Abstract
The efficacy of nicotine vaccines for smoking cessation is dependent upon their ability to elicit sufficiently high serum antibody concentrations. This study compared two nicotine immunogens representing different hapten presentations, 3′-aminomethyl nicotine conjugated to recombinant Pseudomonas exoprotein A (3′-AmNic-rEPA) and 6-carboxymethlureido nicotine conjugated to keyhole limpet hemocyanin (6-CMUNic-KLH), and assessed whether their concurrent administration would produce additive serum antibody concentrations in rats. Effects of vaccination on nicotine pharmacokinetics were also studied. Vaccination of rats with these immunogens produced non cross-reacting nicotine-specific antibodies (NicAb). Serum NicAb concentrations elicited by each individual immunogen were not affected by whether the immunogens were administered alone as monovalent vaccines or together as a bivalent vaccine. The total NicAb concentration in the bivalent vaccine group was additive compared to that of the monovalent vaccines alone. Higher serum NicAb concentrations, irrespective of which immunogen elicited the antibodies, were associated with greater binding of nicotine in serum, a lower unbound nicotine concentration in serum, and lower brain nicotine concentration. These results demonstrate that it is possible to design immunogens which provide distinct nicotine epitopes for immune presentation, and which produce additive serum antibody levels. The concurrent administration of these immunogens as a bivalent vaccine may provide a general strategy for enhancing the antibody response to small molecules such as nicotine.
Keywords: Nicotine, antibody, immunotherapy, vaccine, bivalent, immunogenicity
Introduction
Nicotine is the principal addictive component of tobacco smoke [1]. Vaccination against nicotine is a potential means of modifying or attenuating nicotine’s addictive effects [2,3]. Vaccination of rats with a suitable nicotine immunogen elicits the production of nicotine-specific antibodies which bind nicotine in serum and extracellular fluid, reduce the concentration of unbound nicotine, reduce or slow nicotine distribution to the brain [4–7], and attenuate a variety of nicotine-induced behaviors in rats including locomotor activation [8], nicotine discrimination [9], and the acquisition, maintenance and reinstatement of nicotine self administration [10,11]. The effects of immunization on nicotine pharmacokinetics in rats are a function of both the serum NicAb concentration and antibody affinity for nicotine. Close correlations have been found between the serum NicAb concentration and the percent of nicotine bound in serum, the concentration of unbound nicotine in serum, and the distribution of nicotine to brain [5,12,13]. Recent studies also show a correlation between the serum antibody concentration and a behavioral outcome, locomotor sensitization to nicotine (unpublished data). Three nicotine vaccines are in Phase II-III clinical trials as adjuncts for smoking cessation and have shown preliminary evidence of efficacy. In each of these clinical trials, efficacy has been closely related to the serum antibody concentration or titer in that increased smoking cessation rates were observed in only those subjects with the highest serum NicAb levels [4,14–17](C. Bunce, personal communication). These highly congruent animal and human data clearly demonstrate the need to achieve high serum NicAb concentrations in order to maximize the efficacy of vaccination for treating tobacco dependence.
Two challenges have emerged from clinical trials regarding the generation of high antibody concentrations from nicotine vaccines. First, the mean serum NicAb concentrations reported have been modest, 32 ug/ml in one trial [14], which is considerably lower than typical levels of 180–250 ug/ml in rats vaccinated with the same immunogen [5,18]. This difference may be due in part to the ability to use Freund’s adjuvant in rats, while alum was used in humans. Second, considerable variability in serum antibody concentrations has occurred following vaccination against nicotine; a 30-fold range in one study, from a high of 100 µg/ml to less than10 µg/ml [14]. Although only limited data have been published, these challenges appear to be common to all three nicotine vaccines currently under clinical investigation [14,15](C. Bunce, personal communication). As a result, only a minority of vaccinated subjects have achieved antibody levels sufficient to enhance smoking cessation rates.
The current study investigated the combined administration of two distinct nicotine immunogens in rats as a means of enhancing the total serum NicAb response, and also reducing individual variability. The two immunogens utilized different linker positions, linker composition, and carrier proteins in order to provide distinct hapten presentations. It was hypothesized that immune responses to the two immunogens would be independent so that combining the two immunogens into a bivalent vaccine would not compromise the immune response to each individual immunogen, and would produce additive serum antibody levels. The effects of these vaccines on the distribution and serum protein binding of a single nicotine dose were also studied.
