Abstract
Sulforaphane (SFN) is a natural isothiocyanate that is present in cruciferous vegetables such as broccoli and cabbage. Previous studies have shown that SFN is effective in preventing carcinogenesis induced by carcinogens in rodents, which is related in part to its potent anti-inflammation properties. In the present study, we compared the anti-inflammatory effect of SFN on LPS-stimulated inflammation in primary peritoneal macrophages derived from Nrf2 (+/+) and Nrf2 mice. Pretreatment of SFN in Nrf2 (+/+) primary peritoneal macrophages potently inhibited LPS-stimulated mRNA expression, protein expression and production of TNFα, IL-1β, Cox-2 and iNOS. HO-1 expression, which is significantly augmented in LPS-stimulated Nrf2 (+/+) primary peritoneal macrophages by SFN. Interestingly, the anti-inflammatory effect was attenuated in Nrf2 (−/−) primary peritoneal macrophages. We concluded that SFN exerts its anti-inflammatory activity mainly via activation of Nrf2 in mouse peritoneal macrophages.
Keywords: Sulforaphane, Nuclear E2-factor related factor 2, Inflammation, Peritoneal macrophage, Tumor necrosis factor-α, Interleukin-1β, Cyclooxygenase-2, inducible nitric oxide synthase
1. Introduction
Sulforaphane (SFN) [1-isothiocyanato-4-(methylsulfinyl)-butane], an isothiocyanate, is a chemopreventive photochemical which is a potent inducer of phase II enzyme involved in the detoxification of xenobiotics[1–3]. The chemopreventive effect of sulforaphane has been attributed to induction of phase II detoxification enzymes and induction of apoptosis [1, 3–6]. In addition, Heiss et al. reported that sulforaphane possesses anti-inflammatory activity, resulting in down-regulation of LPS-stimulated inducible nitric-oxide synthase (iNOS), COX-2 and TNF-α expression in Raw macrophages due to inhibition on DNA binding of NF-kB[7].
Since Rudolf Virchow noted leucocytes in neoplastic tissues and made a connection between inflammation and cancer, substantial evidence demonstrates that chronic inflammation may be a key predisposing factor to cancer. Inflammation could be triggered by an environmental insult such as microbial/viral infection or chemical/toxin irritation. Driven by a cascade of cytokines and chemokines interaction, inflammatory cells such as neutrophils, macrophages and lymphocytes will infiltrate the wounded tissue [8]. Inflammatory cytokines and chemokines have the potential to stimulate tumor-cell proliferation and survival. In addition, reactive oxygen or nitrogen species produced by activated inflammatory cells cause mutations in tumor suppressor genes and modification of proteins involved in essential cellular processes including apoptosis, DNA repair and cell cycle checkpoint [9].
Of particular interest from studies in our laboratory were observations demonstrating that increased susceptibility of Nrf2 deficient mice to DSS-induced colitis was associated with decreased expression of antioxidant/phase II detoxifying enzymes with concomitant increased of pro-inflammatory cytokines/biomarkers [10]. Transcription factor NF-E2-related factor 2 (Nrf2) was demonstrated to regulate the induction of genes encoding antioxidant proteins and phase 2 detoxifying. Such findings delineated the significance of Nrf2 in protecting intestinal integrity upon inflammatory stimuli. Findings in our lab indicated that SFN treatments resulted in decreased levels of pro-inflammatory mediators prostaglandin E2 or leukotriene B4 in intestinal polyps or apparently normal mucosa in ApcMin/+ mice model [1]. We hypothesized that Nrf2 deficiency would attenuate anti-inflammatory effect of sulforaphane in macrophages.
In the current study, we used the peritoneal macrophages derived from Nrf2 (+/+) and Nrf2 (−/−) mice, which can be challenged with bacterial lipopolysaccharides (LPS) to mimic a state of infection and inflammation to delineate anti-inflammatory properties of sulforaphane. Sulforaphane significantly suppressed the LPS-induced inflammation in Nrf2 (+/+) mouse peritoneal macrophages but not in Nrf2 (−/−) peritoneal macrophages, indicating that Nrf2 is a key modulator in anti-inflammatory action of sulforaphane.
2. Materials and Method
Animal, Cell Culture and Reagents
Nrf2 (−/−) mice (C57BL/SV129) have been described previously (13). Nrf2 (−/−) mice were backcrossed with C57BL/6J wild-type mice (The Jackson Laboratory, Bar Harbor, ME). To confirm the genotype from each animal, DNA was extracted from the tail and analyzed by PCR using the following primers: 3′-primer, 5′-GGAATGGAAAATAGCTCCTGCC-3′; 5′-primer, 5′-GCCTGAGAGCTGTAGGCCC-3′; lacZ primer, 5′-GGGTTTTCCCAGTCACGAC-3′ Nrf2 (−/−) and Nrf2 (+/+) mice exhibited one band at 200 and 300 bp, respectively. The second generations (F2) of male Nrf2 knockout mice were used in this study. Age-matched male C57BL/6J mice were purchased from The Jackson Laboratory. 9- to 12-week-old mice were used and housed at Rutgers Animal Facility and maintained under 12-hour light/dark cycles.
