Abstract
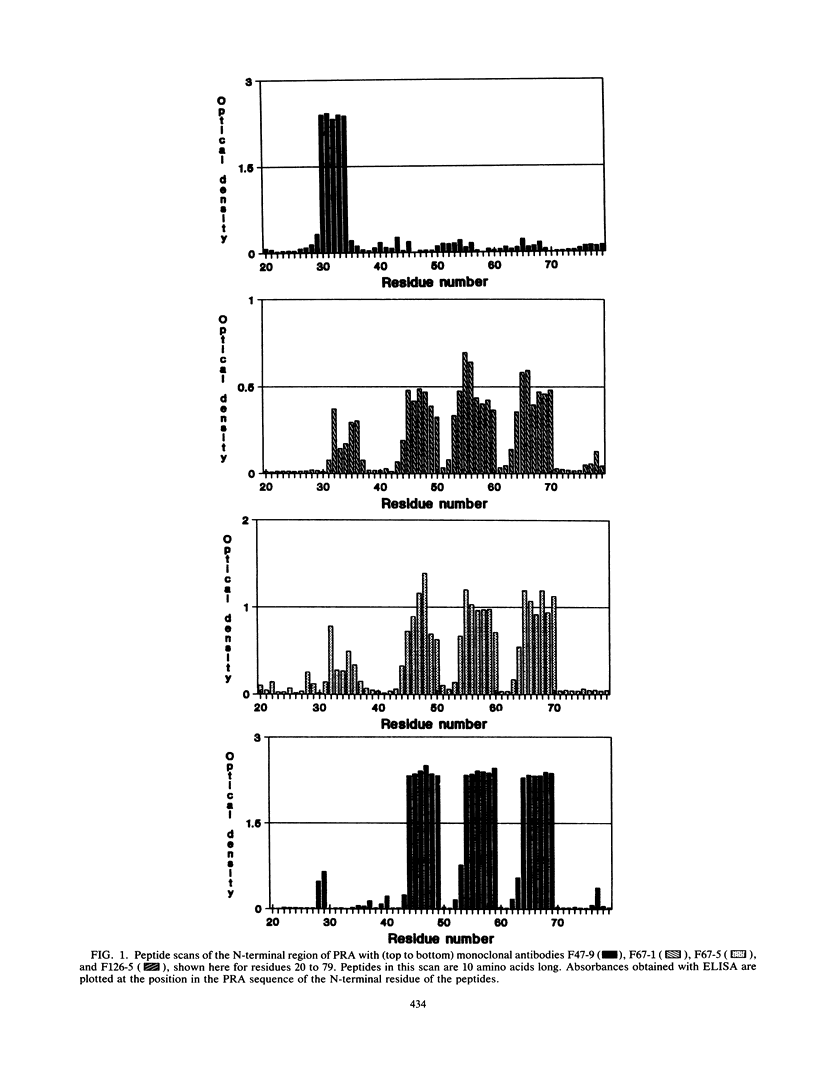
Using synthetic peptides representing overlapping sequences of the 100-amino-acid-long N-terminal region of the proline-rich antigen of Mycobacterium leprae (PRA), we have mapped the epitopes in the primary structure of PRA recognized by four monoclonal antibodies. The M. leprae-specific monoclonal antibody F47-9 recognized the amino acid sequence LGSAYP (residues 34 to 39). Both monoclonal antibodies F67-1 and F67-5 recognized the sequence YPPP within the repeated sequence of PRA at four sites (residues 38 to 41, 50 to 53, 60 to 63, and 70 to 73). Monoclonal antibody F126-5 recognized the sequence SYPPP, also within the repeat, at three sites (residues 49 to 53, 59 to 63, and 69 to 73). All three epitopes appeared to be linear as far as can be determined by this approach.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow D. J., Edwards M. S., Thornton J. M. Continuous and discontinuous protein antigenic determinants. Nature. 1986 Aug 21;322(6081):747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartskeerl R. A., van Rens R. M., Stabel L. F., de Wit M. Y., Klatser P. R. Selection and characterization of recombinant clones that produce Mycobacterium leprae antigens recognized by antibodies in sera from household contacts of leprosy patients. Infect Immun. 1990 Sep;58(9):2821–2827. doi: 10.1128/iai.58.9.2821-2827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin Exp Immunol. 1985 Dec;62(3):468–473. [PMC free article] [PubMed] [Google Scholar]
- Klatser P. R., van Rens M. M., Eggelte T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidase technique (SGIP) using patients' sera. Clin Exp Immunol. 1984 Jun;56(3):537–544. [PMC free article] [PubMed] [Google Scholar]
- Meeker H. C., Williams D. L., Anderson D. C., Gillis T. P., Schuller-Levis G., Levis W. R. Analysis of human antibody epitopes on the 65-kilodalton protein of Mycobacterium leprae by using synthetic peptides. Infect Immun. 1989 Dec;57(12):3689–3694. doi: 10.1128/iai.57.12.3689-3694.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1985 May;48(2):603–605. doi: 10.1128/iai.48.2.603-605.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadiee A. R., Gillis T. P., Shannon E. J. Confirmation of a false-positive result associated with a competition inhibition assay used for detecting antibodies to a protein epitope of Mycobacterium leprae. Clin Exp Immunol. 1990 Mar;79(3):397–402. doi: 10.1111/j.1365-2249.1990.tb08102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Haft D. H., Getzoff E. D., Tainer J. A., Lerner R. A., Brenner S. Identical short peptide sequences in unrelated proteins can have different conformations: a testing ground for theories of immune recognition. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5255–5259. doi: 10.1073/pnas.82.16.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M. Y., Klatser P. R. Purification and characterization of a 36 kDa antigen of Mycobacterium leprae. J Gen Microbiol. 1988 Jun;134(6):1541–1548. doi: 10.1099/00221287-134-6-1541. [DOI] [PubMed] [Google Scholar]


