Abstract
The role of tumor necrosis factor (TNF) as an immune mediator has long been appreciated but its function in the brain is still unclear. TNF receptor 1 (TNFR1) is expressed in most cell types, and can be activated by binding of either soluble TNF (solTNF) or transmembrane TNF (tmTNF), with a preference for solTNF; whereas TNFR2 is expressed primarily by microglia and endothelial cells and is preferentially activated by tmTNF. Elevation of solTNF is a hallmark of acute and chronic neuroinflammation as well as a number of neurodegenerative conditions including ischemic stroke, Alzheimer's (AD), Parkinson's (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). The presence of this potent inflammatory factor at sites of injury implicates it as a mediator of neuronal damage and disease pathogenesis, making TNF an attractive target for therapeutic development to treat acute and chronic neurodegenerative conditions. However, new and old observations from animal models and clinical trials reviewed here suggest solTNF and tmTNF exert different functions under normal and pathological conditions in the CNS. A potential role for TNF in synaptic scaling and hippocampal neurogenesis demonstrated by recent studies suggest additional in-depth mechanistic studies are warranted to delineate the distinct functions of the two TNF ligands in different parts of the brain prior to large-scale development of anti-TNF therapies in the CNS. If inactivation of TNF-dependent inflammation in the brain is warranted by additional pre-clinical studies, selective targeting of TNFR1-mediated signaling while sparing TNFR2 activation may lessen adverse effects of anti-TNF therapies in the CNS.
Introduction
The potent pro-inflammatory cytokine Tumor necrosis factor (TNF) is a member of the TNF superfamily of ligands, many of which promote inflammatory signaling [1-3]. TNF is synthesized as a monomeric type-2 transmembrane protein (tmTNF) that is inserted into the membrane as a homotrimer and cleaved by the matrix metalloprotease TNF alpha converting enzyme (TACE; ADAM17) to a 51 kDa soluble circulating trimer (solTNF); both tmTNF and solTNF are biologically active (reviewed in [4-6]), and can be synthesized in the central nervous system (CNS) by microglia, astrocytes, and some populations of neurons [7-9]. The balance between tmTNF and solTNF signaling is influenced by cell type, activation status of the cell, the stimulus eliciting TNF production, TACE activity, and expression of endogenous TACE inhibitors leading to divergent TNF-mediated effects on cellular viability [10,11].
TNF receptors
TNF receptor 1 (TNFR1, Tnfrsf1a) and TNF receptor 2 (TNFR2, Tnfrsf1b) are membrane glycoprotein receptors that specifically bind TNF and homotrimers of lymphotoxin A, but the two receptors differ in their expression profiles, ligand affinity, cytoplasmic tail structure, and downstream signaling pathway activation (reviewed in [12]). Signaling of TNF through TNFRs requires that receptors preassemble on the cell membrane as trimers prior to ligand binding, this trimerization occurs through the intracellular cytoplasmic tail of the receptors and trimers are composed of like receptors due to the divergent sequence of their intracellular domains [13,14]. TNFR1 is expressed in most cell types, and can be activated by binding of either solTNF or tmTNF, with a preference for solTNF; whereas TNFR2 is expressed primarily by cells of the immune system (including microglia) and by endothelial cells, and is preferentially activated by tmTNF [15,16].
Intracellular signaling pathways activated by TNF receptors
TNF signaling through TNFR1 and TNFR2 can elicit a variety of cellular responses depending on many factors including the metabolic state of the cell and the adaptor proteins present in the cell. These differences then influence the activation of a number of intracellular signaling pathways including nuclear factor kappa-B (NF-κB), p38, c-jun N-terminal kinase (JNK), and the ceramide/sphingomyelinase signaling pathway, resulting in a number of responses including inflammation, proliferation, cell migration, apoptosis, and necrosis [17-20].
TNFR1-mediated signaling
SolTNF signaling is thought to elicit its biological effects primarily through TNFR1 activation. TNFR1 contains a cytoplasmic death domain (DD) characteristic of many members of the TNF superfamily that permits the assembly of the TNFR1 signaling complex through the dissociation of silencer of death domains (SODD) and subsequent binding of TNF receptor associated death domain (TRADD) [21,22]. Binding of TRADD then allows the recruitment of other adaptor proteins including receptor interacting protein (RIP) and TNF receptor associated factor 2 (TRAF2) [23-26]. This complex then leads to RIP-dependent activation of NFκB signaling to initiate pro-survival signaling, cellular proliferation, and cytokine production. This membrane associated complex of ligand-engaged TNFR1 with TRADD, TRAF2, and RIP also recruits cellular inhibitor of apoptosis proteins 1 and 2 (cIAP 1,2) resulting in activation of ERK, JNK, p38 MAP kinase, and ceramide/sphingomyelinase pathways [27-30]. The kinetics of JNK activation is particularly important in determining the functional outcome of a TNF signal. Acute, transient TNF-induced JNK activation, which is cytoprotective, results from TAK1-dependent phosphorylation [31], whereas sustained JNK activation leading to caspase-dependent apoptosis depends upon JNK phosphorylation by apoptosis signal-regulating kinase 1 (ASK1) [32]. Knock-in mice expressing mutant TNF that is no longer a substrate for TACE cleavage (and therefore remains membrane bound) have demonstrated solTNF to be the primary mediator of TNF-dependent inflammatory responses. In these transgenic mice, lymphoid organ development is relatively normal (with the exception of primary B cell follicles), but the initiation of autoimmune pathology is severely compromised [33].
In addition to TNFR1-induced activation of stress-induced kinase signaling in contributing to apoptotic signaling, TNFR1 can be internalized after TNF binding and this leads to dissociation of the TRADD/TRAF2/RIP complex and association of Fas-associated DD (FADD), recruitment of pro-caspase 8, and formation of the death-inducing signaling complex (DISC), triggering activation of the executioner caspases through the extrinsic apoptosis pathway [34,35]. Caspase-8, combined with its ability to induce apoptosis through the extrinsic pathway, also triggers the intrinsic apoptosis pathway by cleaving the pro-apoptotic Bcl-2 family members Bax and Bid to initiate mitochondrial-induced apoptosis [36-38]. The complexity of TNFR1-mediated signaling is what allows many divergent (ie, proliferation, activation, apoptosis) outcomes to occur as a result of TNF signaling and contributes to the difficulties inherent with and the side effects resulting from broad TNF signaling inhibition, particularly in areas including the CNS where TNF signaling has been demonstrated to be functionally important for homeostatic process as discussed in the sections below.
TNFR2-mediated signaling
TNFR2 is restricted to expression in endothelial, hematopoetic, and some neuronal populations and displays preferential binding to tmTNF, both of these characteristic are likely to result in fewer biological effects compared to those mediated by TNFR1-dependent signaling [16]. Signaling through TNFR2 activates inflammatory and pro-survival signaling pathways through recruitment of TRAF1 and TRAF2 adaptor proteins and subsequent activation of cIAPs and the NF-κB pathway [39-41]. TNFR2 has also been shown to activate phosphatidylinositol 3-kinase-dependent signaling to promote neuron survival [42,43]. TNFR2 can promote TNFR1 signaling by enhancing the association between solTNF and TNFR1 via a ligand passing mechanism, and it has been suggested that this ligand passing is the primary contribution of TNFR2 to TNF-mediated signaling in contrast to direct signaling pathway activation through adaptor protein association with the intracellular domain of TNFR2 [44]. TNFR2 does not contain a DD, and thus, unlike signaling through TNFR1, TNFR2 activation does not directly lead to caspase activation. Overall, TNFR2 activation is believed to initiate primarily pro-inflammatory and pro-survival signaling.