Thioglycolate broth-elicited peritoneal macrophages were prepared as previously described [11] and cultured in RPMI 1640 medium containing l-glutamine, penicillin, streptomycin, and 5% FBS. The macrophages were cultured in RPMI 1640 at 37°C in an atmosphere of 5% CO2 and then treated with stimuli for the indicated times as described. Sulforaphane (SFN) was obtained from Sigma (St Louis, MO). Thioglycolate broth was obtained from Edge Biologicals (Memphis, TN). iNOS, COX2 antibodies were purchased from Cayman Chemical (Ann Arbor, MI) and HO-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). TNF-α, IL-1-β, PGE2 ELISA kits were acquired from Calbiochem Technology (San Diego, CA). TNF-α, IL-1β ELISA kits were obtained from Biosource (Camarillo, CA).
Western blotting analysis
After treatment, peritoneal macrophages in six-well plates were washed with ice-cold PBS and lysed with 200 µl of whole cell lyses buffer (10 mM Tris–HCl, pH 7.9, 250 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 0.5% Triton X-100, 10% glycerol, 1 mM proteinase inhibitor mixture, 1 mM phenylmethylsulfonyl fluoride, 100 lM Na3VO4, 5 lM ZnCl2, 2 mM indole acetic acid). The cell lysates were centrifuged at 12,000 g for 10 min at 4°C. The protein concentrations of the supernatants of the whole cell lysate were determined using a Bio-Rad protein assay kit. Protein (20 µg) was loaded onto NuPAGE 4% to 12% electrophoresis gel (Invitrogen, Carlsbad, CA) and, after electrophoresis, transferred onto polyvinylidene difluoride membrane. After incubation with appropriate primary antibodies, the membranes were detected by HRP-conjugated secondary antibodies and ECL reagents (GE Healthcare).
RNA isolation and reverse transcription-PCR
Total RNA from mouse peritoneal macrophages was isolated by Trizol (Invitrogen, Carlsbad, CA). Total RNA samples were converted to single-stranded cDNA by the Superscript First-Strand Synthesis System III (Invitrogen). The resulting cDNA was amplified by the PCR supermix kit (Invitrogen). PCR conditions are as follows: 94°C for 10 min followed by 30 cycles of denaturation at 94°C for 30s, annealing at 50°C for 30s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were resolved on 1% agarose gels and visualized under UV lamps.
Nitrate oxide assay
Nitric oxides secreted by peritoneal macrophages were measured by Griess reagent (Promega). A nitric oxide standard curve (0.1 M sodium nitrite in water, making 100, 50, 25, 12.5, 6.25, 3.13, 1.56 and 0 µM) was prepared. 50 µl of the samples to be measured were pipetted into a 96-well plate. Using a multichannel pipette, 50 µl Sulfanilamide (1% sulfanilamide in 5% phosphoric acid) solution were added to each sample and the mixture was incubated for 10 minutes at room temperature protected from light. Following that, 50 µl NED (0.1% N-1-napthylethylenediamine dihydrochloride in water) solution was added to all wells and the mixture was incubated for 10 minutes at room temperature protected from light. Purple color started to appear and the absorbance was measured at a wavelength between 520–550 nm.
TNF-α, IL--1β and PGE2 ELISA assay
PGE2 assay was performed according to the protocols of Cayman Chemical (Ann Arbor, MI). TNF-α, IL-1β assays were performed protocols of Biosource (Camarillo, CA) For the TNF-α ELISA assay, 50 µl of incubation buffer was first added to all wells. Next, 50 µl standard diluents buffer and 50 µl of samples were added to each well. 50 µl of biotin conjugate was added afterwards, mixed well and the reaction was incubated for 90 min at room temperature. The wells were then aspirated and washed thoroughly 4 times. 100 µl of streptavidin-HRP working solution was then added and incubated for 45 min at room temperature. The reaction was stopped by adding 100 µl stop solution and the reaction was read at 450 nm.
3. Results
We used the primary peritoneal macrophage from Nrf2 (+/+) and Nrf2 (−/−) mice, which releases NO, PGs, and pro-inflammatory cytokines such as TNF-α IL-1, upon stimulation with LPS, hence providing a suitable model for studying anti-inflammatory activity of sulforaphane.