Materials and Methods
Immunogens
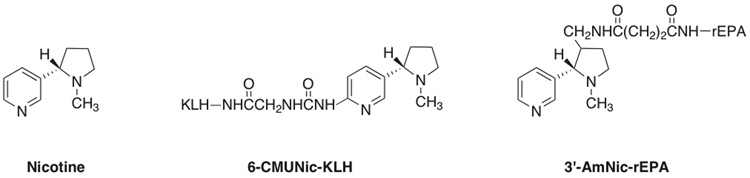
Two previously characterized nicotine immunogens were used which differ in the position and structure of the linker, and the carrier protein used (Figure 1). 3′-Aminomethyl nicotine (3′-AmNic) was provided by Nabi Biopharmaceuticals (Boca Raton, FL) and synthesized and conjugated to recombinant Pseudomonas aeruginosa protein A (rEPA) as previously described. This immunogen has <1% cross reactivity with the major nicotine metabolites cotinine and nicotine-N-oxide, the endogenous nicotinic cholinergic receptor ligand acetylcholine, and a variety of neurotransmitters or medications [18]. 6-Carboxymethylureido nicotine (6-CMUNic) was synthesized and conjugated to keyhole limpet hemocyanin (KLH) as previously described [19]. Control immunogens consisted of unconjugated rEPA or KLH carrier protein alone without hapten.
Figure 1.
Nicotine and immunogens
Vaccination
Groups of 12 rats received nicotine immunogen in 0.4 ml of complete Freund’s adjuvant for the initial injection and 0.4 ml of incomplete Freund’s adjuvant for each boost. The monovalent 3′-AmNic-rEPA vaccine group received 25 µg 3′˜ AmNic-rEPA, the monovalent 6-CMUNic-KLH vaccine group received 25 µg 6-CMUNic-KLH, the bivalent vaccine group received 25 µg 3′-AmNic-rEPA + 25 µg 6-CMUNic-KLH, and controls received 25 µg unconjugated rEPA + 25 µg unconjugated KLH . Rats received 3 vaccine doses i.p. at 3 week intervals.
Antibody concentrations and affinity
Serum concentrations of NicAb elicited by 3′-AmNic-rEPA were measured by quantitative ELISA using 3′-AmNic-polyglutamate as the coating antigen to avoid detecting antibodies directed at carrier protein [18]. A standard curve for NicAb concentration was constructed using sera from rats vaccinated with 3′-AmNic-rEPA in which NicAb concentrations had been independently determined by radioimmunoassay [5]. Serum concentrations of NicAb elicited by 6-CMUNic-KLH were similarly determined using 6-CMUNic-albumin as the coating antigen.
Cross reactivity in the ELISA assays was determined by assaying serum samples separately using either 3′AmNic-polyglutamate as the coating antigen for serum from animals immunized with 6-CMUNic-KLH, or 6-CMUNic-albumin as the coating antigen for serum from animals immunized with 3′-AmNic-rEPA. The percent cross-reactivity was then used to adjust serum NicAb concentrations for the bivalent vaccine group. Antibody affinity for nicotine in the monovalent vaccine groups was measured by soluble RIA of pooled serum [20]. Serum was obtained before nicotine dosing to avoid the presence of nicotine in the sample, and pooling was used because the volume available from each animal was insufficient to perform individual assays.
Measurement of nicotine concentrations
Serum and brain nicotine concentrations were measured by gas chromatography with nitrogen phosphorus detection [21]. Brain nicotine concentrations were corrected for brain blood content [12]. Serum protein binding of nicotine was measured by equilibrium dialysis for 4 h at 37°C using Spectrapor 2 membranes [19]. The fraction unbound was the ratio of the nicotine concentrations on the buffer and serum sides, and the unbound nicotine concentration was the product of that ratio and the total serum nicotine concentration prior to dialysis.