Reverse signaling through transmembrane TNF
Upon receptor binding, tmTNF has been demonstrated to initiate intracellular signaling in the tmTNF-expressing cell [20,45]. This signaling is mediated, at least in part, through casein kinase 1 phosphorylation of the cytoplamsic tail of TNFR2 resulting in increased intracellular calcium levels and activation of p38 and MAP kinase pathways [46-48]. Another type of reverse signaling though tmTNF is possible; after release of solTNF by TACE, the TNF intracellular domain (TNF-ICD) can be released into the cell through regulated intramembrane proteolysis by signal peptide peptidase-like proteases where it is trafficked to the nucleus by virtue of a nuclear localization signal resulting in increased production of pro-inflammatory cytokines [49-51]. A detailed understanding of the signaling that occurs through tmTNF is still underway and the in vivo significance of these reverse signaling pathways remains to be established.
TNF signaling in the brain
TNF signaling has been shown to have several important functions within the CNS [52] including injury-mediated microglial and astrocyte activation, and regulation of blood brain barrier permeability, febrile responses, glutamatergic transmission, and synaptic plasticity and scaling [53-58]. Excitatory synapse scaling resulting from activity blockade has been shown to be mediated by TNF-dependent increases in AMPA receptors at the cell surface and decreases in GABAA receptor cell surface expression, suggesting that TNF may control synaptic strength at excitatory synapses by increasing excitatory synaptic transmission and reducing inhibitory transmission [57,59,60]. TNF was demonstrated to increase the mean frequency of miniature excitatory postsynaptic currents (mEPSCs) [57,59]. In addition TNF has been shown to increase mEPSC amplitude and decrease mIPSC (miniature inhibitory postsynaptic currents) amplitude [60]. Pharmacologically, TNFR or anti-TNF antibody treatment could prevent basal and tetrototoxin-induced increases in surface expression of AMPA receptors as well as increases in mEPSC amplitude and decreases in mIPSC amplitude, further supporting solTNF as an important mediator of synaptic scaling [57,59]. Synaptic scaling was also examined in hippocampal cultures from TNF deficient mice and chronic activity blockade did not result in increased mEPSC amplitude which is seen in wildtype cultures confirming the TNF dependence of this increase [59].
The function of TNF signaling through TNFR1 and TNFR2 in the hippocampus and cortex has not been fully elucidated. On the one hand, TNF appears to regulate hippocampal neuronal development as TNF-deficient mice display accelerated maturation of the dentate gyrus region and smaller dendritic trees in the hippocampus [61]. In contrast, TNF can potentiate excitotoxicity by two mechanisms. In combination with sub-threshold glutamate levels, TNF can potentiate glutamate excitotoxicity directly through activation of glutamate-NMDA receptors [62] and localization of AMPA receptors to synapses [63-65], and indirectly by inhibiting glial glutamate transporters on astrocytes [66]. However, findings that suggest TNFR2 signaling protects against excitotoxicity have also been reported. Specifically, cortical neurons from mice that overexpress TNF in the CNS were protected from glutamate toxicity as were wildtype and TNFR1-deficient mice pretreated with TNF or agonistic TNFR2 antibodies; consistent with those findings, neurons derived from TNFR2-deficient mice were susceptible to both TNF and glutamate-induced death [43]. In these studies, protein kinase B/Akt dependent activation of NFκB was necessary for neuronal survival after glutamate exposure [43].
Genetic manipulation of TNF or TNF receptors in mouse models of disease have provided valuable insight into the biological roles of TNF in the CNS [52]. Elevated levels of TNF are evident in a large number of neurological disorders including ischemia [67,68], traumatic brain injury [69], multiple sclerosis [70-74], Alzheimer's disease [75-77], and Parkinson's disease [78-83], but whether TNF signaling actively contributes to or limits neuronal injury in these disorders has yet to be established. A number of pre-clinical and clinical studies in various disease models and in chronic neurodegenerative conditions suggest that targeting TNF action in the brain may be an attractive disease-modifying strategy to slow progression or attenuate severity of the disease and are discussed in sections below.
TNF inhibitors
Endogenous TNF inhibitors
A number of endogenous mechanisms limit TNF activity during inflammatory responses as part of resolution of those responses (reviewed in [84]). Identified endogenous inhibitors of TNF include prostaglandins and cyclic AMP which limit TNF production and glucocorticoids which are produced when TNF levels are high by activation of the hypothalamus pituitary adrenal axis to inhibit TNF production [84-88]. Anti-inflammatory cytokines including IL-10, IL-13 and IL-4 can inhibit TNF production [89-92]. TACE cleaves tmTNF to generate solTNF and it can also cleave TNF receptors to generate solTNFRs which can bind solTNF in the circulation. In fact, soluble forms of TNF receptors were first identified in urine and their discovery lead to the development of anti-TNF antibody-based biologics [93-96]. Efferent activity of the vagus nerve has also been shown to inhibit TNF production through cholinergic activation of muscarinic receptors [97-99].
Biologics
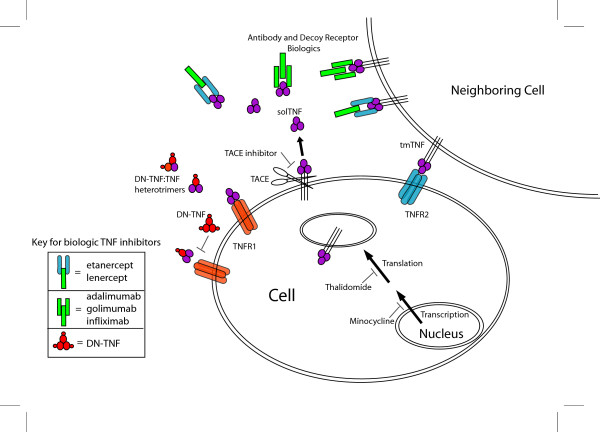
A number of protein-based TNF inhibitors which we will refer to as biologics are available or currently under development (Table 1), and a subset of these have been approved for use in the treatment of peripheral autoimmune disorders including rheumatoid and juvenile arthritis, ankylosing spondylitis, and Crohn's disease. Non-selective biologic inhibitors include, but are not limited to, infliximab, etanercept, and adalimumab, all of which bind to soluble and tmTNF and prevent binding to TNF receptors (Figure 1). Infliximab is a chimeric bivalent IgG1 monoclonal antibody composed of a human constant region and murine variable regions, adalimumab is a humanized bivalent mouse IgG1 monoclonal antibody, and etanercept is a fusion protein comprised of human IgG fused to a dimer of the extracellular regions of TNFR2. A number of important differences exist between TNF inhibitors in their mode of action and specificity that are worth noting (Table 1). Infliximab and adalimumab, by virtue of being IgGI antibodies, can activate complement and bind Fc Receptor and they also can bind both monomeric and trimeric solTNF whereas etanercept, like TNFR2, only binds TNF trimers, however all three inhibitors bind tmTNF and therefore inhibit signaling through both TNF receptors [100-103]. Another difference between etanercept and infliximab and adalimumab is the ability of etanercept to neutralize lymphotoxin, a property that is shared by its parent receptor TNFR2 [100,104]. In addition to these approved inhibitors several similar antibody and receptor based TNF inhibitors are currently or were previously under clinical investigation including lenercept, a dimeric TNFR1 receptor extracellular domain fused to a human IgG1 heavy chain fragment, and the fully human monoclonal antibody golimumab (Table 1).
