (PFA). To detect CD25 positive cells after PC61 treatment, FITC-conjugated anti-CD25 antibody (clone 7D4) was used. Intracellular staining was performed for Foxp3 staining with the PE Anti-Mouse Foxp3 Staining Set (eBioscience) according to the manufacturer’s instructions. After staining of cell surface antigens, Fix/Perm buffer was added and cells were incubated 4°C overnight in the dark. After washing the cells with PBS, permeabilization buffer was added and cells were washed twice. After blocking for 10 minutes with blocking antibody, PE-conjugated Foxp3 antibody (1ìg/1 × 106cells) was added and incubated for 30 minutes on ice. After washing the cells twice with permeabilization buffer, cells were fixed with 1% PFA. Data acquisition was performed by flow cytometry (FACScan or FACSCalibur; BD Biosciences) within 24hours after sample fixation, and data analysis was performed with CellQuest software (BD Biosciences).
ELISA
To test tumor antigen-specific IFN-γ production by lymphocytes, ELISA was performed with Mouse IFN-γ ELISA Set (BD Biosciences). Mice were injected with either PBS or PC61 i.p., followed 3 days later with mice vaccinated with OVA257–264 peptide-pulsed DC twice at 7-day interval. Five days after the final vaccination, spleens from the vaccinated mice or naïve mice were removed and splenocytes were collected and suspended in CM at 5 × 105/ml. The splenocytes were left unstimulated or stimulated with unpulsed DC, TRP2180–188-pulsed DC or OVA257–264-pulsed DC. The ratio of splenocytes to DC was 10:1. After a 48-hour incubation, supernatants were collected and IFN-γ was measured with ELISA.
Statistical analysis
For the comparison of treatment groups, one-way ANOVA was performed. Student’s t-test was performed to compare results between the two groups. All statistical evaluations of data were performed by GraphPad Prism (GraphPad Software, Inc. San Diego, CA). Data was considered to be statistically significant once a p-value of <0.05 was achieved.
Results
Regulatory T (Treg) cells in tumor bearing mice
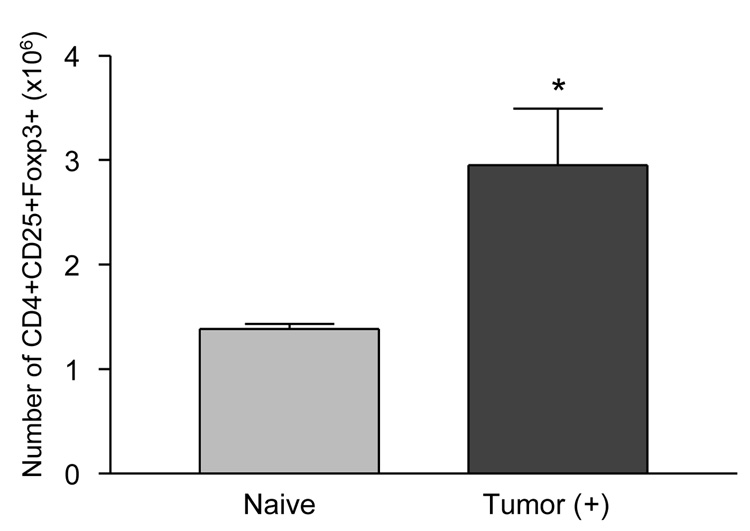
To assess immune suppression in tumor bearing mice, we first measured the number of CD4 (+)/CD25(+)/Foxp3(+) cells within the spleens of tumor bearing mice. B16 melanoma cells (1×105) were injected s.c. into mice. Mice were sacrificed at 21 days after tumor injection, and spleens were removed from select tumor-bearing and tumor-näive mice. CD4(+)/CD25(+)/Foxp3(+) splenocytes were measured by flow cytometry and we found a statistically significantly higher number of Treg cells in the tumor bearing mice compared to naïve mice (Fig. 1) (*P<0.05).
Figure 1. CD4(+)/CD25(+)/Foxp3(+) T cells are increased in tumor bearing mice.
B16 melanoma cells (1×105) were injected into the mice s.c. and 21days after the injection, the numbers of CD4(+)/CD25(+)/Foxp3(+) cells in spleens from naïve mice (n=4) and tumor bearing mice (n=4) were assessed by flow cytometry (*p<0.05).
The effect of cyclophosphamide on Treg cell reduction
To test the optimal concentration of CY necessary to reduce Treg cells in vivo, we injected various amounts of CY into the mice and examined the number of each cell subsets in the spleens utilizing flow cytometry. CD19(+) cells were markedly reduced in total numbers at low concentrations of CY (Fig. 2A). We also observed a significant reduction in CD4(+) and CD8(+) cells when 200mg/kg of CY was used, with an 85% reduction in CD4(+) cells and 79% reduction in CD8(+) cells from naïve mice. The largest decrease of CD4(+) and CD8(+) cells was observed 3 days after CY injection, with the baseline levels of each cell type returning to previous levels 5 days after the initial CY administration (data not shown). An overall decrease of CD4(+)/CD25(+) cells was observed when any concentration over 100mg/kg of CY was utilized (Fig. 2B) (*P<0.01; CY100mg/kg, **P<0.001; CY200 mg/kg). The respective decrease in Tregs was 41.2% (100mg/kg) and 80.4% (200mg/kg) from naïve mice. The largest decrease in CD4(+)/CD25(+)/Foxp3(+) cells was observed 3 days after the injection, with Treg cell counts returning to the same level as in naïve mice 7 days after CY administration (Figure 2C). We therefore determined that 200mg/kg of CY was the optimal concentration for further studies involved with the depletion of Tregs with higher concentrations of CY resulting in toxicity in treated mice. We began by first injecting 200mg/kg of CY 2 days prior to B16 tumor injection. Although Treg cells were decreased by CY administration, we found that the time frame of tumor progression was similar to that seen in the control group, with no significant difference in tumor growth or metastatic spread noted (Figure 2D). Autoimmune vitiligo or other adverse side effects were not observed in mice treated with CY.