Experimental protocol
Rats were immunized as described above. One week after the final vaccine dose, rats were anesthetized with droperidol/fentanyl, left femoral and right jugular venous catheters were placed, and blood was removed for measurement of baseline serum NicAb concentrations. Rats then received nicotine 0.03 mg/kg over 10 sec via the jugular cannula. Rats were decapitated 3 min later and trunk blood and brain were collected. Serum was stored at 4°C and brain was stored at −20°C until processed.
Data analysis
Serum and brain nicotine concentrations, nicotine protein binding parameters, and serum NicAb concentrations were compared among groups by one way ANOVA and individual comparisons were analyzed by t-test with Bonferroni adjustments. If variances differed significantly among groups, a nonparametric Kruskal-Wallis test was also performed. The relationship of brain nicotine concentration and the log serum NicAb concentration was analyzed by linear regression. The relationship of serum NicAb concentrations elicited by each of the 2 individual immunogens in the bivalent vaccine group was assessed by correlation analysis.
Results
Antibody cross reactivity
Serum from rats immunized with 3′-AmNic-rEPA showed 7.6% cross-reactivity when assayed with the ELISA used to quantitate antibodies elicited by 6-CMUNic-KLH. Serum from rats immunized with 6-CMUNic-KLH showed <1% cross reactivity when assayed with the ELISA used to quantitate antibodies elicited by 3′-AmNic-rEPA.
Serum NicAb
Both immunogens elicited substantial serum antibody concentrations (Table1). The concurrent administration of the two immunogens as a bivalent vaccine did not compromise their immunogenicity; serum concentrations of antibodies elicited by each individual immunogen were at least as high when they were combined as when they were administered separately. The total serum NicAb concentration produced by the bivalent vaccine was significantly higher than that produced by either of the monovalent vaccines alone (p <0.01). The same result was obtained when the data were analyzed using nonparametric methods.
Table 1.
Serum NicAb concentrations (mean±SD), corrected for ELISA cross reactivity.
| Antibody Concentration (µg/ml) | |||
|---|---|---|---|
| Group | 3′-AmNic | 6-CMUNic | Total 3′-AmNic + 6-CMUNic |
| Control | <1 | <1 | <1 |
| 3′-AmNic-rEPA | 184 ± 118 | <1 | 184 ± 118 |
| 6-CMUNic-KLH | <1 | 149 ± 84 | 149 ± 84 |
| Bivalent | 224 ± 131 | 227 ± 102 | 451±205* |
p<0.001 compared to monovalent 3′AmNic-rEPA alone or monovalent 6-CMUNic-KLH alone.
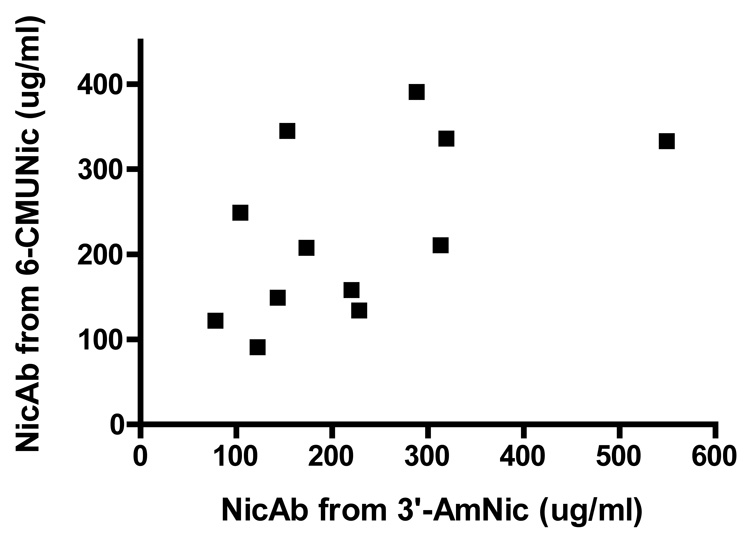
The serum concentrations of NicAb elicited by each of the individual immunogens in the bivalent vaccine group were not significantly correlated with each other (p = 0.07, Figure 2). The Kd for nicotine of the pooled sera in the 3′-AmNic-rEPA group was 11 nM. The Kd for nicotine of the pooled sera in the 6-CMUNic-KLH group was 27 nM.
Figure 2.