Table 1.
Selected TNF inhibitors.
| Inhibitor (Trade name) | Class | Description/Mode of Action | Specificity | Developer and Stage | References |
| Adalimumab (Humira) | Monoclonal antibody | Humanized bivalent mouse IgG1 monoclonal antibody; binds to ligands | solTNF, tmTNF | Abbott FDA approved 2005 | [100-103,179,180] |
| Apratastat | Small molecule | Dual TACE and MMP inhibitor | MMPs, TACE | Wyeth Phase II terminated 2006 | [121] |
| BMS-561392 | Small molecule | Specific TACE inhibitor | TACE | Bristol-Myers Squibb Phase II | [121] |
| Certolizumab (Cimzia) | Monoclonal antibody | PEGylated antibody fragment | solTNF, tmTNF | UCB FDA approved 2008 | [182] |
| CYT007-TNFQb | Vaccine | Anti-TNF vaccine | TNF | Cytos Biotechnology Phase I-IIa | [181] |
| DN-TNF (XPro1595) | Inactive TNF variant | TNF monomers engineered with point mutations to disrupt binding to TNFRs; exchange with solTNF to form dominant-negative heterotrimers; tmTNF-sparing | solTNF | Xencor Preclinical | [105,106] |
| ESBA105 | Antibody fragment | Single-chain antibody fragment directed against TNF | TNF | Esbatech (Zurich) Preclinical | [181] |
| Etanercept (Enbrel) | Receptor biologic | Human IgG fused to a dimer of the extracellular regions of TNFR2; binds to ligands | solTNF, tmTNF, Lympotoxin A |
Amgen; Wyeth; Takeda FDA approved 1998 | [179,180] |
| Golimumab (CNTO148) | Monoclonal antibody | Human monoclonal antibody; binds to ligands | solTNF, tmTNF | Centocor, Schering-Plough Phase III | [101] |
| GW333 | Small molecule | Dual TACE and MMP inhibitor | MMPs, TACE | GlaxoSmithKline Preclinical | [121] |
| Infliximab (Remicade) | Monoclonal antibody | Murine-human Chimeric bivalent IgG1 monoclonal antibody; binds to ligands | solTNF, tmTNF | Centocor Schering-Plough FDA approved 1998 | [179,180] |
| Lenercept | Receptor biologic | Dimeric TNFR1 receptor extracellular domain fused to a human IgG1heavy chain fragment; binds to ligands | solTNF, tmTNF, Lympotoxin A |
Roche Phase III terminated | [101,131,183] |
| Minocycline | Small molecule | Broad-spectrum tetracycline antibiotic; inhibits synthesis of TNF and other inflammatory mediators | solTNF, tmTNF, MMPs, COX-2, prostaglandin E2 | Off patent | [110-117] |
| tgAAC94 | Gene therapy | AAV vectors containing the TNFR2:Fc fusion | solTNF, tmTNF, Lympotoxin A |
Targeted Genetics Phase I-II | [184] |
| Thalidomide | Small molecule | Immunomodulatory drug; increases degradation of mRNA of a number of inflammatory genes | TNF, COX-2, IL-1β, TGF-β, IL-12 and IL-6. | Celgene Corporation FDA approved 1998 | [118,119] |
Abbreviations: solTNF, soluble tumor necrosis factor; tmTNF, transmembrane tumor necrosis factor; TACE, tumor necrosis factor alpha converting enzyme; MMP, matrix metalloprotease; DN-TNF, dominant-negative tumor necrosis factor; TNFR, tumor necrosis factor receptor; COX-2, cyclo-oxygenase-2; IL1-β, interleukin-1beta; TGF-β, transforming growth factor beta; IL-12, interleukin-12; IL-6, interleukin-6.
Figure 1.
Schematic of TNF inhibitors and their mode of action. Receptor (i.e. etanercept, lenercept) and antibody based (i.e. infliximab, adalimumab, golimumab) anti-TNF biologics inhibit both solTNF and tmTNF. TNF variants (DN-TNFs) exchange with native solTNF monomers to form heterotrimers with drastically reduced abilities to bind TNF receptors, making them selective for solTNF signaling inhibition. Small molecule inhibitors of TNF signaling include minocycline which decreases TNF synthesis, thalidomide which enhances degradation of TNF mRNA, and TACE inhibitors which prevent TACE induced release of solTNF.
A new class of anti-TNF biologic with a dominant-negative (DN) mechanism of action has emerged and was developed using structure-based computational design leading to the generation of TNF variants referred to as DN-TNFs which are able to exchange with native soluble TNF monomers to form heterotrimers (Figure 1) with drastically reduced abilities to bind TNF receptors [105,106]. Due to this novel mechanism of action, DN-TNFs selectively inhibit solTNF and do not inhibit tmTNF signaling [105,107]. The ability of DN-TNFs to be tmTNF-sparing and solTNF-selective may be ideal in clinical conditions where solTNF signaling mediates pathology, and/or to ensure that the role of tmTNF in immune function is not compromised.
Small molecule TNF inhibition
A small molecule TNF inhibitor that has been described is composed of trifluoromethylphenyl indole and dimethyl chromone moieties linked by a dimethylamine spacer [108]. This small molecule inhibitor binds to trimerized TNF and accelerates subunit dissociation to lead to a TNF dimer/small molecule complex that can not bind to and activate TNFR1 [108].
Immunomodulatory drugs with TNF inhibitory action
Less specific inhibitors of TNF include minocycline and thalidomide which have been used in the treatment of inflammatory conditions. Minocycline, a broad-spectrum tetracycline antibiotic, has been shown to have both bacteriostatic and anti-inflammatory actions [109]. Minocycline not only decreases TNF synthesis, but also inhibits matrix metalloproteases, reduces COX-2 activity and prostaglandin E2 production, and attenuates apoptosis [110-117]. Thalidomide is an immunomodulatory drug which means it can attenuate a variety of cytokines and immune cell-mediated responses. Thalidomide inhibits TNF through enhanced degradation of TNF mRNA [118,119], however is can also alter the expression of COX-2, IL-1β, TGF-β, IL-12 and IL-6 to affect immune cell regulation and migration separate from its TNF-dependent effects (reviewed in [118]).