Figure 2. The effect of CY on lymphocyte subsets and tumor growth.
Three days after the i.p. injection of different concentrations of CY, the numbers of each lymphocyte subset (A) and Treg cells (B) in spleens were assessed by flow cytometry (*p<0.01 at 100mg/kg, **p<0.001 at 200mg/kg). (C) The number of CD4(+)/CD25(+)/Foxp3(+) T cells in the spleen were measured 1–7 days after injection of 200 mg/kg CY. (D) Two days before B16 tumor subcutaneous injection (1×105), 200mg/kg CY or PBS was injected into the mice (n=6). The mean tumor area was then measured. Open triangle: CY 200mg/kg, Closed square: PBS
Optimal administration of DD necessary to reduce Treg cells in vivo
We first determined the optimal concentration of DD in vivo, based upon in vitro data that showed a reduction in Treg cells treated with 10ng/ml or more of DD 24 hours after the treatment (data not shown). We then measured the effect of DD in vivo 24 hours after the final injection of DD. We utilized a time course of DD injections consecutively for 5 days, finding a reduction in Treg cells in vivo in both lymph nodes and splenocytes. We then injected DD for periods longer than 5-days consecutively, with no additional effects observed (data not shown). Treatment with DD at any concentration did not result in adverse side effects or the development of autoimmune vitiligo.
We then examined the optimal concentration of DD necessary to reduce Treg populations, without reducing either CD4(+) or CD8(+) T cells. Utilizing the same consecutive 5-day time course, we injected 30µg/kg DD each time, showing a significant reduction of Tregs in both lymph nodes and splenocytes, with higher concentrations of DD not showing any additional effects (data not shown). Furthermore, we found there was no difference in Treg reduction utilizing DD via an intraperitoneal or intravenous approach. Combining these results, we therefore decided to use a consecutive 5-day i.p. injection of 30µg/kg of DD for the in vivo studies. In contrast to the results obtained by CY administration, DD did not affect the overall CD4(+) or CD8(+) cell numbers (Fig. 3A), additionally reducing the population of Tregs by 59% in naïve mice (Fig. 3B) (*P<0.05). In order to verify that the depleted cells consisted primarily of Tregs, we measured CD4(+)/CD25(+)/Foxp3(+) expression in the cells. As shown in Figure 3C, treatment with DD resulted in a significant decrease in this cell population (p<0.05 compared to PBS-treated mice). However, we observed early recovery of Treg cells after treatment with DD in vivo (Fig. 3D).
Figure 3. The effect of DD administration to the number of lymphocyte subsets.
DD administration did not affect the number of CD8(+) cells (A) and showed significant Treg cell reduction (B). Twenty-four hours after the final injection of 30µg/kg 5-day DD i.p. injection, the numbers of each lymphocyte subsets in the spleens were assessed by flow cytometry (*P<0.05). (C) DD administration reduced the percentage of CD4(+)/CD25(+)/Foxp3(+) cells in the spleens of mice as assessed with flow cytometry. Bar graph indicates the result from 3 individual experiments (*p<0.05). (D) Treg recovery of Treg cells after DD administration. Mice were injected with 30µg/kg of DD for 5days i.p. and CD4(+)/CD25(+) cells in the spleens were measured by flow cytometry at days 1, 3, 5, 7, and 10 from the last day of DD injection.
The effect of DD on tumor regression
In order to examine the possible relationship between the depletion of Tregs and tumor regression, we treated mice with a 5-day consecutive course of DD followed by inoculation of mice with B16 melanoma 1 day after the final DD injection. Although we found an over 50% reduction in Tregs, we did not observe a significant regression or delay in tumor growth compared to control groups (Fig. 4A). We hypothesized that the concomitant induction of cytotoxic T lymphocytes (CTLs) with a depleted number of Tregs was not induced in this model to cause significant tumor regression. Thus, we further added DC vaccination to this treatment model, treating groups with either PBS or DD for 5 consecutive days followed by vaccination with TRP2180–188-pulsed DC 1 day after tumor injection. Again, we did not observe any significant difference between the DD+DC and PBS+DC groups (Fig. 4B). This may possibly be explained by the observation of the rapid recovery time of the Treg populations, returning to near-baseline levels only 3days after the final injection of DD (Fig. 3D).
Figure 4. DD administration does not delay tumor growth.
(A): Mice (n=6) received consecutive 5-day injections of 30µg/kg DD or PBS i.p.. One day after the final injection, 1×105 B16 cells were injected into the flank and mean tumor area was measured. Closed square: PBS, Open triangle: DD
(B): In addition to the schedule in experiment (A), mice in both groups were vaccinated with 1×106 TRP2180–188-pulsed dendritic cells 1 day after the B16 tumor inoculation, and mean tumor area was measured. Closed square: PBS+TRP2180–188-DC, Open triangle: DD+TRP2180–188-DC.
The effect of anti-CD25 antibody (PC61) on Treg cell reduction
To examine the effect of PC61 upon Tregs, we injected 100 µl of PC61 ascites fluid into mice i.p., showing a 75.5% decrease in Tregs from naïve mice (*P<0.05) without a parallel reduction of CD4(+) or CD8(+) cells (Fig. 5A and 5B). Furthermore, this effect continued for at least 10 days after PC61 injection (55.4% decrease at day 10, preliminary data). Despite significant reduction in Tregs, we did not observe autoimmune vitiligo in mice treated with PC61.