Relationship of serum NicAb concentration elicited by each of the individual immunogens in the bivalent vaccine group. There was no significant correlation between these antibody concentrations (p = 0.07).
Nicotine distribution and binding
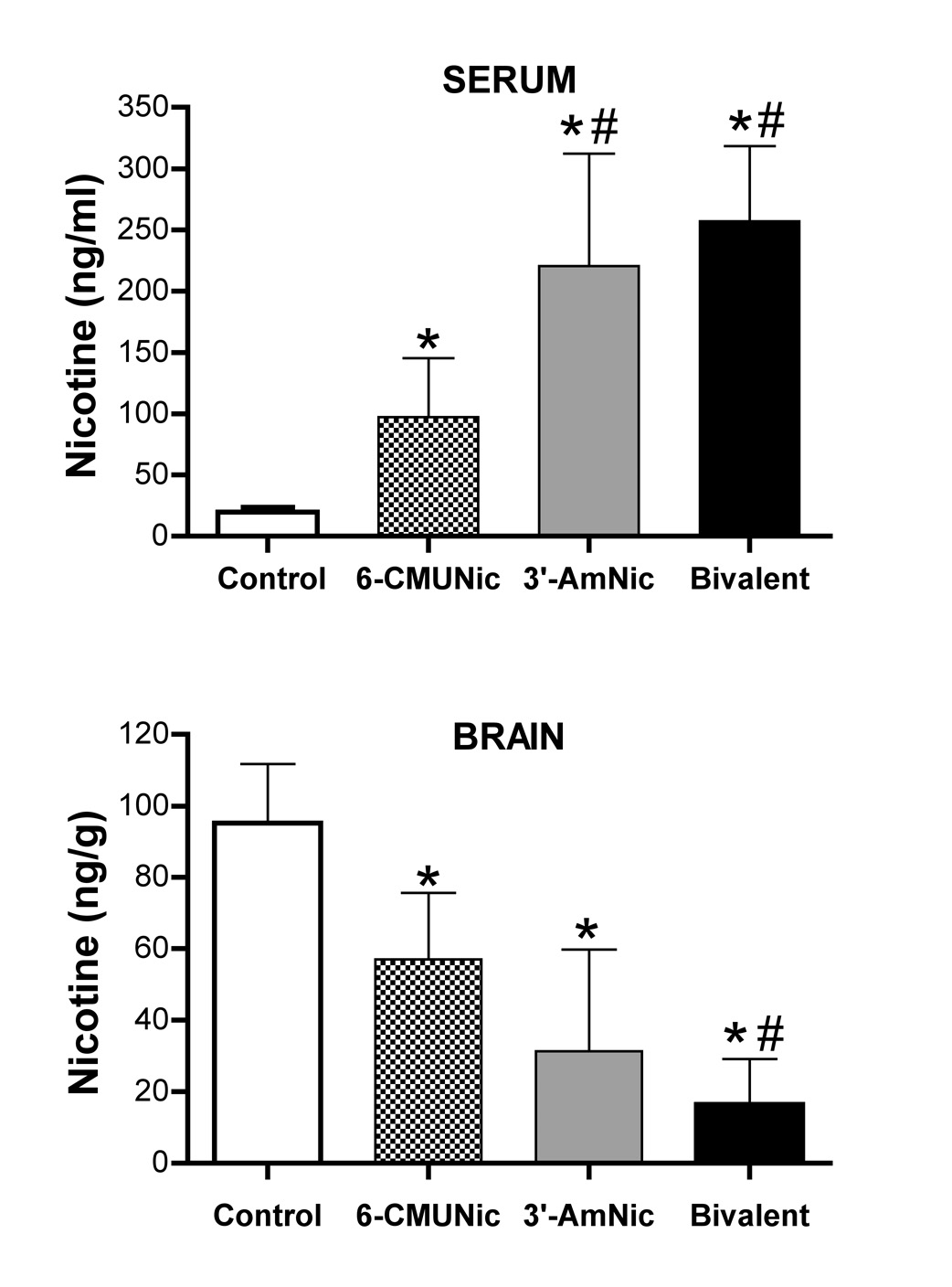
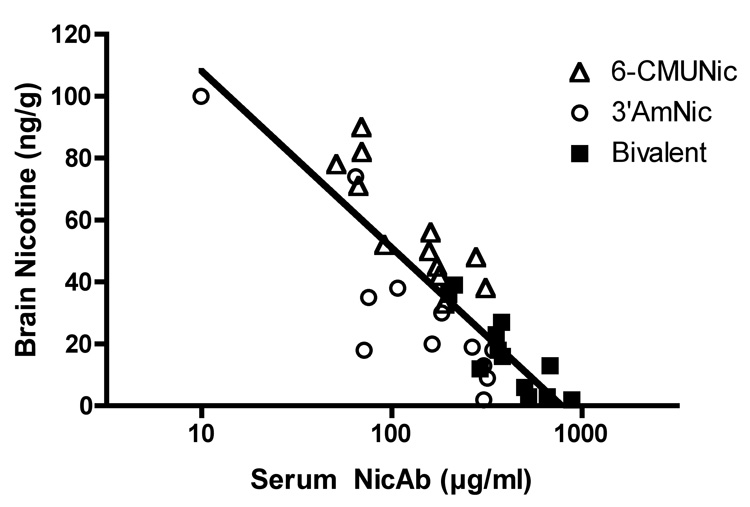
Each of the vaccines increased the total serum nicotine concentration, increased the binding of nicotine in serum, and reduced the unbound serum nicotine concentration compared to controls (Table 2). The bivalent vaccine was more effective than 6-CMUNic-KLH alone for each of these measures. Differences in pharmacokinetic efficacy between the bivalent vaccine and 3’-AmNic-rEPA alone were not significant, although differences from the control group were numerically greater for the bivalent vaccine in every instance. Each of the vaccines also significantly increased nicotine retention in serum and reduced nicotine distribution to brain (Figure 3); the bivalent vaccine was more effective than 6-CMUNic-KLH alone, but not more effective than 3′-AmNic-rEPA alone. However, analyzing all vaccinated groups together, there were significant correlations between the total serum NicAb concentration and total nicotine concentration in serum (r2 = 0.55, p <0.001), the fraction of nicotine bound in serum (r2 = 0.42, p <0.001), the unbound nicotine concentration in serum (r2 = 0.37, p <0.001), and the brain nicotine concentration (r2 = 0.71, p <0.001, Figure 4).