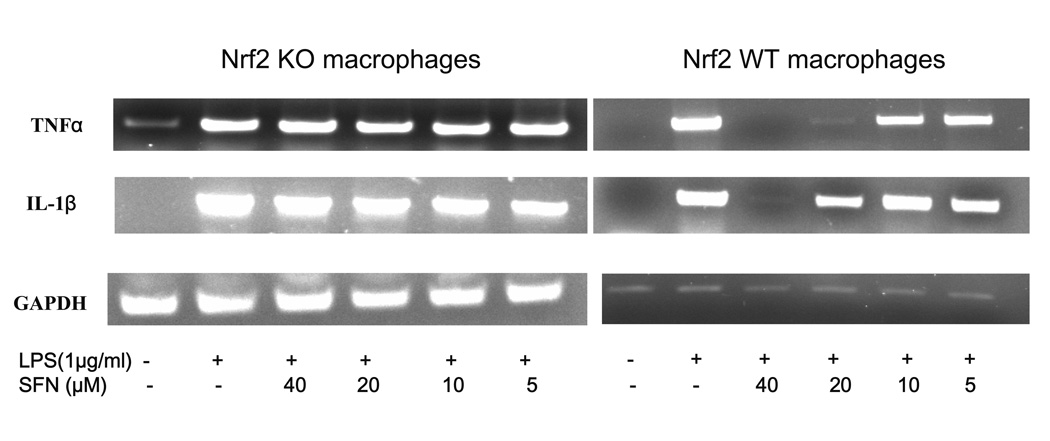
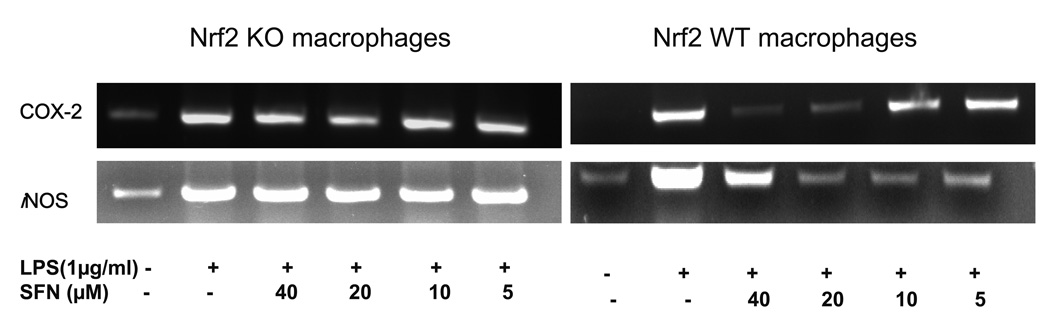
3.1. Sulforaphane significantly inhibited LPS-induced IL-1β, TNF-α, Cox-2 and iNOS mRNA in Nrf2 (+/+) peritoneal macrophages but not in Nrf2 (−/−) peritoneal macrophages
To assess the role of Nrf2 in LPS stimulated inflammation in macrophages, Nrf2 KO primary peritoneal macrophages and their wild type counterparts were pretreated with sulforaphane (5–40 µM) for 6 hrs and challenged with LPS (1µg/ml) for 6 hrs subsequently. Using a RT-PCR method, we determined the mRNA expression of TNFα and IL-1β and found that their expression was abolished by sulforaphane at 40 µM in wild type peritoneal macrophages (Fig. 1A). Sulforaphane at 10, 20 µM significantly inhibited mRNA expression of TNFα, IL-1β, Cox-2 and iNOS in Nrf2 (+/+) peritoneal macrophages (Fig. 1A & 1B). Interestingly, the inhibition of TNF, IL-1, Cox-2 and iNOS by sulforaphane (5–40 µM) was not significant in Nrf2 (−/−) peritoneal macrophages. These data indicated that suppression of inflammation by sulforaphane is significantly more potent in LPS-stimulated Nrf2 (+/+) peritoneal macrophages than LPS-stimulated Nrf2 (−/−) peritoneal macrophages.
Figure 1.
The mRNA expression of pro-inflammatory cytokines and mediators in the LPS-stimulated Nrf2 (−/−) mouse peritoneal macrophages compared with the LPS-stimulated Nrf2 (+/+) mouse peritoneal macrophages. (A) Expression of TNF and IL-1 was significantly attenuated in Nrf2 (+/+) mouse peritoneal macrophages by sulforaphane. (B) Expression of Cox-2 and iNOS was significantly decreased in Nrf2 (+/+) mouse peritoneal macrophages by sulforaphane. Total RNA were extracted and pooled from peritoneal macrophages of at least four mice.