Figure 5. PC61 administration reduced Treg cells without influencing other lymphocyte subsets.
Mice (n=3) received 100µl of anti-CD25 antibody ascitic fluid (PC61) and the numbers of lymphocyte subsets (A) and Treg cells (B) in the spleens were measured 5 days after PC61 i.p. injection (*p<0.05).
The effect of PC61 on melanoma growth and survival
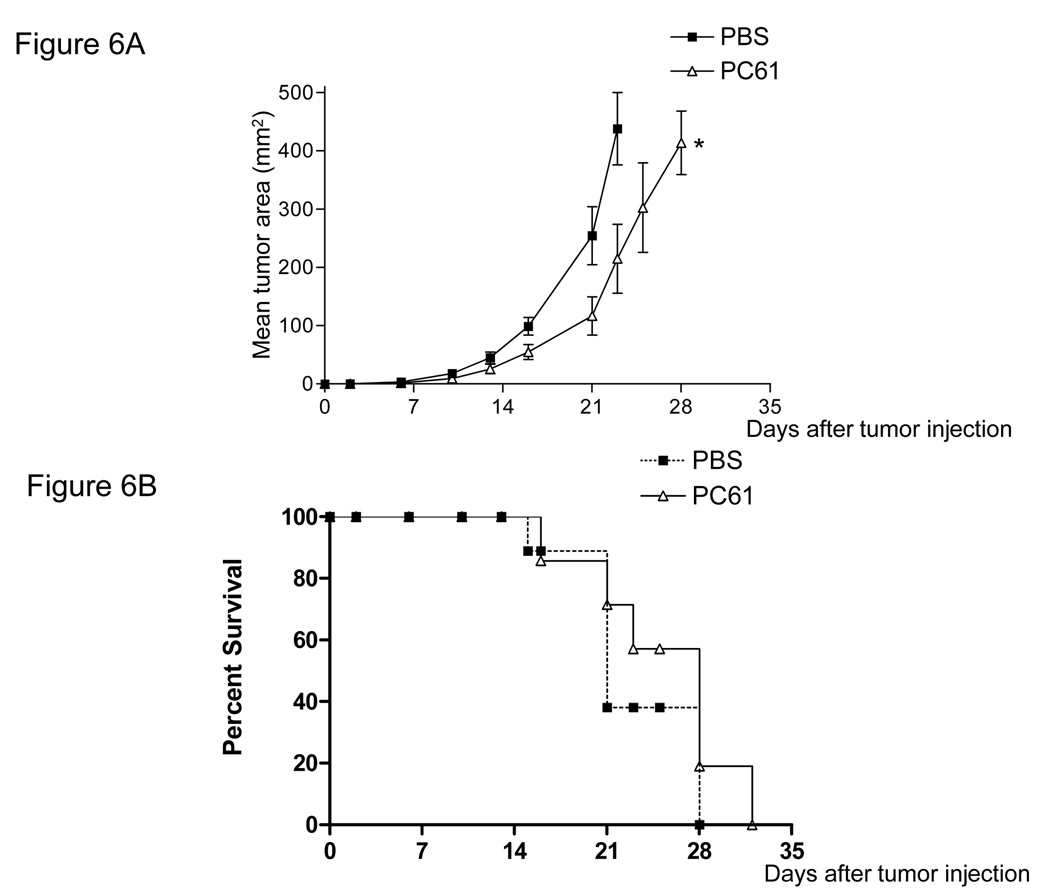
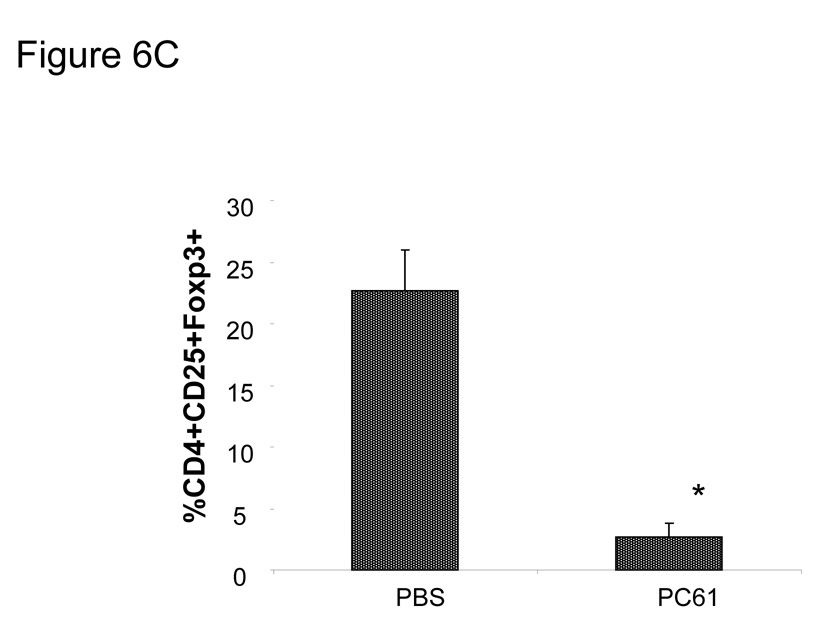
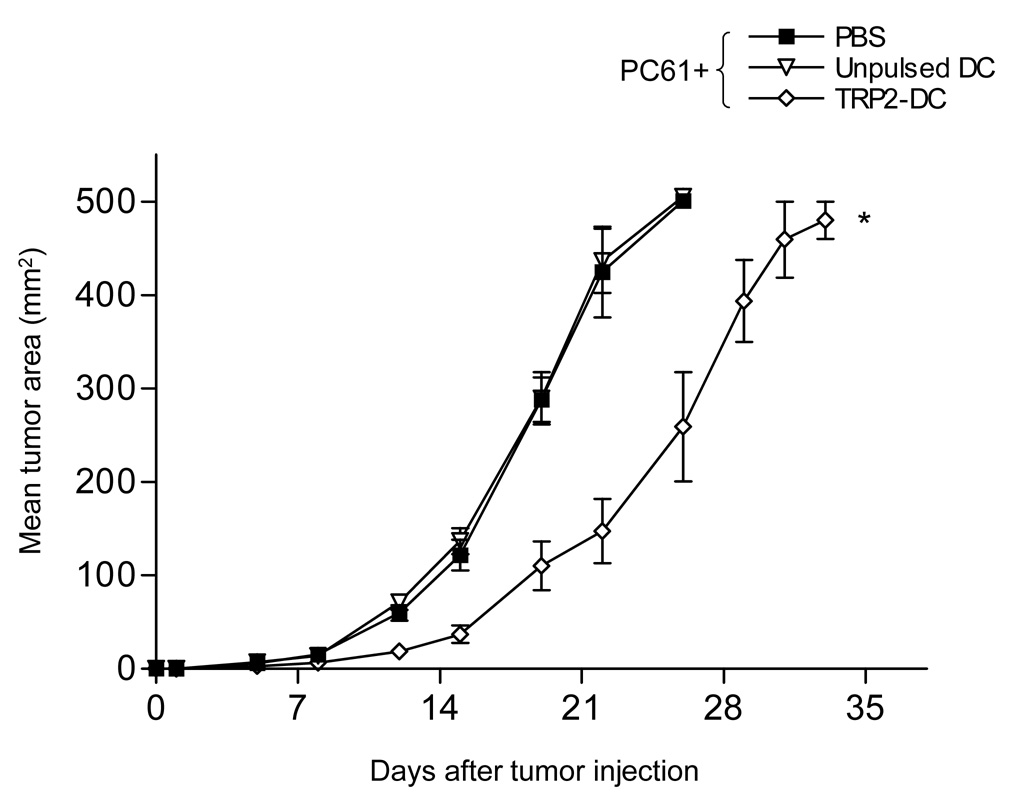
We measured the levels of CD25 expression on B16 melanoma and MO5 tumor cells and found no detectable levels noted on either cell populations (data not shown). We next examined the effect of PC61 on tumor regression in vivo after Treg reduction with PC61. We injected PC61 1day prior to B16 tumor inoculation into B6 mice, finding that a single injection of PC61 resulted in a significant delay in tumor growth (Fig. 6A *P<0.05 at day 23). However, we did not observe a significant prolongation in overall survival time in PC61 treated mice (PBS; 21.2±4.1days, PC61; 24.7±5.8days, P>0.05) (Fig. 6B). We verified that CD4(+)/CD25(+)/Foxp3(+) cells were reduced in the population of tumor infiltrating lymphocytes of these mice at 15 days after PC61 injection (Fig. 6C). We then treated mice with PC61 1 day prior to B16 melanoma cell s.c. injection, followed by vaccination with TRP2180–188-pulsed DC 3 times at 2, 4 and 6 days after tumor injection. We found that unpulsed DC did not result in any significant tumor regression compared to the control (PBS) group in the presence of PC61. However, TRP2180–188-pulsed DC showed a significant delay in tumor growth, even when PC61 was injected prior to DC vaccination (Fig. 7) (*P<0.01). Thus, treatment with PC61 did not hinder, and may in fact have a synergistic effect upon the anti-tumor effect elicited by DC-based vaccination.