Table 2.
Nicotine protein binding in serum (mean±SD)
| Total Nicotine | Nicotine | Unbound Nicotine | |
|---|---|---|---|
| Group | ng/ml | % Bound | ng/ml |
| Control | 20 ± 4 | 11 ± 8 | 17.7 ± 3.8 |
| 6-CMUNic-KLH | 97 ± 48 | 85 ± 14 | 9.0 ± 3.0* |
| 3′-AmNic-rEPA | 221 ± 92 | 94 ± 11 | 5.1 ± 3.2* |
| Bivalent | 257 ± 61 | 98 ± 2 | 3.8 ± 2.1* # |
p<0.001 compared to Control
p <0.01 compared to 6-CMUNic-KLH
Figure 3.
Serum and brain nicotine concentrations measured 3 min after nicotine dosing. * p <0.05 compared to controls. # p<0.05 compared to 6-CMUNic-KLH
Figure 4.
Relationship between the log total serum NicAb concentration and the brain nicotine concentration (r2= 0.71, p <0.001).
Discussion
The main findings of this study were that 1) the use of nicotine haptens differing in the location and structure of the linker, as well as the carrier protein to which they are conjugated, resulted in distinct immunogens which elicited non cross-reacting antibodies, 2) the concurrent administration of these two nicotine immunogens in a bivalent vaccine did not compromise their immunogenicity, and 3) the resulting total serum antibody concentration correlated with the magnitude of vaccine effects on nicotine pharmacokinetics. These results show that it is possible to design more than one immunologically distinct hapten from a small molecule such as nicotine.