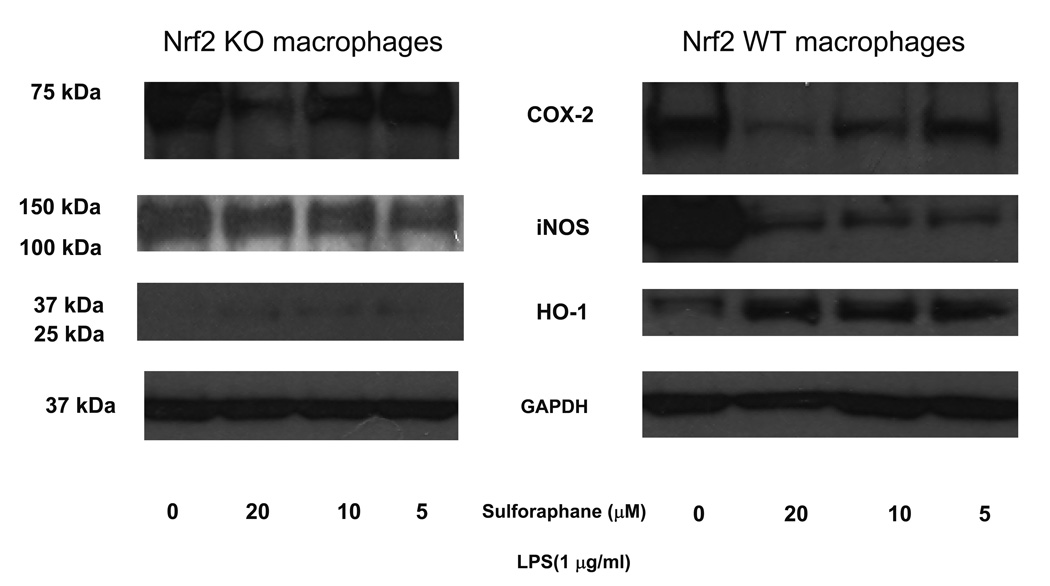
3.2. Sulforaphane reduced protein expression levels of Cox2 and iNOS but induced HO-1 protein expression
To further confirm the anti-inflammatory effect of sulforaphane, protein expression of iNOS and Cox-2 was examined in Nrf2 (+/+) peritoneal macrophages and Nrf2 (−/−) peritoneal macrophages by Western blot analyses. As demonstrated in Fig. 2, sulforaphane at 5, 10, 20 µM significantly attenuated protein level of Cox-2 and iNOS in Nrf2 (+/+) peritoneal macrophages. In Nrf2 (−/−) peritoneal macrophages, attenuation on Cox-2 protein was observed only with 20 µM of sulforaphane. Sulforaphane at 5, 10, 20 µM did not inhibit iNOS protein expression in Nrf2 (−/−) peritoneal macrophages. This data is in good agreement with marked decrease of mRNA expression by sulforaphane in Nrf2 (+/+) peritoneal macrophages and Nrf2 (−/−) peritoneal macrophages. HO-1 expression was not detectable in LPS-stimulated Nrf2 (−/−) peritoneal macrophages but its expression was significantly induced in LPS-stimulated Nrf2 (+/+) peritoneal macrophages by sulforaphane (5, 10, 20 µM)