Figure 6. A single injection of PC61 delayed tumor growth but did not prolong survival time.
Mice (n=6) received a single 100µl PC61 injection i.p. 1 day before 1×105 B16 tumor s.c. injection. Slight tumor growth delay was observed (A) (*P<0.05 at day23), although survival time was not prolonged (B). Closed square: PBS, Open triangle: PC61 (C) The percentage of CD4(+)/CD25(+)/Foxp3(+) cells in the tumors of mice receiving PC61 was measured by flow cytometry (*p<0.05). The bar graph represents results from 3 individual mice.
Figure 7. Administration of PC61 did not hinder the tumor antigen-specific immunity induced by DC.
For in vivo tumor experiments, mice (n=8) in all the groups received 100µl PC61 i.p. 1 day before the 1×105 B16 tumor injection. PBS or 1×106 unpulsed DC or 1×106 TRP2180–188-pulsed DC was injected s.c. at days 2, 4, and 6 after the tumor injection. Mean tumor area was then measured (*P<0.01 at day26). Closed square: PC61+PBS, Inverted open triangle: PC61+Unpulsed DC, Open diamond: PC61+TRP2180–188-pulsed DC
PC61 treatment enhances tumor reactivity of T cells
In order to analyze the induction of tumor antigen-specific T cells after treatment with PC61 and DC, we injected mice with PC61 24 hours prior to injection of MO5 cells. Mice received 2 injections of OVA257–264 peptide-pulsed DC on days 2 and 9 after tumor injection. We found that vaccination with OVA257–264 peptide-pulsed DC+PC61 induced a significant delay in tumor growth (Fig. 8A) (*P<0.001) in addition to a statistically significant prolongation in overall survival in the treatment group (PBS+DC; 23.1±3.9days, PC61+DC; 31.5±7.1days, *P<0.05) (Fig. 8B).
Figure 8. PC61 administration demonstrated tumor growth delay and prolonged survival time.