TACE inhibitors
Another method by which inhibition of soluble TNF production can be achieved is by inhibition of TACE, the protease that cleaves and converts tmTNF into its circulating form. TACE, a member of the ADAM (A Disintegrin And Metalloproteinase protein) family of matrix metalloproteases, is the primary enzyme responsible for TNF shedding in vivo [120]. Unlike what has been possible to achieve with anti-TNF biologics, development of orally available brain-permeant TACE inhibitors has been successful. TACE inhibitors are in various stages of clinical development, but there are limitations with current inhibitors including non-specific inhibition of other matrix metalloproteases, and inhibition of other TACE substrates; both properties may lead to undesired side-effects. Current TACE inhibitors include two small-molecule dual TACE and MMP inhibitors, GW333 and apratastat (TMI-005), and a more specific TACE inhibitor BMS-561392 (reviewed in [121]). Development of apratastat for the treatment of rheumatoid arthritis was terminated after phase II clinical trials due to lack of efficacy [122]. Other TACE inhibitors including BMS-561392 have been delayed from clinical trials due to the potential risk of hepatoxicity resulting from the accumulation of tmTNF and the inhibition of TNFR1 and TNFR2 shedding which was demonstrated in rats treated with TACE inhibitors (reviewed in [121]).
TNF in multiple sclerosis (MS)
Evidence that implicates TNF in the underlying pathology of multiple sclerosis (MS) includes: the observation that at autopsy MS patients have elevated TNF levels at the site of active MS lesions [70]; reports that CSF and serum TNF levels in individuals with MS are elevated compared to unaffected individuals and TNF levels correlate to the severity of the lesions [72,123,124]; and evidence that peripheral blood mononuclear cells from MS patients just prior to symptom exacerbation have increased TNF secretion after stimulation compared to cells form the same patients during remission [73,125]. These characteristics of TNF involvement are shared with peripheral autoimmune diseases. Based on these strong clinical parameters implicating TNF signaling in contributing to MS disease severity, the effects of manipulation of the TNF pathway were investigated in mouse models of MS. Specifically, overexpression of TNF leads to demyelinating disease and neutralization of TNF with anti-TNF antibodies or receptor fusion proteins is protective in experimental autoimmune encephalomyelitis (EAE) transgenic mouse models [126-130]. Logically, based upon promising results in these animal models, lenercept, a dimeric TNFR1 receptor extracellular domain fused to a human IgG1heavy chain fragment, was evaluated in MS patients. Unfortunately, a phase II randomized, multi-center, placebo-controlled study had to be halted due to a dose-dependent increase in MS attack frequency and a trend towards increased attack duration and severity [131]. Although the reasons for unanticipated failure were not immediately clear, the failed lenercept clinical trial served to prompt further investigations into the action of TNF in nerve myelination in animal models of MS and in transgenic mice with alterations in TNF and its receptors. Subsequently, TNFR1 signaling has been demonstrated to mediate nerve demyelination whereas TNFR2 signaling appears to be crucial for remyelination in the cuprizone mouse model of MS. Specifically, it was shown that the absence of TNF resulted in delayed remyelination, depletion of the oligodendrocyte progenitors, and reduction in mature astrocytes after cuprizone withdrawal with TNFR2 expression alone being sufficient to restore oligodendrocyte regeneration [132]. Interestingly, the importance of TNF signaling in mediating nerve demyelination was found to occur independently of the adaptive immune response as the Tg6074 line of TNF overexpressing mice, even when backcrossed to mice deficient in CD4 (to ablate CD4+ lympocytes), β2-microglobulin (MHC I restricted T lymphocytes), immunoglobulin μ (Ig+ lymphocytes), or Rag-1 (T Cell receptor and Ig positive lymphocytes), still develop primary demyelination to the same extent as mice with an intact adaptive immune response [133]. These important findings in pre-clinical models have clinical implications and suggest that inhibition of tmTNF/TNFR2 signaling by lenercept was the molecular basis for the worsening symptoms in MS patients. Experiments to block solTNF/TNFR1 signaling with the solTNF-selective DN-TNF inhibitors in the cuprizone model should yield further support for this hypothesis. Transgenic animal models in which tmTNF is expressed exclusively demonstrated that tmTNF, in the absence of solTNF, suppresses EAE disease onset and progression, while still maintaining the ability of TNF signaling to suppress autoimmune properties [134,135]. This ability of tmTNF to maintain immune functions such as self-tolerance and resistance to infection while limiting other TNF functions including primary demyelination and oligodendrocyte apoptosis [135] opens the possibility of selective inhibition of solTNF/TNFR1 signaling as a therapeutic strategy to prevent relapsing-remitting MS in patients afflicted with this chronic inflammatory disease of the nervous system.
TNF in ischemia (stroke) models
There is conflicting evidence concerning the role of TNF in ischemia (stroke) models (reviewed in [136]). For example, TNF action has been inhibited in acute stroke through intraventricular infusion of TNFR1 decoy receptor or anti-TNF antibody, and through systemic administration of a TACE inhibitor and both approaches resulted in reduced ischemic injury [137-139]. However, hippocampal neurogenesis after ischemic injury resulting from stroke was abolished when animals were treated with anti-TNF antibodies two weeks after the ischemic event, suggesting that TNF signaling may be necessary for repair processes after an ischemic insult [140]. In addition, epileptic seizures resulting from focal cerebral ischemia worsened in mice deficient for TNF or TNF receptors, further suggesting that TNF signaling is important for hippocampal function following ischemic injury [141]. The mechanism of TNF-induced protection from epileptic development was investigated in TNF and TNF receptor transgenic mouse models. Specifically, astrocyte restricted overexpression of TNF or intrahippocampal injection of TNF was found to reduce susceptibility to kainate-induced seizure activity, whereas single TNFR2 or double TNFR1/TNFR2-deficient mice displayed prolonged seizure activity, implicating TNFR2 and tmTNF signaling in protection against seizure [142]. In summary, studies with TNF antibodies in stroke and seizure models raise the interesting possibility that TNF action through TNFR2 is important in hippocampal repair and neurogenesis, and suggest that use of anti-TNF drugs that do not spare TNFR2-dependent signaling may result in untoward effects in this brain region.
TNF in Parkinson's disease (PD)
As in many other neurodegenerative diseases, TNF and solTNFR1 levels are elevated in cerebrospinal fluid and tissues of PD patients as well as in postmortem PD brains with the highest TNF levels present in areas that have the greatest loss of dopaminergic neurons [78-81]. Nevertheless, the importance of TNF signaling in contributing to DA neuron dysfunction and death has only recently been appreciated. In animal models of PD increased TNF mRNA and protein are detectable in the midbrain within hours of in vivo administration of a neurotoxic dopamine analog, 6-hydroxydopamine (6-OHDA) [143], the mitochondrial complex I inhibitor 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [144-146], and the bacterial endotoxin lipopolysaccharide (LPS) [147]. In MPTP-treated non-human primates, serum TNF levels have been reported to remain elevated up to one year after a single MPTP injection [148]. Multiple studies indicate that TNF is highly toxic to dopaminergic neurons in both in vitro primary cultures [149-151] and in vivo [152,153]. The strongest genetic evidence implicating TNF in initiation and progression of PD is that a polymorphism (-1031 C) in the TNF promoter that drives transcriptional activity and results in higher than normal TNF production is over-represented in a cohort of Japanese early-onset PD patients compared to late-onset PD patients and unaffected controls [154]. Importantly, this -1031 C polymorphism has been associated with PD risk in an additional study [155]. A second polymorphism in the TNF gene promoter (-308 G/A) that results in elevated serum TNF levels has also been found to be over-represented in early onset sporadic PD [156,157]. Lastly, TNFR1 polymorphisms TNFR1-609 and TNFRI+36 have been found to be significantly decreased in PD patients [156]. In summary, the association of TNF and TNFR1 polymorphisms with PD risk strongly suggests involvement of TNF signaling in PD progression. Meta-analyses will be needed to assess overall genetic association of TNF with PD risk.