Figure 2.
The protein expression of Cox-2 and iNOS was significantly attenuated by sulforaphane in the LPS-stimulated Nrf2 (+/+) mouse peritoneal macrophages compared with the LPS-stimulated Nrf2 (−/−) mouse peritoneal macrophages. HO-1 expression was elevated by sulforaphane in LPS-stimulated Nrf2 (+/+) mouse peritoneal macrophages. Total protein were extracted and pooled from peritoneal macrophages of at least four mice.
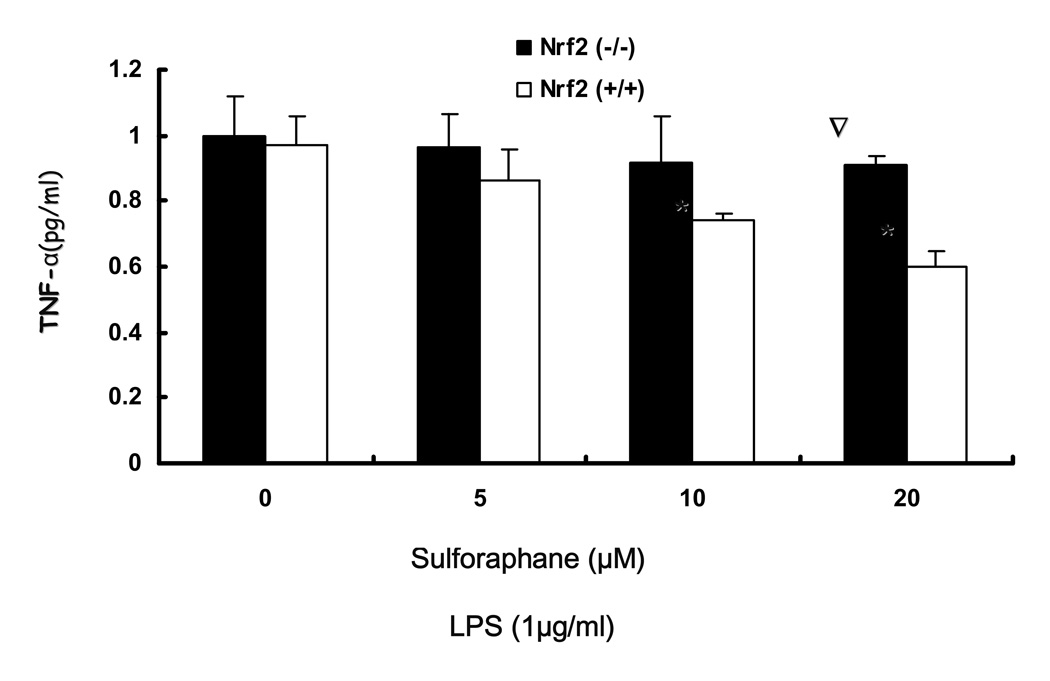
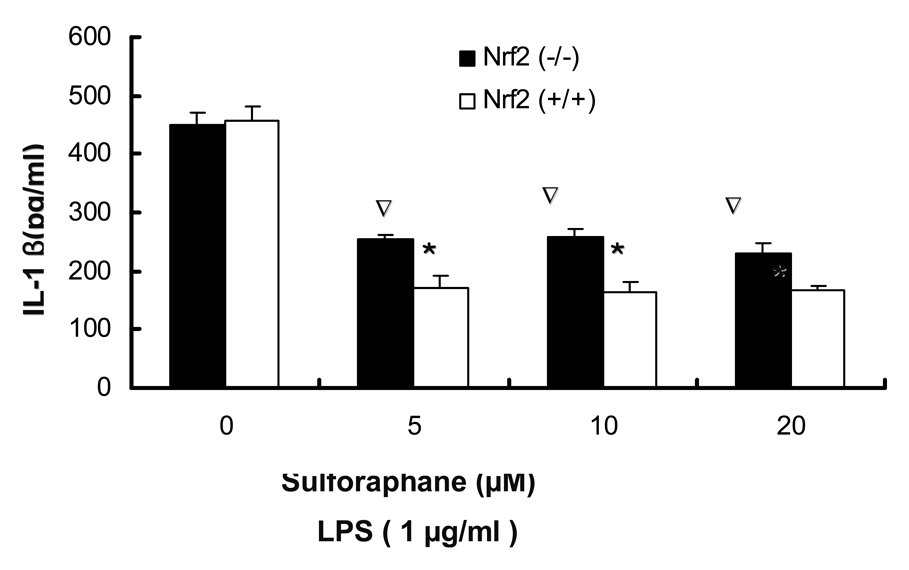
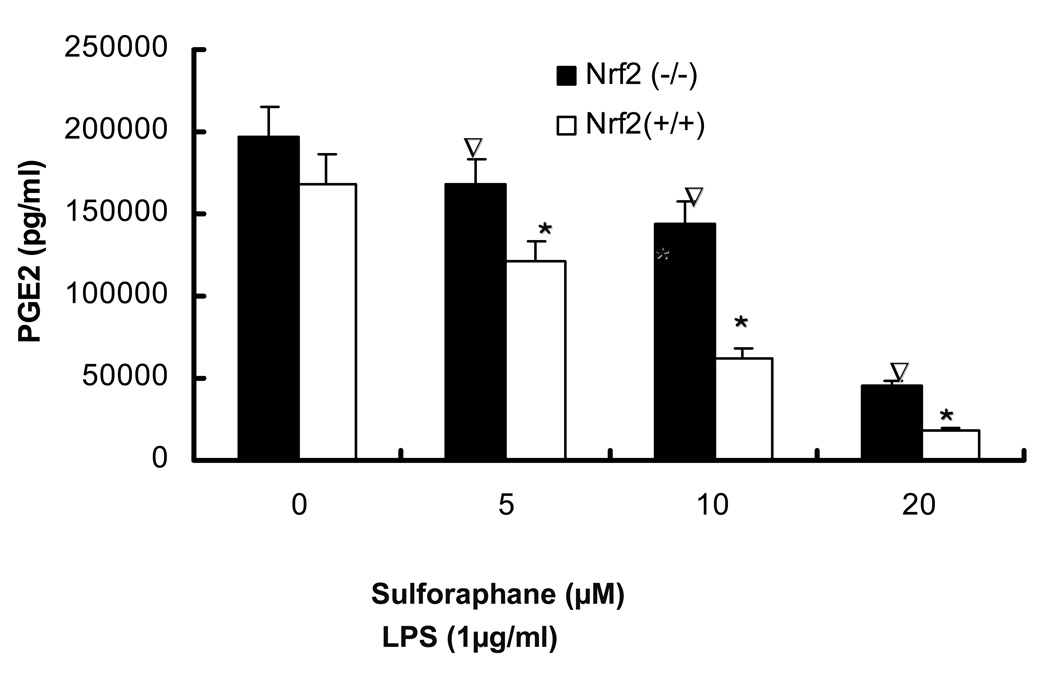
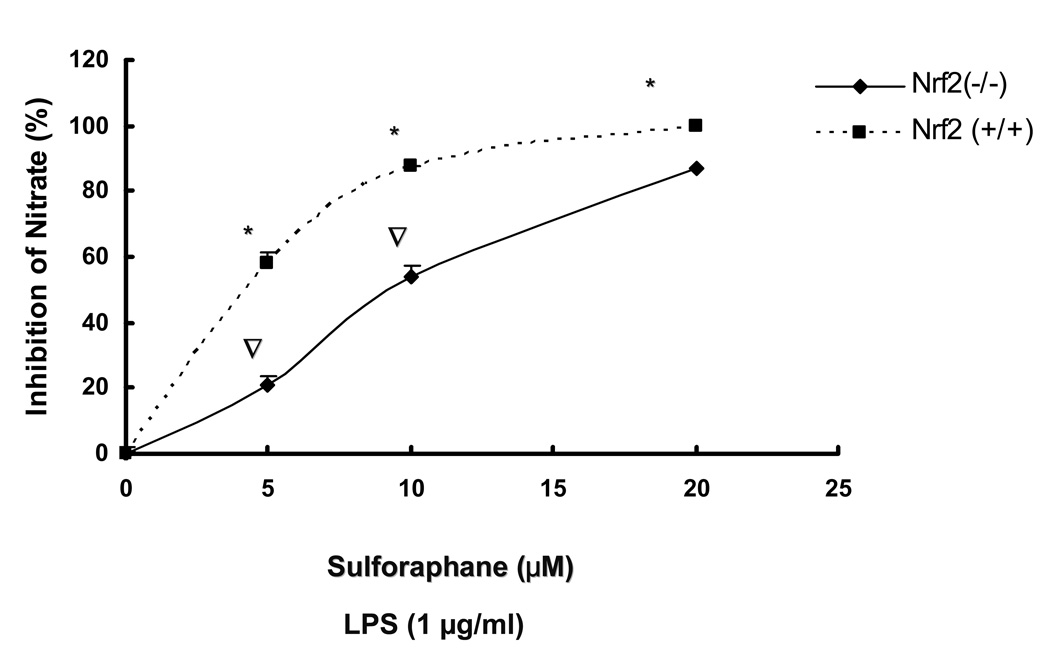
3.3. LPS-induced production of PGE2, nitrite production and secretion of TNFα, IL-1β by sulforaphane is significantly inhibited in Nrf2 (+/+) peritoneal macrophages as compared to Nrf2 (−/−) peritoneal macrophages
The observed inhibition of mRNA expression of TNF, IL-1, Cox-2 and iNOS by sulforaphane in Nrf2 (+/+) peritoneal macrophages suggested that such inhibition may have occurred in the secretion of TNFα, IL-1β, PGE2 and nitrite in Nrf2 (+/+) peritoneal macrophages. TNFα, IL-1β, PGE2 levels were measured by ELISA and nitrite level was measured by Griess reagent after 24 hrs of LPS stimulation in peritoneal macrophages. Fig. 3A, showed that SFN (10, 20 µM) significantly attenuated TNFα secretion in Nrf2 (+/+) peritoneal macrophages and the inhibition is significantly more potent than the inhibition in Nrf2 (−/−) peritoneal macrophages. Fig. 3B indicated that SFN (5, 10, 20µM) significantly suppressed IL-1β secretion in Nrf2 (+/+) peritoneal macrophages, relative to Nrf2 (−/−) peritoneal macrophages. PGE2 and nitrite are downstream products of arachidonic acid metabolism involving COX-2 and iNOS. Fig. 3C indicated the production of PGE2 was significantly down-regulated by sulforaphane at 5, 10, 20 µM in Nrf2 (+/+) peritoneal macrophages. Such inhibition was significantly more potent than the inhibition in Nrf2 (−/−) peritoneal macrophages. Fig. 3D indicated that sulforaphane at 10, 20 µM In close correlation with the results obtained at the protein level and mRNA level, SFN potently down-regulated LPS-stimulated pro-inflammatory mediator PGE2, nitrite and pro-inflammatory cytokines TNFα, IL-1β.
Figure 3.