Mice (n=8) received 100µl PC61 or PBS i.p. 1 day before the 1×106 MO5 OVA-expressing tumor injection followed by the vaccination with 1×106 H-2Kb-restricted OVA257–264 peptide-pulsed DC at days 2 and 9. (A) Mean tumor area was measured and (B) survival recorded (*P<0.001 at day28 in (A), *P<0.05 in (B)). Closed triangle: PBS+OVA257–264-DC, Open square: PC61+OVA257–264-DC
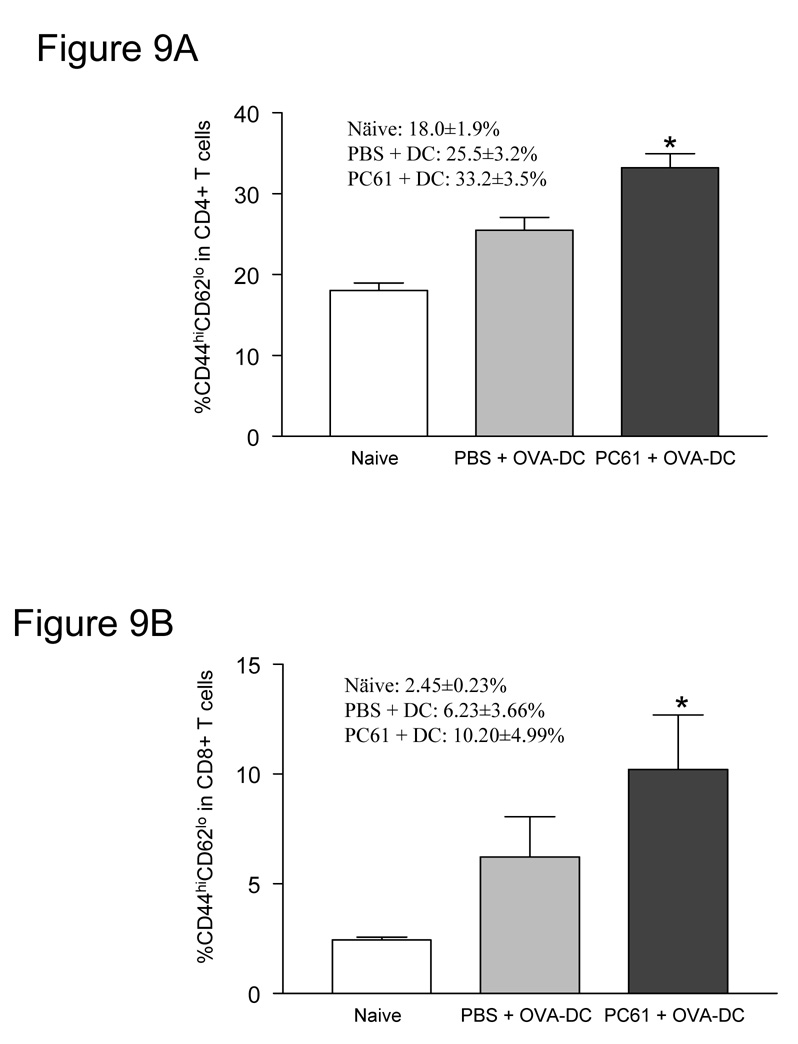
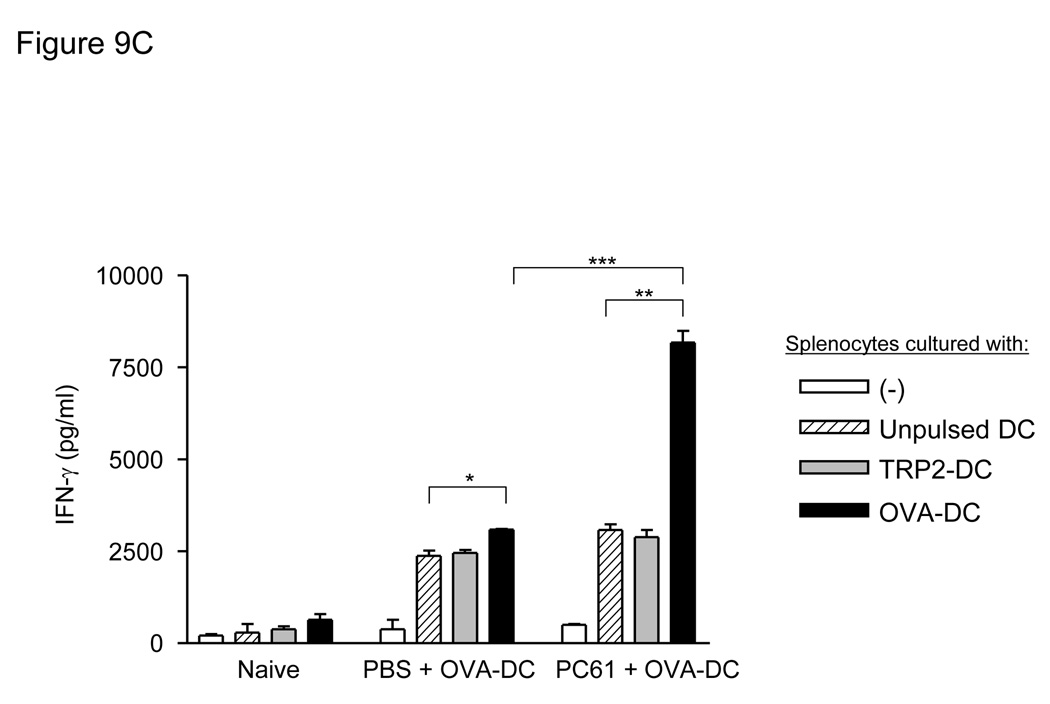
Since we observed a marked enhancement of protection in mice in response a DC-based vaccination strategy, we further investigated the specific phenotypes of the various T cell populations and the antigen-specific cytokine production after vaccination. As shown in figure 9A and 9B, the activated effector memory T cell phenotype (CD44 high and CD62L low) was increased in both CD4(+) and CD8(+) T cell populations only when mice were treated with PC61 followed by DC vaccination (*P<0.001 for CD4(+), *P<0.05 for CD8(+) T cells). We then measured the tumor antigen-specific IFN-γ production after combined vaccination with PC61 and pulsed-DC. Splenocytes were prepared from naïve mice and vaccinated mice with or without PC61 treatment and IFN-γ production was measured (Fig. 9C). OVA257–264 peptide-specific IFN-γ production was up-regulated by DC vaccination without PC61 administration (PBS-treated splenocytes co-cultured with unpulsed DC: 2376.7±240.1pg/ml, PBS-treated splenocytes co-cultured with OVA257–264 peptide-pulsed DC: 3086.7±32.1pg/ml, *P<0.05). The addition of PC61 to DC vaccination resulted in a high production of OVA257–264 peptide-specific IFN-γ (PC61-treated splenocytes co-cultured with unpulsed DC: 3050.0±255.1 pg/ml, PC61-treated splenocytes co-cultured with OVA257–264 peptide-pulsed DC: 8160.0±572.4 pg/ml, **P<0.001). Moreover, PC61 treatment significantly improved OVA257–264 peptide-specific IFN-γ production compared to PBS treatment (***P<0.005). Taken together, these results strongly indicate that Treg reduction by PC61 treatment improves tumor antigen-specific immunity elicited by DC vaccination in vivo.