Genetic approaches to investigate the role of TNF signaling in the nigrostriatal pathway have involved use of mice deficient in TNF and TNFRs but have yielded conflicting results. In some studies TNFR1 or TNFR2 single-knockout mice or double receptor deficient mice are not protected from MPTP-induced striatal terminal damage or nigral dopaminergic neuron loss [144,158]; whereas in other studies receptor double-knockout mice have less striatal damage than non-transgenic mice [145,159]; and in mice lacking TNF MPTP-induced striatal dysfunction is reduced, but dopaminergic neuron loss is not [146]. Different extents of MPTP lesioning may account for the conflicting results regarding the role of TNF and its receptors in contributing to dopaminergic neuron loss.
Use of non-specific TNF inhibitors including thalidomide, a potent anti-inflammatory and sedative, and minocycline, a tetracycline antibiotic that inhibits TNF synthesis, has also been investigated in MPTP intoxication paradigms in mice and in intranigral LPS rat models of PD with protective outcomes in some [146,160] but not all studies [161]. Due to potential genetic compensation and/or TNF-independent anti-inflammatory effects of these compounds, the direct role of TNF in dopaminergic neuron death could not be established but was certainly inferred. Only recently, a direct role for solTNF signaling in mediating the in vivo degeneration of DA neurons in both the 6-OHDA oxidative neurotoxin and chronic LPS models of PD has been demonstrated by the ability of dominant-negative (DN) TNF inhibitors or a lentivirus encoding DN-TNF to attenuate nigral DA neuron loss when delivered intranigrally [162,163]. In summary, combined evidence from histopathologic, epidemiologic, and pharmacologic studies supports a role for TNF in eliciting dopaminergic neuron loss and nigrostriatal degeneration, suggesting TNF neurotoxicity may underlie the progressive loss that occurs in humans with PD.
TNF in Alzheimer's disease (AD)
The first indication of a contribution for TNF signaling in AD was the presence of TNF at amyloidogenic plaques in post-mortem analysis of AD brains [164]. In pre-clinical studies, transgenic Tg2576 mice which overexpress a mutated form of human amyloid precursor protein (APP) and develop amyloid plaque pathology display elevated TNF levels around fibrillar plaques consistent with findings in human tissue [165-167]. The localization of TNF within plaques in both animal models and human brains prompted investigations into genetic associations between AD and TNF and its receptors. A genome-wide screen found that three TNF polymorphisms (-308, -238 promoter polymorphisms and a 10.5 kb upstream microsatellite TNFa) which result in elevated TNF secretion, formed a haplotype that was associated with AD [168]. However, others have found that these polymorphisms increase the age of AD onset [169]. In addition to genetic linkage with TNF, the genetic association between the TNF receptors (TNFR1 and TNFR2) and AD was investigated. Specifically, the genes for TNFR1 and TNFR2 reside on chromosome 1p and chromosome 12p, respectively and these regions show genetic linkage to late-onset AD; in addition, a genetic polymorphism in exon 6 of the TNFR2 gene was associated with late-onset AD, while no significant association was found between AD and three genetic polymorphisms in the TNFR1 gene [170]. Although these genetic studies implicate TNF and its receptors in modifying AD risk, independent confirmation by additional studies as well as meta-analyses of genetic association studies will be needed to assess the overall genetic effect of these TNF related genes on AD [171,172].
Other evidence of increased inflammation and elevated TNF levels in AD pathology includes the dysregulation of TNF levels and other pro-inflammatory cytokines in AD patients and in transgenic mouse models of AD. Elevated TNF levels appear to correlate with disease progression as higher serum levels of TNF, as well as an increase in the TNF/IL-1β ratio, are present in patients with severe AD compared to individuals with mild-to-moderate disease [76]. However, similar to the case with genetic linkage studies, not all studies found differences in TNF levels between mild and severe disease [76,173]. Transgenic mouse models of AD have provided evidence that inflammation and TNF contribute to disease progression and onset. In three month old mice harboring three familial AD-linked mutations (APPSwe, tauP301L, and PS1M146V) which lead to accumulation of intraneuronal amyloid immunoreactivity in regions that include entorhinal cortex, elevated TNF mRNA levels were reported in the same regions and correlated with the onset of cognitive deficits in these mice [174,175]. Inhibition of solTNF signaling in triple transgenic mice (APPSwe, tauP301L, and PS1M146V) by either hippocampal infusion of DN-TNF inhibitors or intracerebroventricular injection of a lentivirus encoding DN-TNF reduced inflammation-induced accumulation of C-terminal APP fragments in the hippocampus, cortex, and amygdale. In triple (3xTgAD) transgenic mice lacking TNFR1, exposure to chronic systemic inflammation did not result in intraneuronal accumulation of amyloid immunoreactivity, suggesting that solTNF/TNFR1 signaling may be an important regulator of APP processing and that pathological elevation of TNF may contribute to pre-plaque pathology and acceleration of cognitive deficits (McAlpine et al., under review). In support of this idea, the contribution of TNFR1 signaling to AD pathogenesis was recently shown in APP23 transgenic mice which overexpress APPKM670/671NL. In these transgenic mice, TNFR1 deletion reduces Aß pathology, microglia activation, BACE1 activity, neuron loss, and memory deficits compared to transgenic APP23 mice expressing normal levels of TNFR1 [176]. Although far from conclusive, a recent report of a single-center, open-label clinical pilot study involving patients with mild-to-severe AD who received semi-weekly perispinal infusion of the anti-TNF biologic etanercept for six months claimed detectable improvement of cognitive performance in patients who received etanercept compared to those that received placebo, raising the interesting possibility that modulation of TNF signaling peripherally or centrally may have effects on cognitive performance in patients with AD [177]. These observations are not surprising in light of published studies that demonstrate TNF is a potent glio-transmitter. However, additional double-blinded placebo-controlled studies will be needed to confirm these promising findings.
Conclusions and considerations for the future
TNF exerts pleiotropic effects in the CNS and its role in normal brain functions, in particular synaptic scaling, has yet to be fully elucidated. While it is clear that solTNF is toxic to dopaminergic neurons and compromises their survival during chronic inflammatory stimuli in the ventral midbrain, tmTNF and TNFR2 appear to have important roles in myelination and may contribute to hippocampal neurogenesis after ischemic injury. These observations strongly suggest that one potential approach to lessen adverse effects of anti-TNF therapies in the CNS may be to selectively target TNFR1 signaling with localized delivery of inhibitors which spare TNFR2-mediated signaling. As solTNF signals preferentially through TNFR1, selective inhibition of solTNF signaling may be advantageous. This approach may allow efficient neutralization of solTNF in the desired region (i.e. substantia nigra) without eliciting collateral damage to TNFR2/tmTNF-dependent processes in regions of the brain where tmTNF has important homeostatic functions. An additional benefit to selective targeting of solTNF would be sparing tmTNF-dependent functions in the immune system, including proper development and maintenance of immune cell populations as well as self-tolerance and resistance to infectious agents (such as Mycobacterium and Listeria). Given the growing evidence that TNFR2 may antagonize TNFR1 signaling, a second strategy, which may bestow beneficial effects and allow protection of neuronal populations highly sensitive to solTNF/TNFR1-mediated toxicity, may be to increase TNFR2 expression levels through gene therapy. Lastly, it is important to note that while brain-permeant small molecule inhibitors of TNF, including the immunomodulatory drugs thalidomide and minocycline, may be attractive for CNS applications, their lack of selectivity for solTNF over tmTNF and their inhibitory effects on other inflammatory mediators may contribute to undesirable effects as TNF signaling and other inflammatory cascades would be suppressed globally in the CNS [178].
