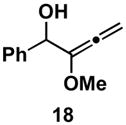
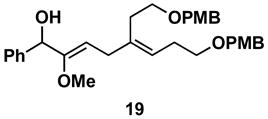
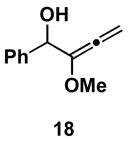
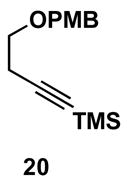
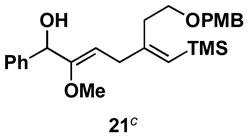
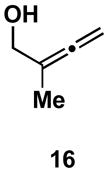
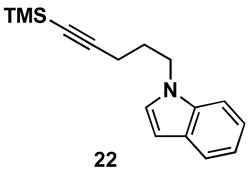
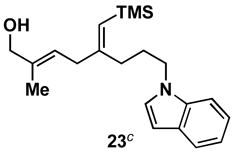
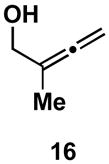
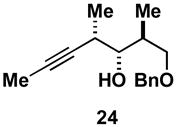
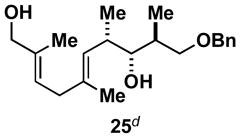
Table 1.
Typical reaction conditions: alkyne (1.4 eq), ClTi(Oi-Pr)3 (2.1 eq), c-C5H9MgCl (4.2 eq),PhMe (−78 to −30 °C), cool to −78 °C then add allenyl alkoxide (1 eq), (−78 to 0 °C).
Selectivity refers to the olefin formed from the allene coupling partner.
rs = 4:1.
rr = 2:1.