Figure 9. PC61 administration enhanced activation of T cells and induced tumor antigen-specific immune response in vivo.
Activated T cell subsets increased after administration of PC61. Mice received 100µl PC61 or PBS i.p. followed by OVA-DC vaccination, and activated effector memory phenotype (CD44 high and CD62L low) was measured in CD4(+) (A) or CD8(+) (B) splenocytes. Graphs indicate results from 4 individual samples (*P<0.001 in CD4(+), *P<0.05 in CD8(+)). (C): PC61 administration with tumor antigen-loaded DC vaccination increased tumor antigen specific IFN-γ production. Mice received PBS or 100µl PC61 injection i.p. and were vaccinated with 1×106 OVA257–264-pulsed DC 3 and 10 days after PC61 injection. Five days after the final vaccination, lymphocytes were isolated from the spleens and the cells were left unstimulated or co-cultured with unpulsed DC or TRP2180–188-pulsed DC or OVA257–264-pulsed DC. After 48-hour incubation, supernatants were collected and IFN-γ production was measured by ELISA (*P<0.05, **P<0.001, ***P<0.005).
Discussion
Targeted therapy for patients with metastatic melanoma has developed rapidly over the last 10 years as our knowledge has grown in regards to specific targets and immunologic breakthroughs. For instance, although Tregs have been realized for some time, their key role in potential tumor escape mechanisms has only recently been discovered. Curiel et al. have identified a potent tumor-induced escape mechanism for patients with advanced ovarian cancer whereby the tumor cells are able to produce a chemokine, CCL22, capable of attracting regulatory T cells to the tumor site and malignant ascites (Curiel et al., 2004). They also showed that as the number of Tregs increased at the tumor site, the overall survival for these patients decreased dramatically, highlighting the powerful immunosuppressive effects of the Tregs upon the host immune system. Several researchers have thus identified the potential importance of suppressing and/or eliminating Tregs as a first step in an effective treatment strategy for patients with advanced melanoma (Viguier et al., 2004; Ribas et al., 2005).
We have begun to realize the complex nature of the host immune system in response to growing tumor, with many groups highlighting the central role of Tregs in regulating the immune response. Tregs are considered to play an important role in maintaining self-tolerance (Sakaguchi, 2005). By this mechanism, auto-immune phenomenon could be explained in part via the loss of Treg function in humans and mouse (Bennett et al., 2001; Wildin et al., 2001; Fontenot et al., 2003; Khattri et al., 2003). Since auto-immunity and tumor immunity are linked to each other, Tregs were found to suppress tumor immunity as well being involved in the development of auto-immunity (Shimizu et al., 1999; Sutmuller et al., 2001). Tregs were found to be increased in tumor bearing rodents as we verified in our studies (Fig. 1) as well as by others (Ghiringhelli et al., 2004). Other researchers have shown that Treg populations are increased in human cancer patients (Wolf et al., 2003; Curiel et al., 2004; Viguier et al., 2004).
Tregs are identified by their expression of CD4, CD25, CTLA-4, GITR and Foxp3, with their known immune suppressive activity via direct cell-cell contact (Ge et al., 2002). Treg cells suppress IL-2 production from CD4(+)/CD25(−) cells (Takahashi et al., 1998; Thornton and Shevach, 1998) or show the suppression through CTLA-4 (Read et al., 2000; Takahashi et al., 2000). Many researchers have reported an improvement of anti-tumor immunity after the pharmacologic depletion of Tregs. Several methods were reported to reduce Treg function in vivo such as CY (Ghiringhelli et al., 2004; Lutsiak et al., 2005), DD (denileukin diftitox) (Dannull et al., 2005), anti-CD25 antibody (PC61) (Onizuka et al., 1999; Shimizu et al., 1999; Tanaka et al., 2002; Tawara et al., 2002; Oldenhove et al., 2003; Haeryfar et al., 2005; Prasad et al., 2005), anti-CTLA-4 antibody (Sutmuller et al., 2001; Ribas et al., 2005) or GITR stimulation (McHugh et al., 2002; Shimizu et al., 2002; Ji et al., 2004).