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The manuscript was written by MKM as part of her doctoral thesis. MGT provided historical perspectives and editorial assistance.
Contributor Information
Melissa K McCoy, Email: mccoym@mail.nih.gov.
Malú G Tansey, Email: malu.tansey@utsouthwestern.edu.
References
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. Faseb J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Samanta A, Feldmann M. TNFα. In: Oppenheim JJaFM, editor. Cytokine Reference. London: Academic Press; 2000. pp. 414–434. [Google Scholar]
- Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc Res Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Smookler DS, Mohammed FF, Kassiri Z, Duncan GS, Mak TW, Khokha R. Tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J Immunol. 2006;176:721–725. doi: 10.4049/jimmunol.176.2.721. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Samanta A, Feldmann M. TNF Receptors. In: Oppenheim JJaFM, editor. Cytokine Reference. London: Academic Press; 2000. pp. 1620–1632. [Google Scholar]
- Engelmann H, Holtmann H, Brakebusch C, Avni YS, Sarov I, Nophar Y, Hadas E, Leitner O, Wallach D. Antibodies to a soluble form of a tumor necrosis factor (TNF) receptor have TNF-like activity. J Biol Chem. 1990;265:14497–14504. [PubMed] [Google Scholar]
- Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm. 1995;47:8–17. [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Eissner G, Kirchner S, Lindner H, Kolch W, Janosch P, Grell M, Scheurich P, Andreesen R, Holler E. Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages. J Immunol. 2000;164:6193–6198. doi: 10.4049/jimmunol.164.12.6193. [DOI] [PubMed] [Google Scholar]
- Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15:353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Harashima S, Horiuchi T, Hatta N, Morita C, Higuchi M, Sawabe T, Tsukamoto H, Tahira T, Hayashi K, Fujita S, Niho Y. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. J Immunol. 2001;166:130–136. doi: 10.4049/jimmunol.166.1.130. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Ware CF, VanArsdale S, VanArsdale TL. Apoptosis mediated by the TNF-related cytokine and receptor families. J Cell Biochem. 1996;60:47–55. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C47::AID-JCB8%3E3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Huang Q, Oikemus S, Shank J, Ventura JJ, Cusson N, Vaillancourt RR, Su B, Davis RJ, Kelliher MA. The death domain kinase RIP1 is essential for tumor necrosis factor alpha signaling to p38 mitogen-activated protein kinase. Mol Cell Biol. 2003;23:8377–8385. doi: 10.1128/MCB.23.22.8377-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston BW, Lange-Carter CA, Gardner AM, Johnson GL, Riches DW. Tumor necrosis factor alpha rapidly activates the mitogen-activated protein kinase (MAPK) cascade in a MAPK kinase kinase-dependent, c-Raf-1-independent fashion in mouse macrophages. Proc Natl Acad Sci USA. 1995;92:1614–1618. doi: 10.1073/pnas.92.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schievella AR, Chen JH, Graham JR, Lin LL. MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem. 1997;272:12069–12075. doi: 10.1074/jbc.272.18.12069. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuls SR, Hoek RM, Ngo VN, McNeil T, Lucian LA, Janatpour MJ, Korner H, Scheerens H, Hessel EM, Cyster JG, et al. Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity. 2001;15:533–543. doi: 10.1016/s1074-7613(01)00215-1. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]
- Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Rao P, Hsu KC, Chao MV. Upregulation of NF-kappa B-dependent gene expression mediated by the p75 tumor necrosis factor receptor. J Interferon Cytokine Res. 1995;15:171–177. doi: 10.1089/jir.1995.15.171. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993;268:18542–18548. [PubMed] [Google Scholar]
- Higuchi M, Nagasawa K, Horiuchi T, Oike M, Ito Y, Yasukawa M, Niho Y. Membrane tumor necrosis factor-alpha (TNF-alpha) expressed on HTLV-I-infected T cells mediates a costimulatory signal for B cell activation – characterization of membrane TNF-alpha. Clin Immunol Immunopathol. 1997;82:133–140. doi: 10.1006/clin.1996.4291. [DOI] [PubMed] [Google Scholar]
- Pocsik E, Duda E, Wallach D. Phosphorylation of the 26 kDa TNF precursor in monocytic cells and in transfected HeLa cells. J Inflamm. 1995;45:152–160. [PubMed] [Google Scholar]
- Watts AD, Hunt NH, Wanigasekara Y, Bloomfield G, Wallach D, Roufogalis BD, Chaudhri G. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in 'reverse signalling'. EMBO J. 1999;18:2119–2126. doi: 10.1093/emboj/18.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner S, Boldt S, Kolch W, Haffner S, Kazak S, Janosch P, Holler E, Andreesen R, Eissner G. LPS resistance in monocytic cells caused by reverse signaling through transmembrane TNF (mTNF) is mediated by the MAPK/ERK pathway. J Leukoc Biol. 2004;75:324–331. doi: 10.1189/jlb.0703343. [DOI] [PubMed] [Google Scholar]
- Domonkos A, Udvardy A, Laszlo L, Nagy T, Duda E. Receptor-like properties of the 26 kDa transmembrane form of TNF. Eur Cytokine Netw. 2001;12:411–419. [PubMed] [Google Scholar]
- Fluhrer R, Grammer G, Israel L, Condron MM, Haffner C, Friedmann E, Bohland C, Imhof A, Martoglio B, Teplow DB, Haass C. A gamma-secretase-like intramembrane cleavage of TNFalpha by the GxGD aspartyl protease SPPL2b. Nat Cell Biol. 2006;8:894–896. doi: 10.1038/ncb1450. [DOI] [PubMed] [Google Scholar]
- Friedmann E, Hauben E, Maylandt K, Schleeger S, Vreugde S, Lichtenthaler SF, Kuhn PH, Stauffer D, Rovelli G, Martoglio B. SPPL2a and SPPL2b promote intramembrane proteolysis of TNFalpha in activated dendritic cells to trigger IL-12 production. Nat Cell Biol. 2006;8:843–848. doi: 10.1038/ncb1440. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Wyss-Coray T. Cytokines in CNS inflammation and disease. In: Lane TE, Carson M, C B, T W-C, editor. Central Nervous System Diseases and Inflammation. W-C: Springer; 2008. [Google Scholar]
- Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990;144:129–135. [PubMed] [Google Scholar]
- Merrill JE. Effects of interleukin-1 and tumor necrosis factor-alpha on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13:130–137. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-[alpha] Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential Regulation of AMPA Receptor and GABA Receptor Trafficking by Tumor Necrosis Factor-{alpha} J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex. 2004;14:97–105. doi: 10.1093/cercor/bhg108. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Braithwaite SP, Beattie MS, Beattie EC. TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol. 2004;1:263–273. doi: 10.1017/S1740925X05000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6:341–360. [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30:213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, Luer W, Helwig A, Poser S. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann Neurol. 1995;37:82–88. doi: 10.1002/ana.410370115. [DOI] [PubMed] [Google Scholar]
- Raine CS, Bonetti B, Cannella B. Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev Neurol (Paris) 1998;154:577–585. [PubMed] [Google Scholar]