Vaccination with 3′-AmNic-rEPA or 6-CMUNic-KLH alone produced serum antibody concentrations and antibody affinities for nicotine similar to those previously reported [18,19]. The minimal cross-reactivity between the antibodies elicited by these two immunogens indicates that these antibodies arise largely from distinct B cell populations and that antibody production from these immunogens should be largely independent. In support of this, the serum NicAb levels elicited by each immunogen in the bivalent vaccine were equal to or higher than those generated by the same immunogens administered individually as monovalent vaccines, and the total serum antibody concentration in the bivalent vaccine group was significantly higher than that generated by either of its individual immunogen components. Because serum NicAb levels are strongly correlated with vaccine efficacy in animals and in humans, these data provide a rationale for the use of bivalent vaccines to enhance the efficacy of immunotherapy for tobacco dependence.
Although not the primary goal of this study, effects of vaccines on the distribution and serum protein binding of a single nicotine dose were used to asses vaccine efficacy. These parameters reflect the mechanism of action of nicotine vaccines and serve as predictors of behavioral efficacy [13,18]. A limitation of this study was that, as previously reported, 3′-AmNic-rEPA was more effective in altering nicotine pharmacokinetics than 6-CMUNic-KLH, most likely because 3′-AmNic-rEPA produced NicAb with a higher affinity for nicotine. The importance of NicAb affinity for nicotine has been studied using monoclonal nicotine-specific antibodies and, within a range of Kd’s from 1.6 to 250 nM, affinity markedly influences the ability of these antibodies to reduce nicotine distribution to brain. The discrepancy in efficacy between immunogens made it difficult to detect differences between the bivalent vaccine and the monovalent 3′-AmNic-rEPA vaccine alone, since the contribution of antibodies generated by 6-CMUNic-KLH to the pharmacokinetic efficacy of the bivalent vaccines was smaller than that of antibodies generated by 3′-AmNic-rEPA. Nevertheless, in a pooled analysis of all vaccinated animals, the total serum NicAb concentration (irrespective of which immunogen stimulated the antibodies) was correlated with the total serum nicotine concentration, the fraction of nicotine bound in serum, the unbound nicotine concentration in serum, and the brain nicotine concentration. These data are entirely consistent with previous studies of nicotine vaccines and nicotine-specific monoclonal antibodies showing correlations between the serum NicAb concentration and each of these pharmacokinetic measures, and strongly suggest that the effects of the 6-CMUNic-KLH and 3′-AmNic-rEPA vaccines on nicotine pharmacokinetics were additive.
The use of bi-or multivalent vaccines to prevent infectious diseases is well established [22]. For these applications, multivalent vaccines combine immunogens derived from different pathogens (e.g. measles, mumps and rubella, or diphtheria, pertussis and tetanus) or immunogens derived from different strains of the same pathogen (e.g. polyvalent pneumococcal vaccines). The titers of antibodies directed at each individual component are in some cases lower than when the individual components are administered separately, due to various mechanisms including incompatibility of preservatives, one immunogen leading to desorption of another from the alum adjuvant, or competition by viral components for mucosal receptors or lymphocyte binding sites [23–26]. However, in most cases antibody titers are still substantial and sufficient so that vaccine efficacy in preventing disease is not compromised [22].
Even for a single pathogen, antibody responses are often directed at two or more antigenic determinants contained on the pathogen because these are large enough to provide multiple distinct epitopes [27]. The minimum molecular size required for a compound to contain multiple epitopes is not known. The current study suggests that, with appropriate hapten design, even a small molecule such as nicotine (molecular weight 162 Da) can provide distinct epitopic presentations which elicit independent antibody responses. Two factors may have contributed to these distinct responses. The different linker positions may have presented different aspects of the nicotine to immune cell receptors, or the differing linkers and carrier proteins themselves may have been included in the epitopes presented and contributed to immunogen identity. However a contribution from carrier protein is unlikely because the ELISA specificity assay used different carrier proteins for the coating antigen than those used in the vaccine immunogens. Presumably the two nicotine immunogens stimulated distinct populations of B cells even though both elicited the production of nicotine-specific antibodies. A related finding was reported by Castro et al [28], who reported that antibodies produced using a 6-aminonicotine immunogen did not crossreact with a 2-aminonicotine hapten. However, this study used 125I-tyrosine methyl ester linked to nicotine through urea, rather than nicotine, as the radioligand for cross reactivity determination, which complicates interpretation of cross-reactivity data, and did not test the cross reactivity of the antibodies produced by the 2-aminonicotine immunogen. The current data extend these findings by showing lack of cross-reactivity of both immunogens, and by showing in vivo additivity of serum nicotine-specific antibody levels and effects on nicotine pharmacokinetics.