Secretion of TNF-, IL-1 and production of PGE2, nitrites were significantly attenuated by sulforaphane in the LPS-stimulated Nrf2 (+/+) mouse peritoneal macrophages compared with the LPS-stimulated Nrf2 (−/−) mouse peritoneal macrophages. A: Secretion of TNFα in peritoneal macrophages; B: Secretion of IL-1 in peritoneal macrophages; C: production of PGE2 in peritoneal macrophages; D: production of nitrite in peritoneal macrophages. Columns represent the mean ± SD. The experiments were repeated three times with replicates. One-way ANOVA were used to compare the means between groups. * Significantly different (pÃ0.05) in Nrf2 (+/+) mouse peritoneal macrophages; ∇ significantly different (p<0.05) between Nrf2 (+/+) mouse peritoneal macrophages and Nrf2 (−/−) mouse peritoneal macrophages
4. Discussion
SFN is a dietary chemopreventive compound extracted from cruciferous vegetables such as broccoli and cabbage. It possesses potent anti-inflammation and anti-cancer properties. There is emerging evidence that chronic inflammation plays an important role in the carcinogenesis of various cancers. Heiss et al. reported that sulforaphane possesses anti-inflammatory activity in Raw macrophages due to inhibition on DNA binding of NF-κB [7]. Other findings suggest that sulforaphane effectively suppressed the LPS-induced COX-2 protein via modulation of multiple core promoter elements (NF-kappaB, C/EBP, CREB and AP-1) in the COX-2 transcriptional regulation [12]. We hypothesized that sulforaphane exert its anti-inflammatory activity via activation of transcription factor Nrf2 in mouse peritoneal macrophages.
SFN can directly react with sulhydryl groups, oxidize cysteine residues and deplete reduced cellular glutathione (GSH) thus mimickinging an oxidative insult and release Nrf2 from Keap-1 binding [13]. Inhibition of NO, PGE2 production and TNFα, IL-1β has been proposed to be a useful approach for the treatment of various inflammatory diseases as well as potential chemoprevention strategy [14, 15]. Our results clearly indicated that inhibitions of mRNA expression of pro-inflammatory cytokines such as TNF-α, IL-1β and pro-inflammatory mediators such as Cox-2, iNOS are more significant in Nrf2 (+/+) peritoneal macrophages relative to Nrf2 (−/−) peritoneal macrophages. Such observations strongly suggested Nrf2 is involved in the anti-inflammatory activity of SFN. In good agreement with results in mRNA expression, inhibitions on protein expression of TNF-α, IL-1β, Cox-2 and iNOS are more significant in Nrf2 (+/+) peritoneal macrophages. Unpublished data in our lab indicated that no significant difference in inhibition of LPS-stimulated inflammation between Nrf2 (+/+) and Nrf2 (−/−) peritoneal macrophages treated with sulforaphane and LPS simultaneously. Hence, antioxidants/phase-II detoxifying enzymes induced by sulforaphane via activation of Nrf2 might confer protection upon LPS stimuli. Interestingly, the expression of heme oxygenase-1 (HO-1) was increased in the peritoneal macrophages of wild type peritoneal macrophages but almost undetectable in Nrf2 KO mouse peritoneal macrophages after LPS treatment. It is clear that Nrf2 is involved in the induction of biological defense proteins such as antioxidant proteins, such a notion is supported by the finding that disruption of Nrf2 resulted in a drastic increase in lethality during LPS-induced septic shock [16]. It is not surprising that HO-1 was induced in Nrf2 (+/+) mouse peritoneal macrophages upon LPS challenge, in order to counteract LPS-induced injury. Pretreatment of SFN in Nrf2 (+/+) peritoneal macrophages significantly induced HO-1 expression, which confers anti-inflammatory activity in LPS-stimulated macrophages. Our finding was in accordance with anti-inflammatory activity of phenolic antioxidants in LPS induced inflammation in macrophages [17, 18]. NF-κB is a redox-sensitive transcription factor strongly regulated by the intracellular redox status, which is maintained mainly by the ratio of rGSSG/GSH [19, 20]. It is plausible that HO-1 and other antioxidants induced by SFN via activation of Nrf2 inhibit the activity of NF- κB after LPS stimulation thus exerting its anti-inflammatory activities. Recent finding revealed that NF-κB antagonizes Nrf2-ARE pathway via deprivation of coactivator CREB binding protein (CBP) and promotion of recruitment of histone deacetylase 3 to ARE [21]. This notion serves to possibly explain that the activation of Nrf2 by pretreatment with SFN inhibited interaction between p65 and CBP in peritoneal macrophages, consequently, down-regulated NF-κB signaling pathway in inflammation. Beside NF-κB, Activator protein-1 (AP-1) is another LPS-induced proinflamatory transcription factor in peritoneal macrophages. Zhu et al. revealed that SFN inhibited UVB-induced AP-1 activation in human keratinocytes[22]. Their findings also suggested that the induction of phase II enzymes may have limited role on UVB-induced AP-1 activation. Given the fact that SFN at high concentration displayed a moderate inhibition in LPS-stimulated Nrf2(−/−) peritoneal macrophages, it is possible that SFN might impact AP-1 activity, which is not related to Nrf2-ARE pathway.