- Fillit H, Ding WH, Buee L, Kalman J, Altstiel L, Lawlor B, Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- Paganelli R, Di Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Di Gioacchino M, Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer's disease patients. Exp Gerontol. 2002;37:257–263. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Cacabelos R, Sanpedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm. 2000;107:335–341. doi: 10.1007/s007020050028. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson's disease. Biomed Pharmacother. 1999;53:141–145. doi: 10.1016/S0753-3322(99)80079-1. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Corti A, Ghezzi P. Tumor Necrosis Factor: Methods and Protocols. First. Totowa, New Jersey: Humana Press; 2004. [Google Scholar]
- Beutler B, Krochin N, Milsark IW, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Perlstein RS, Whitnall MH, Abrams JS, Mougey EH, Neta R. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology. 1993;132:946–952. doi: 10.1210/endo.132.3.8382602. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Matta SG, Peterson PK, Newton R, Chao C, McAllen K. Tumor necrosis factor-alpha is a potent ACTH secretagogue: comparison to interleukin-1 beta. Endocrinology. 1989;124:3131–3133. doi: 10.1210/endo-124-6-3131. [DOI] [PubMed] [Google Scholar]
- Waage A, Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988;63:299–302. [PMC free article] [PubMed] [Google Scholar]
- Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S, Tebo JM, Hamilton TA. IL-4 suppresses cytokine gene expression induced by IFN-gamma and/or IL-2 in murine peritoneal macrophages. J Immunol. 1992;148:1725–1730. [PubMed] [Google Scholar]
- de Waal Malefyt R, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, de Vries JE. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- Seckinger P, Isaaz S, Dayer JM. A human inhibitor of tumor necrosis factor alpha. J Exp Med. 1988;167:1511–1516. doi: 10.1084/jem.167.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger P, Isaaz S, Dayer JM. Purification and biologic characterization of a specific tumor necrosis factor alpha inhibitor. J Biol Chem. 1989;264:11966–11973. [PubMed] [Google Scholar]
- Novick D, Engelmann H, Wallach D, Rubinstein M. Soluble cytokine receptors are present in normal human urine. J Exp Med. 1989;170:1409–1414. doi: 10.1084/jem.170.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann H, Novick D, Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990;265:1531–1536. [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2007 doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Horiuchi T, Tsukamoto H. Binding activities of infliximab and etanercept to transmembrane tumor necrosis factor-alpha. Gastroenterology. 2004;126:934–935. doi: 10.1053/j.gastro.2004.01.036. author reply 935–936. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Horiuchi T, Hatta N, Tsukamoto H, Harashima S, Kikuchi Y, Otsuka J, Okamura S, Fujita S, Harada M. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology. 2005;128:376–392. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Gudbrandsdottir S, Larsen R, Sorensen LK, Nielsen S, Hansen MB, Svenson M, Bendtzen K, Muller K. TNF and LT binding capacities in the plasma of arthritis patients: effect of etanercept treatment in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2004;22:118–124. [PubMed] [Google Scholar]
- Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, Szymkowski DE, Abbott C, Carmichael D, Chan C, Cherry L, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- Szymkowski DE. Creating the next generation of protein therapeutics through rational drug design. Curr Opin Drug Discov Devel. 2005;8:590–600. [PubMed] [Google Scholar]
- Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O'Brien C, Eivazi A, Kung J, Nguyen DH, Doberstein SK, et al. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol. 2007;179:1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- He MM, Smith AS, Oslob JD, Flanagan WM, Braisted AC, Whitty A, Cancilla MT, Wang J, Lugovskoy AA, Yoburn JC, et al. Small-Molecule Inhibition of TNF-{alpha} Science. 2005;310:1022–1025. doi: 10.1126/science.1116304. [DOI] [PubMed] [Google Scholar]
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Gabler WL, Creamer HR. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26:52–58. doi: 10.1111/j.1600-0765.1991.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Halliwell B. Prevention of peroxynitrite-dependent tyrosine nitration and inactivation of alpha1-antiproteinase by antibiotics. Free Radic Res. 1997;26:49–56. doi: 10.3109/10715769709097783. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki S, Iuchi Y, Ikeda Y, Sasagawa I, Tomita Y, Fujii J. Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Biophys Res Commun. 2003;312:843–849. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279:19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu du C, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- Melchert M, List A. The thalidomide saga. Int J Biochem Cell Biol. 2007;39:1489–1499. doi: 10.1016/j.biocel.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Moss ML, Sklair-Tavron L, Nudelman R. Drug Insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008;4:300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- Thabet MM, Huizinga TW. Drug evaluation: apratastat, a novel TACE/MMP inhibitor for rheumatoid arthritis. Curr Opin Investig Drugs. 2006;7:1014–1019. [PubMed] [Google Scholar]
- Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Maimone D, Gregory S, Arnason BGW, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. Journal of Neuroimmunology. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, Luer W, Poser S. Cytokine mRNA levels in mononuclear blood cells from patients with multiple sclerosis. Neurology. 1994;44:1523. doi: 10.1212/wnl.44.8.1523. [DOI] [PubMed] [Google Scholar]
- Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1995;92:11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Cross AH. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991;30:694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Papierz W, Glabinski A, Kohno T. Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol. 1995;56:135–141. doi: 10.1016/0165-5728(94)00139-f. [DOI] [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Experimental autoimmune encephalomyelitis: immunotherapy with anti-tumor necrosis factor antibodies and soluble tumor necrosis factor receptors. Neurology. 1995;45:S44–49. doi: 10.1212/wnl.45.6_suppl_6.s44. [DOI] [PubMed] [Google Scholar]
- TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457. [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Bauer J, Akassoglou K, Lassmann H, Kollias G, Probert L. A tumor necrosis factor-induced model of human primary demyelinating diseases develops in immunodeficient mice. Eur J Immunol. 1999;29:912–917. doi: 10.1002/(SICI)1521-4141(199903)29:03<912::AID-IMMU912>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Kollias G. Uncoupling the Proinflammatory from the Immunosuppressive Properties of Tumor Necrosis Factor (TNF) at the p55 TNF Receptor Level: Implications for Pathogenesis and Therapy of Autoimmune Demyelination. J Exp Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L, Kranidioti K, Xanthoulea S, Denis M, Kotanidou A, Douni E, Blackshear PJ, Kontoyiannis DL, Kollias G. Transmembrane TNF protects mutant mice against intracellular bacterial infections, chronic inflammation and autoimmunity. European Journal of Immunology. 2006;36:2768–2780. doi: 10.1002/eji.200635921. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Seri M, Yin L, Barone V, Bolino A, Celli I, Bocciardi R, Pasini B, Ceccherini I, Lerone M, Kristoffersson U, et al. Frequency of RET mutations in long- and short-segment Hirschsprung disease. Hum Mutat. 1997;9:243–249. doi: 10.1002/(SICI)1098-1004(1997)9:3<243::AID-HUMU5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Martin D, Hallenbeck JM. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res. 1997;778:265–271. doi: 10.1016/s0006-8993(97)00981-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Feuerstein GZ, Xu L, Wang H, Schumacher WA, Ogletree ML, Taub R, Duan JJ, Decicco CP, Liu RQ. Inhibition of tumor necrosis factor-alpha-converting enzyme by a selective antagonist protects brain from focal ischemic injury in rats. Mol Pharmacol. 2004;65:890–896. doi: 10.1124/mol.65.4.890. [DOI] [PubMed] [Google Scholar]