In addition to enhancing vaccine efficacy through additive antibody responses, a bivalent nicotine vaccine could serve to reduce individual variability and the number of non-responders. The mechanisms responsible for the large variability in serum antibody concentrations produced by nicotine vaccines in both animals and humans are unclear [4,5,14], but wide ranges in antibody response have been reported with vaccines for cocaine addiction [29] and many infectious diseases [23,30] as well. Genetic factors controlling HLA, cytokine and T cell surface receptor expression contribute a portion of this variability [31]. It is not clear whether individuals with a poor response to one vaccine necessarily have poor responses to others. In the current study, serum antibody concentrations elicited by the two immunogens in the bivalent vaccine group showed only a nonsignificant trend toward a correlation (p = 0.07). These results suggest a potential role for a bivalent vaccine in reducing variability in response to these immunogens, but further studies with larger groups would be helpful.
The vaccines used in this study were administered in Freund’s adjuvant, rather than alum which is the most commonly chosen adjuvant for clinical use, and CMUNic was conjugated to the carrier protein KLH which is not used in any currently marketed vaccines. These reagents were used because of our prior experience with them, and because the goal of this study was primarily to establish proof of principle for the bivalent vaccine strategy. The same 3′-AmNic-rEPA immunogen used in the current study has been used in clinical trials administered in alum adjuvant, with immunogenicity similar to that of the Freund’s mixture in rats except for lower serum antibody concentrations [14]. While a given carrier protein or adjuvant may be more effective for one immunogen than another [32], there is no reason to expect that the additivity of antibody levels found in the current study would not extend to different carrier proteins or adjuvants provided that the hapten presentations remain immunologically distinct.
In summary, these data suggest that it is possible to design immunologically distinct epitopes from small molecules and use them in combination to stimulate independent and additive antibody responses. This approach could be helpful when the magnitude of antibody response to a small molecule is critical to efficacy, as appears to be the case with existing vaccines for nicotine or cocaine addiction [14,29].
Acknowledgments
The 3′-AmNic-rEPA immunogen and rEPA carrier protein were gifts of Nabi Biopharmaceuticals. Internal standard for the nicotine assay was a gift from P Jacob (University of California, San Francisco). Supported by PHS grants DA10714, F31-DA021946, T32-DA07097, and P50-DA013333.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services. Nicotine addiction: Health consequences of smoking. DHHS. 1999
- 2.Vocci FJ, Chiang CN. Vaccines against nicotine: how effective are they likely to be in preventing smoking? CNS Drugs. 2001;15:505–514. doi: 10.2165/00023210-200115070-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pentel PR, LeSage MG, Keyler DE, Hatsukami DE. Immunologic approaches to nicotine addiction. In: George TP, editor. Medication treatments for nicotine dependence. New York: Taylor & Francis; 2007. pp. 151–166. [Google Scholar]
- 4.Maurer P, Jennings GT, Willers J, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 5.Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol. 2003;3:957–970. doi: 10.1016/S1567-5769(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 6.de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine alters the distribution of nicotine but not the metabolism to cotinine in the rat. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:299–304. doi: 10.1007/s00210-004-0960-3. [DOI] [PubMed] [Google Scholar]
- 7.Cerny EH, Levy R, Mauel J, et al. Preclinical development of a vaccine 'Against Smoking'. Onkologie. 2002;25:406–411. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- 8.Carrera MR, Ashley JA, Hoffman TZ, et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem. 2004;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Malin DH, Alvarado CL, Woodhouse KS, et al. Passive immunization against nicotine attenuates nicotine discrimination. Life Sciences. 2002;70:2793–2798. doi: 10.1016/s0024-3205(02)01523-0. [DOI] [PubMed] [Google Scholar]