Oldenhove et al. reported that Tregs are capable of suppressing DC function, leading to an increase in Th1 responses in vivo (Oldenhove et al., 2003). Dendritic cells are potent antigen presenting cells capable of eliciting immune responses to tumor antigens (Steinman, 1991; Hart, 1997). Peptide-pulsed (Mayordomo et al., 1995) and tumor-lysate-pulsed DC have been shown to induce tumor antigen-specific CTLs in murine tumor models and human (Fields et al., 1998; Geiger et al., 2001; Chang et al., 2002). B16 melanoma is poorly immunogenic. However concomitant tumor regression in B16 melanoma was reported after reducing Tregs in vivo (Turk et al., 2004). The mechanisms controlling DC-based immunity by Tregs in humans need to be further investigated. To this end, we investigated various methods to transiently eliminate Tregs in vivo for DC-based immunotherapy against melanoma.
We have attempted to identify a method of improving upon current immunotherapeutic treatment strategies for patients with metastatic melanoma. To this end, we have found an effective methodology of first eliminating Tregs followed by DC vaccination in a murine model. As shown in this report, 200mg/kg of CY was necessary to reduce Tregs in vivo. At this concentration, CY showed the highest reduction of Tregs among the 3 agents tested. However, we also found that CY (200mg/kg) unavoidably reduces the total number of CD8(+) T cells as well. At least 5 days were necessary to recover CD8(+) cells up to the level of naïve mice after the administration of CY. During this recovery period, CD8(+) cells are capable of being primed with known tumor antigens. However, in the B16 melanoma model, there was no significant difference between the untreated and CY-treated group. The timing of CY administration has been examined by others, showing that if CY is administrated 4 days prior to tumor inoculation, there is a significant prolongation in survival time (Turk et al., 2004). This lends support to the notion that T cell priming under Treg-depleted conditions should be performed at an early stage of tumor progression. As shown in other reports, the specific induction of tumor antigen-specific T cell after treatment with CY may be ultimately necessary in such a model in humans (Dudley et al., 2002; Rosenberg et al., 2003).
Denileukin diftitox (DD) is an immunotoxin that is specific for the IL-2 receptor. DD has current FDA approval for treating patients with cutaneous T cell lymphoma, with an overall response rate of approximately 30–40% (Olsen et al., 2001). Clinical trials utilizing DD have also been completed in patients with other cancer types, such as non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Our initial experiments have confirmed that DD induced cell death of Tregs in vitro (data not shown) and, contrary to CY, DD treatment did not affect the numbers of CD8(+) and CD4(+) cells in vivo. Interestingly, after treatment with DD, the reported remaining number of Tregs had a decrease in Foxp3 expression (Fig. 3C), a gene implicated as a critical regulator of Treg development and function (Fontenot et al., 2003).
Given these data, we hypothesized that the elimination of Tregs by DD would result in an improvement of tumor-specific immunity in DD-treated mice. However, even when we combined DD administration with tumor antigen-loaded DC, we saw no difference in tumor growth or survival between the groups, despite a 50% reduction in the overall number of Tregs in the DD-treated group. We hypothesized that this may be due to the short half-life of DD (Attia et al., 2005) combined with the early and rapid recovery of Tregs after treatment with DD (Fig. 3D). In comparison to CY treatment, the Treg reduction with DD was not substantial. Moreover, Tregs exhibited a more rapid recovery in total numbers compared to other treatments. One possible explanation may be that the time period of Treg reduction may not have been long enough to prime concomitant tumor antigen-specific immunity in vivo.
Recently, Dannull et al. have shown that the up-front elimination of Tregs with DD followed by vaccination with RNA-transfected DC significantly improved the stimulation of tumor-specific T cell responses in renal cell carcinoma patients (Dannull et al., 2005). However, compelling data from Attia et al. has revealed a much different conclusion as to the ability of DD to eliminate Tregs (Attia et al., 2005). They have extensively examined the functional utility of DD, failing to demonstrate that it was capable of eliminating the absolute number of Tregs or diminishing their suppressive activity. Thus, the current utility of DD in modulating Tregs in cancer patients remains in question at this time. The administration of PC61, revealed an intermediate reduction in the absolute number of Tregs compared to the other treatments (Fig. 5B). The overall effect of PC61 administration was longer than the effect seen with the other agents, CY and DD. Moreover, PC61 did not decrease the concomitant CD8(+) T cell populations (Fig. 5A). Applying PC61 to an in vivo murine melanoma tumor model revealed an enhanced anti-tumor immunity with the poorly immunogenic B16 tumor (Fig. 6A). Therefore, we decided to further pursue the utility of PC61 as part of a multimodal approach involving DC-based immunotherapy under Treg-depleted conditions. We found that ~30% of CD11c(+) cells, a receptor present on DC, also have a functional CD25 receptor (data not shown), thus initially raising concerns about the possibility of cross-reactivity with the PC61 antibody with DC. However, PC61 treatment did not hinder the priming of TRP2180–188-specific T cells by DC (Fig. 7), with the treatment showing additional effects upon the activated effector memory T cell phenotype (CD44 high and CD62L low) (Fig. 9A and B). We also showed that tumor antigen-specific T cell responses were enhanced in vivo compared to the DC vaccination only group (Fig. 9C). Thus, the data suggests that addition of PC61 administration may have several advantages over the other tested agents, possibly adding a degree of synergism that may be beneficial when combined with a multimodal immunotherapeutic approach to treating patients with metastatic melanoma.