- Heldmann U, Thored P, Claasen JH, Arvidsson A, Kokaia Z, Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp Neurol. 2005;196:204–208. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor (TNF)-alpha in 6-hydroxydopamine-lesioned striatum in rats without influence of systemic L-DOPA on the TNF-alpha induction. Neurosci Lett. 1999;268:101–104. doi: 10.1016/s0304-3940(99)00388-2. [DOI] [PubMed] [Google Scholar]
- Rousselet E, Callebert J, Parain K, Joubert C, Hunot S, Hartmann A, Jacque C, Perez-Diaz F, Cohen-Salmon C, Launay JM, Hirsch EC. Role of TNF-alpha receptors in mice intoxicated with the parkinsonian toxin MPTP. Exp Neurol. 2002;177:183–192. doi: 10.1006/exnr.2002.7960. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. Faseb J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Barcia C, de Pablos V, Bautista-Hernandez V, Sanchez-Bahillo A, Bernal I, Fernandez-Villalba E, Martin J, Banon R, Fernandez-Barreiro A, Herrero MT. Increased plasma levels of TNF-alpha but not of IL1-beta in MPTP-treated monkeys one year after the MPTP administration. Parkinsonism Relat Disord. 2005;11:435–439. doi: 10.1016/j.parkreldis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM. Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol. 2001;169:219–230. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- Gayle DA, Ling Z, Tong C, Landers T, Lipton JW, Carvey PM. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Brain Res Dev Brain Res. 2002;133:27–35. doi: 10.1016/s0165-3806(01)00315-7. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Branton RL. A role for tumor necrosis factor alpha in death of dopaminergic neurons following neural transplantation. Exp Neurol. 2002;176:154–162. doi: 10.1006/exnr.2002.7911. [DOI] [PubMed] [Google Scholar]
- Aloe L, Fiore M. TNF-alpha expressed in the brain of transgenic mice lowers central tyroxine hydroxylase immunoreactivity and alters grooming behavior. Neurosci Lett. 1997;238:65–68. doi: 10.1016/s0304-3940(97)00850-1. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chen EY, Lipton JW, Tong CW, Chang QA, Ling ZD. Intra-parenchymal injection of tumor necrosis factor-alpha and interleukin 1-beta produces dopamine neuron loss in the rat. J Neural Transm. 2005;112:601–612. doi: 10.1007/s00702-004-0222-z. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, Kuno S. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson's disease. Neurosci Lett. 2001;311:1–4. doi: 10.1016/s0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- Wu YR, Feng IH, Lyu RK, Chang KH, Lin YY, Chan H, Hu FJ, Lee-Chen GJ, Chen CM. Tumor necrosis factor-alpha promoter polymorphism is associated with the risk of Parkinson's disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:300–304. doi: 10.1002/ajmg.b.30435. [DOI] [PubMed] [Google Scholar]
- Kruger R, Hardt C, Tschentscher F, Jackel S, Kuhn W, Muller T, Werner J, Woitalla D, Berg D, Kuhnl N, et al. Genetic analysis of immunomodulating factors in sporadic Parkinson's disease. J Neural Transm. 2000;107:553–562. doi: 10.1007/s007020070078. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Leng A, Mura A, Feldon J, Ferger B. Tumor necrosis factor-alpha receptor ablation in a chronic MPTP mouse model of Parkinson's disease. Neurosci Lett. 2005;375:107–111. doi: 10.1016/j.neulet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. Faseb J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL. Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis. 2004;16:190–201. doi: 10.1016/j.nbd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O'Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral Lentiviral Delivery of Dominant-negative TNF Attenuates Neurodegeneration and Behavioral Deficits in Hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, Chin JE. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res Bull. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- Munch G, Apelt J, Rosemarie Kientsch E, Stahl P, Luth HJ, Schliebs R. Advanced glycation endproducts and pro-inflammatory cytokines in transgenic Tg2576 mice with amyloid plaque pathology. J Neurochem. 2003;86:283–289. doi: 10.1046/j.1471-4159.2003.01837.x. [DOI] [PubMed] [Google Scholar]
- Collins JS, Perry RT, Watson B, Jr, Harrell LE, Acton RT, Blacker D, Albert MS, Tanzi RE, Bassett SS, McInnis MG, et al. Association of a haplotype for tumor necrosis factor in siblings with late-onset Alzheimer disease: the NIMH Alzheimer Disease Genetics Initiative. Am J Med Genet. 2000;96:823–830. doi: 10.1002/1096-8628(20001204)96:6<823::aid-ajmg26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Harrell LE, Acton RT, Go RC. Investigation of association of 13 polymorphisms in eight genes in southeastern African American Alzheimer disease patients as compared to age-matched controls. Am J Med Genet. 2001;105:332–342. doi: 10.1002/ajmg.1371. [DOI] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer's disease. Neurobiol Aging. 2001;22:873–883. doi: 10.1016/s0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Fernandez-Novoa L, Lombardi V, Kubota Y, Takeda M. Molecular genetics of Alzheimer's disease and aging. Methods Find Exp Clin Pharmacol. 2005;27:1–573. [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, Marksteiner J, Humpel C. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dement Geriatr Cogn Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid {beta} generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobinick E, Gross H, Weinberger A, Cohen H. TNF-alpha Modulation for Treatment of Alzheimer's Disease: A 6-Month Pilot Study. MedGenMed. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Jackson JM. TNF- alpha inhibitors. Dermatol Ther. 2007;20:251–264. doi: 10.1111/j.1529-8019.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- Rigby WF. Drug insight: different mechanisms of action of tumor necrosis factor antagonists-passive-aggressive behavior? Nat Clin Pract Rheumatol. 2007;3:227–233. doi: 10.1038/ncprheum0438. [DOI] [PubMed] [Google Scholar]
- Sheridan C. Cimzia's setback paves way for other TNF inhibitors in Crohn's disease. Nat Biotechnol. 2007;25:487–488. doi: 10.1038/nbt0507-487. [DOI] [PubMed] [Google Scholar]
- Kaushik VV, Moots RJ. CDP-870 (certolizumab) in rheumatoid arthritis. Expert Opin Biol Ther. 2005;5:601–606. doi: 10.1517/14712598.5.4.601. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Hohlfeld R. Therapeutic approaches in multiple sclerosis: lessons from failed and interrupted treatment trials. BioDrugs. 2002;16:183–200. doi: 10.2165/00063030-200216030-00003. [DOI] [PubMed] [Google Scholar]
- Williams DA. RAC Reviews Serious Adverse Event Associated with AAV TherapyTrial. Mol Ther. 2007;15:2053–2054. doi: 10.1038/sj.mt.6300352. [DOI] [PubMed] [Google Scholar]