- 10.LeSage MG, Keyler DE, Hieda Y, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006;184:409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 11.Lindblom N, De Villiers SH, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active Immunization against Nicotine Prevents Reinstatement of Nicotine-Seeking Behavior in Rats. Respiration. 2002;69:254–260. doi: 10.1159/000063629. [DOI] [PubMed] [Google Scholar]
- 12.Hieda Y, Keyler DE, VanDeVoort JT, et al. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology. 1999;143:150–157. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- 13.Keyler DE, Roiko SA, Benlhabib E, et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose-and affinity-response relationships. Drug Metab Dispos. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- 14.Hatsukami DK, Rennard S, Jorenby D, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Maurer P, Bachmann MF. Vaccination against nicotine: an emerging therapy for tobacco dependence. Expert Opin Investig Drugs. 2007;16:1775–1783. doi: 10.1517/13543784.16.11.1775. [DOI] [PubMed] [Google Scholar]
- 16.McNeil C. Nicotine vaccines move toward pivotal trials. J Natl Cancer Inst. 2006;98:301. doi: 10.1093/jnci/djj113. [DOI] [PubMed] [Google Scholar]
- 17.Hatsukami D, Rennard S, Gonzales D, et al. NicVAX® demonstrated proof of concept at 6 months, an evaluation of a conjugate nicotine vaccine in smokers who want to quit. Madrid, Spain: European Society for Research on Nicotine and Tobacco; 2007. Oct, [Google Scholar]
- 18.Pentel PR, Malin DH, Ennifar S, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 19.Hieda Y, Keyler DE, Vandevoort JT, et al. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997;283:1076–1081. [PubMed] [Google Scholar]
- 20.Muller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods of Enzymology. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- 21.Jacob P, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatog. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 22.Rennels MB, Lagos RM, Edwards KM. Combination vaccines for routine infant immunization. In: Levine MM, Kaper JB, Rappuoli R, Liu MA, Good MF, editors. New Generation Vaccines. 3rd ed. New York: Marcel Dekker, Inc.; 2004. pp. 413–419. [Google Scholar]
- 23.Greenberg DP, Wong VK, Partridge S, et al. Immunogenicity of a Haemophilus influenzae type b-tetanus toxoid conjugate vaccine when mixed with a diphtheria-tetanus-acellular pertussis-hepatitis B combination vaccine. Pediatr Infect Dis J. 2000;19:1135–1140. doi: 10.1097/00006454-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Halperin SA, Langley JM, Eastwood BJ. Effect of inactivated poliovirus vaccine on the antibody response to Bordetella pertussis antigens when combined with diphtheria-pertussis-tetanus vaccine. 1996;22:59–62. doi: 10.1093/clinids/22.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Andre FE, Peetermans J. Effect of simultaneous administration of live measles vaccine on the "take rate" of live mumps vaccine. Dev Biol Stand. 1986;65:101–107. [PubMed] [Google Scholar]
- 26.Corkill JM. Symposia Series in Immunoloical Standardization. Basel: Karger; 1967. The stability of the components in DTP-poliiomyelitis and DPT-poliomyelitis-measles vaccines; pp. 165–178. [Google Scholar]
- 27.Mulupuri P, Zimmerman J, Hermann J, et al. Antigen-specific B-cell responses to porcine reproductive and respiratory syndrome virus infection. J. Virology. doi: 10.1128/JVI.01023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro A, Monji N, Ali H, Yi M, Bowman ER, McKennis H., Jr Nicotine antibodies: Comparison of ligand specificities of antibodies produced against two nicotine conjugates. European Journal of Biochemistry. 1980;104:331s–340s. doi: 10.1111/j.1432-1033.1980.tb04433.x. [DOI] [PubMed] [Google Scholar]
- 29.Kosten TR, Gonsai K, St Clair Roberts J, et al. Phase II human study of cocaine vaccine TA-CD. Quebec City: CPDD Annual Meeting; 2002. [Google Scholar]
- 30.O'Brien KL, Moisi J, Moulton LH, et al. Predictors of pneumococcal conjugate vaccine immunogenicity among infants and toddlers in an American Indian PnCRM7 efficacy trial. J Infect Dis. 2007;196:104–114. doi: 10.1086/518438. [DOI] [PubMed] [Google Scholar]
- 31.Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther. 2007;82:653–664. doi: 10.1038/sj.clpt.6100415. [DOI] [PubMed] [Google Scholar]
- 32.Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20 Suppl 3:S56–S64. doi: 10.1016/s0264-410x(02)00174-3. [DOI] [PubMed] [Google Scholar]