Abstract
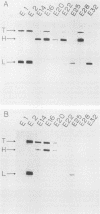
We examined the reactivities of Clostridium butyricum neurotoxin to nine monoclonal antibodies against Clostridium botulinum type E neurotoxin which recognize the light chain or the amino-terminal half (H-1 fragment) or the carboxyl-terminal half (H-2 fragment) of the heavy chain of botulinum neurotoxin. Butyricum neurotoxin and its derived chains did not react to two of four monoclonal antibodies recognizing the light chain, one of three recognizing the H-1 fragment, and one of two recognizing the H-2 fragment. The results indicate that the immunological difference between the two neurotoxins is not attributable to a particular portion of the toxin molecule. The fragment of butyricum neurotoxin obtained by prolonged tryptic treatment was found to comprise the light chain and H-1 fragment linked together by a disulfide bond.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aureli P., Fenicia L., Pasolini B., Gianfranceschi M., McCroskey L. M., Hatheway C. L. Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J Infect Dis. 1986 Aug;154(2):207–211. doi: 10.1093/infdis/154.2.207. [DOI] [PubMed] [Google Scholar]
- Binz T., Kurazono H., Wille M., Frevert J., Wernars K., Niemann H. The complete sequence of botulinum neurotoxin type A and comparison with other clostridial neurotoxins. J Biol Chem. 1990 Jun 5;265(16):9153–9158. [PubMed] [Google Scholar]
- Bittner M. A., DasGupta B. R., Holz R. W. Isolated light chains of botulinum neurotoxins inhibit exocytosis. Studies in digitonin-permeabilized chromaffin cells. J Biol Chem. 1989 Jun 25;264(18):10354–10360. [PubMed] [Google Scholar]
- Giménez J. A., Sugiyama H. Comparison of toxins of Clostridium butyricum and Clostridium botulinum type E. Infect Immun. 1988 Apr;56(4):926–929. doi: 10.1128/iai.56.4.926-929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez J. A., Sugiyama H. Simplified purification method for Clostridium botulinum type E toxin. Appl Environ Microbiol. 1987 Dec;53(12):2827–2830. doi: 10.1128/aem.53.12.2827-2830.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M., Sakaguchi S., Sakaguchi G. Purification and some properties of Clostridium botulinum type-E toxin. Biochim Biophys Acta. 1968 Oct 21;168(2):207–217. doi: 10.1016/0005-2795(68)90144-x. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Kamata Y., Nagai T., Ogasawara J., Sakaguchi G. The use of monoclonal antibodies to analyze the structure of Clostridium botulinum type E derivative toxin. Infect Immun. 1986 Jun;52(3):786–791. doi: 10.1128/iai.52.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Miki A., Kamata Y., Ogasawara J., Sakaguchi G. Immunological characterization of papain-induced fragments of Clostridium botulinum type A neurotoxin and interaction of the fragments with brain synaptosomes. Infect Immun. 1989 Sep;57(9):2634–2639. doi: 10.1128/iai.57.9.2634-2639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Ogasawara J., Shimote Y., Kamata Y., Sakaguchi G. Antigenic structure of Clostridium botulinum type B neurotoxin and its interaction with gangliosides, cerebroside, and free fatty acids. Infect Immun. 1987 Dec;55(12):3051–3056. doi: 10.1128/iai.55.12.3051-3056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCroskey L. M., Hatheway C. L., Fenicia L., Pasolini B., Aureli P. Characterization of an organism that produces type E botulinal toxin but which resembles Clostridium butyricum from the feces of an infant with type E botulism. J Clin Microbiol. 1986 Jan;23(1):201–202. doi: 10.1128/jcm.23.1.201-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midura T. F., Arnon S. S. Infant botulism. Identification of Clostridium botulinum and its toxins in faeces. Lancet. 1976 Oct 30;2(7992):934–936. doi: 10.1016/s0140-6736(76)90894-1. [DOI] [PubMed] [Google Scholar]
- Pickett J., Berg B., Chaplin E., Brunstetter-Shafer M. A. Syndrome of botulism in infancy: clinical and electrophysiologic study. N Engl J Med. 1976 Sep 30;295(14):770–772. doi: 10.1056/NEJM197609302951407. [DOI] [PubMed] [Google Scholar]
- Thompson D. E., Brehm J. K., Oultram J. D., Swinfield T. J., Shone C. C., Atkinson T., Melling J., Minton N. P. The complete amino acid sequence of the Clostridium botulinum type A neurotoxin, deduced by nucleotide sequence analysis of the encoding gene. Eur J Biochem. 1990 Apr 20;189(1):73–81. doi: 10.1111/j.1432-1033.1990.tb15461.x. [DOI] [PubMed] [Google Scholar]