Abstract
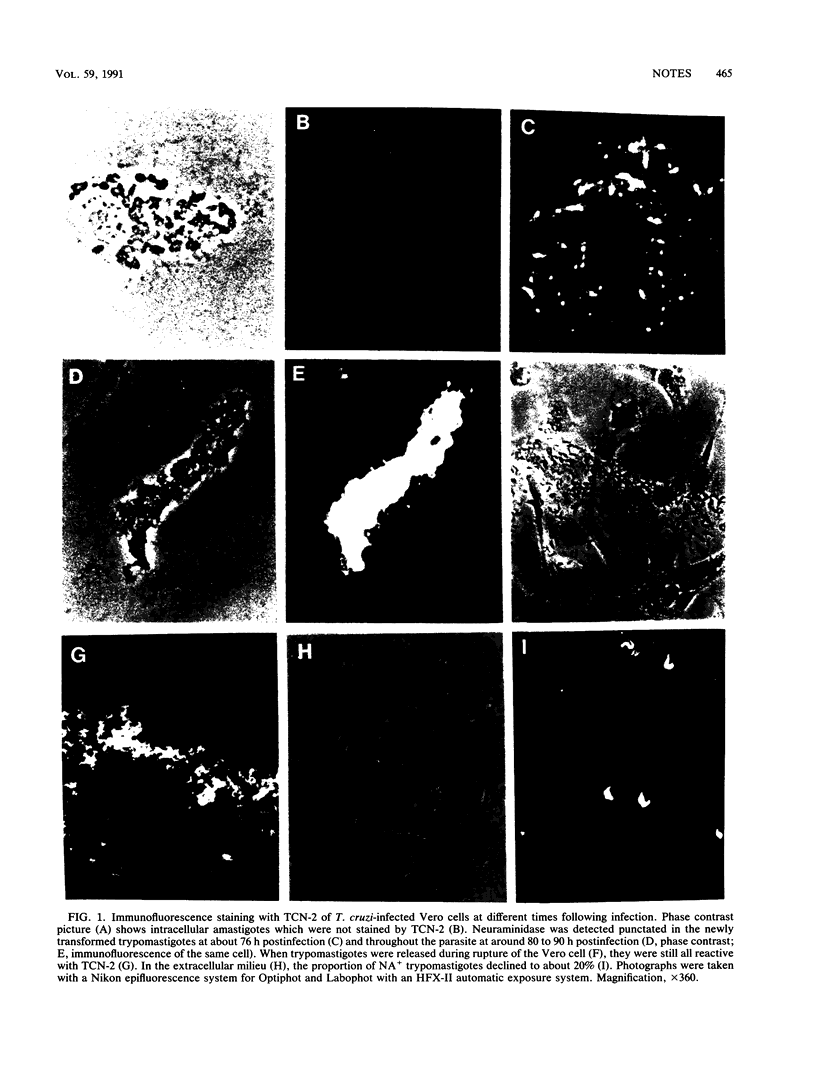
The developmentally regulated expression of Trypanosoma cruzi neuraminidase in culture cells was monitored by immunofluorescence with a monoclonal antibody (TCN-2) against the enzyme. The results showed that TCN-2 reacted with all intracellular trypomastigote forms (NA+) but not with amastigotes. Immunoprecipitation of radiolabeled neuraminidase confirmed TCN-2 reactivity with trypomastigotes and the specificity of the antibody binding. During exiting from the host cells, all trypomastigotes were still NA+. However, when free in the extracellular environment, the relative proportion of NA+ parasites declined from 100% to about 20%, thereby establishing a subpopulation of trypomastigotes which did not express enzyme (NA-). The expression of neuraminidase in all intracellular trypomastigotes and in only a subpopulation of the extracellular counterpart suggests that the enzyme may play a role in parasite exiting from infected cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavallesco R., Pereira M. E. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J Immunol. 1988 Jan 15;140(2):617–625. [PubMed] [Google Scholar]
- Colman P. M., Ward C. W. Structure and diversity of influenza virus neuraminidase. Curr Top Microbiol Immunol. 1985;114:177–255. doi: 10.1007/978-3-642-70227-3_5. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Hoff R. Heterogeneous distribution of neuraminidase activity in strains and clones of Trypanosoma cruzi and its possible association with parasite myotropism. Mol Biochem Parasitol. 1986 Aug;20(2):183–189. doi: 10.1016/0166-6851(86)90030-7. [DOI] [PubMed] [Google Scholar]
- Postan M., Dvorak J. A., McDaniel J. P. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN- mice with two clones isolated from a common source. Am J Trop Med Hyg. 1983 May;32(3):497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Mejia J. S., Pereira M. E. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J Immunol. 1990 Jun 1;144(11):4384–4391. [PubMed] [Google Scholar]
- Siekevitz M., Gefter M. L., Brodeur P., Riblet R., Marshak-Rothstein A. The genetic basis of antibody production: the dominant anti-arsonate idiotype response of the strain A mouse. Eur J Immunol. 1982 Dec;12(12):1023–1032. doi: 10.1002/eji.1830121208. [DOI] [PubMed] [Google Scholar]
- de Titto E. H., Araujo F. G. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin Immunol Immunopathol. 1988 Jan;46(1):157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]