Currently, there are only 2 FDA-approved monoclonal antibodies, basiliximab (Simulect) and daclizumab (Zenapax) directed against the human CD25 receptor. Both of these agents are chimeric monoclonal antibodies that are used for prophylaxis of acute organ rejection in patients who received renal transplantation. Recently, Dresske et al. reported that the initial interruption of daclizumab medication in renal transplant recipients increased the percentage of Tregs in patients and increased Foxp3 mRNA expression in the same cells (Dresske et al., 2006). Others have reported that daclizumab caused a decrease in the number of Tregs in renal transplant recipients (Bielekova et al., 2006), while treatment with basiliximab resulted in the abrogation of proliferative activity and CTLA-4 expression of Tregs (Game et al., 2005). Thus, there is accumulating evidence that the manipulation and elimination of Tregs with an anti-human CD25 antibody may be very important in tilting the immune response either towards suppression or activation. Recently, Banerjee et al. demonstrated that treatment of myeloma patients with cytokine-treated DC induced the proliferation of Tregs in vivo (Banerjee et al., 2006). The induction of Tregs was dependent upon Treg-DC direct cellular contact and IL-2 presence, with the induced Tregs capable of suppressing T cell proliferation. This phenomenon can be translated to the experience with murine tumor models, where anti-tumor immunity is induced in the absence of Tregs with the subsequent development of auto-immune phenomenon (Shimizu et al., 1999; Sutmuller et al., 2001). Since most tumors express self-antigens, DC-based immunotherapy may also be capable of inducing autoimmunity in addition to tumor immunity. It is hypothesized that in order to prevent the development of auto-immunity in vivo, Tregs may need to be activated in order to suppress DC function as part of the normal homeostatic function (Antony and Restifo, 2005). It is possible that activated Tregs suppress the anti-tumor effects induced by DC, since in the absence of Tregs, DC vaccination demonstrated an overall improvement in anti-tumor response as compared to treatment with DC vaccination alone. Thus, this is the first study to directly compare the effects of Treg reduction/elimination via the use of CY, DD or PC61, with our results suggesting that the selective depletion of Tregs followed by DC-based immunotherapy may be a novel approach to treating patients more effectively with stage IV melanoma.
Acknowledgements
This work was supported by grant from National Institute of Health/National Cancer Institute, K12 Institutional Training Grant (AIR, CA 87989-02) and the American Cancer Society (SPT, PF-07-106-01-LIB).
This work has been supported in part by the Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center and Research Institute.
Abbreviations
- Treg
regulatory T cell
- CY
cyclophosphamide
- DD
denileukin diftitox
- IL-2
interleukin-2
- DC
dendritic cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- Banerjee D, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar K. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after DC injection of cytokine matured DCs in myeloma patients. Blood. 2006 doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mulé JJ. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiacomo A, North RJ. T cell suppressors of antitumor immunity. The production of Ly-1-,2+ suppressors of delayed sensitivity precedes the production of suppressors of protective immunity. J Exp Med. 1986;164:1179–1192. doi: 10.1084/jem.164.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresske B, Haendschke F, Lenz P, Ungefroren H, Jenisch S, Exner B, El Mokhtari NE, Lu T, Zavazava N, Faendrich F. WOFIE stimulates regulatory T cells: a 2-year follow-up of renal transplant recipients. Transplantation. 2006;81:1549–1557. doi: 10.1097/01.tp.0000210538.93861.ae. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falo LD, Jr, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1:649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- Fields RC, Shimizu K, Mulé JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Game DS, Hernandez-Fuentes MP, Lechler RI. Everolimus and basiliximab permit suppression by human CD4+CD25+ cells in vitro. Am J Transplant. 2005;5:454–464. doi: 10.1111/j.1600-6143.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- Ge Q, Palliser D, Eisen HN, Chen J. Homeostatic T cell proliferation in a T cell-dendritic cell coculture system. Proc Natl Acad Sci U S A. 2002;99:2983–2988. doi: 10.1073/pnas.052714199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mulé JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- Haeryfar SM, DiPaolo RJ, Tscharke DC, Bennink JR, Yewdell JW. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol. 2005;174:3344–3351. doi: 10.4049/jimmunol.174.6.3344. [DOI] [PubMed] [Google Scholar]
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- Hu HY, Huynh PD, Murphy JR, vanderSpek JC. The effects of helix breaking mutations in the diphtheria toxin transmembrane domain helix layers of the fusion toxin DAB389IL-2. Protein Eng. 1998;11:811–817. doi: 10.1093/protein/11.9.811. [DOI] [PubMed] [Google Scholar]
- Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- Kelley VE, Bacha P, Pankewycz O, Nichols JC, Murphy JR, Strom TB. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc Natl Acad Sci U S A. 1988;85:3980–3984. doi: 10.1073/pnas.85.11.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ. Recombinant toxins for the treatment of cancer. Curr Opin Mol Ther. 2003;5:44–51. [PubMed] [Google Scholar]
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- North RJ. Models of adoptive T-cell-mediated regression of established tumors. Contemp Top Immunobiol. 1984a;13:243–257. doi: 10.1007/978-1-4757-1445-6_12. [DOI] [PubMed] [Google Scholar]
- North RJ. The murine antitumor immune response and its therapeutic manipulation. Adv Immunol. 1984b;35:89–155. doi: 10.1016/s0065-2776(08)60575-1. [DOI] [PubMed] [Google Scholar]
- Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Robbins PF, Wunderlich JR, Hwu P, Sherry RM, Schwartzentruber DJ, Topalian SL, Restifo NP, Filie A, Chang R, Dudley ME. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother. 2003;26:385–393. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207–217. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Tawara I, Take Y, Uenaka A, Noguchi Y, Nakayama E. Sequential involvement of two distinct CD4+ regulatory T cells during the course of transplantable tumor growth and protection from 3-methylcholanthrene-induced tumorigenesis by CD25-depletion. Jpn J Cancer Res. 2002;93:911–916. doi: 10.1111/j.1349-7006.2002.tb01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]