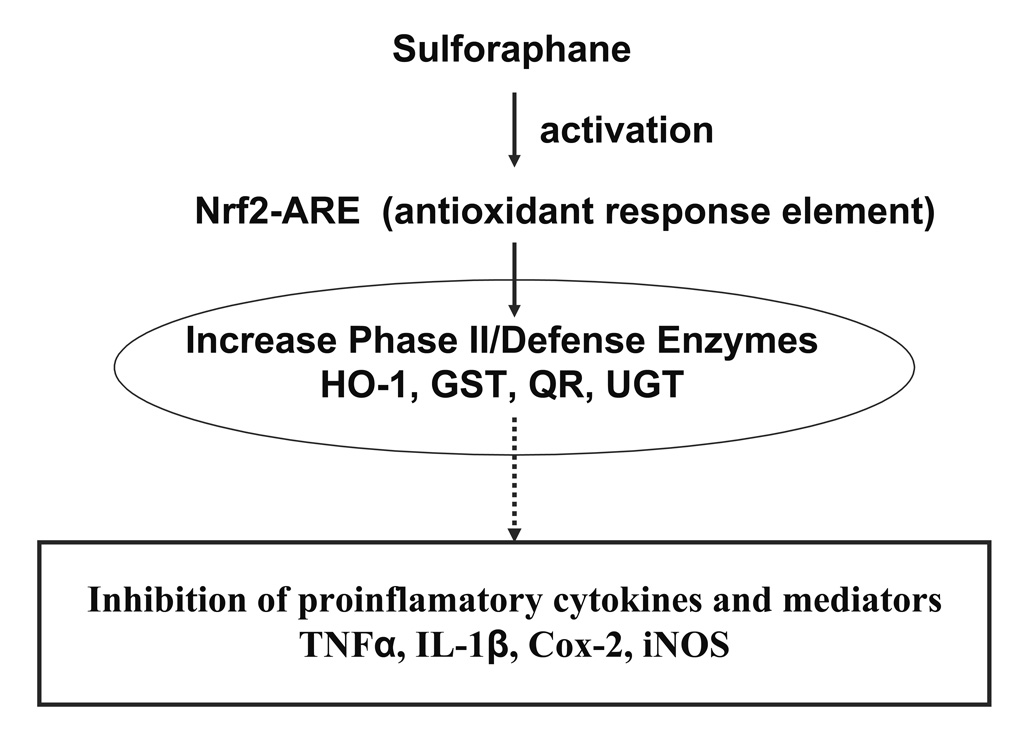
In summary, the present data demonstrated that SFN displayed more significant down-regulation of LPS-induced TNF, IL-1, Cox-2 and iNOS expression in Nrf2 (+/+) peritoneal macrophages than Nrf2 (−/−) peritoneal macrophages, at least in part due to activation of Nrf2. Furthermore, HO-1 expression is marked increased by sulforaphane in Nrf2 (+/+) peritoneal macrophages than Nrf2 (−/−) peritoneal macrophages (Fig. 2). Taken together, the current findings of anti-inflammatory activity of SFN in the mouse peritoneal macrophages will contribute to our overall understanding of potential mechanism of SFN in chemoprevention of inflammatory-associated diseases including cancers (Fig. 4).
Figure 4.
A schematic presentation of the role of Nrf2 in the transcription activation of phase II/antioxidant defense enzymes by SFN, potentially leading to the inhibition of proinflammatory cytokines and other mediators.
Acknowledgement
We thank Dr. Jefferson Chan for his general gift of Nrf2 knockout mice. We thank all current members of Dr. Kong’s laboratory for their contribution and comment. Supported in part by NIH Grant CA-094828.
ABBREVIATIONS
- SFN
sulforaphane
- COX-2
cyclooxygenase-2
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- NF-κB
nuclear 3 factor-κB
- Nrf2
nuclear E2-factor related factor 2
- PGE2
prostaglandin E2
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–9944. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, Chen C, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 4.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 5.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 2005;65:2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 6.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 7.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 8.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–301. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 11.Wang MJ, Jeng KC, Shih PC. Differential expression and regulation of macrophage inflammatory protein (MIP)-1alpha and MIP-2 genes by alveolar and peritoneal macrophages in LPS-hyporesponsive C3H/HeJ mice. Cell Immunol. 2000;204:88–95. doi: 10.1006/cimm.2000.1697. [DOI] [PubMed] [Google Scholar]
- 12.Woo KJ, Kwon TK. Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int Immunopharmacol. 2007;7:1776–1783. doi: 10.1016/j.intimp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 15.Goossens L, Pommery N, Henichart JP. COX-2/5-LOX dual acting anti-inflammatory drugs in cancer chemotherapy. Curr Top Med Chem. 2007;7:283–296. doi: 10.2174/156802607779941369. [DOI] [PubMed] [Google Scholar]
- 16.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon WK, Kim BC. Heme oxygenase-1 mediates the anti-inflammatory effect of propyl gallate in LPS-stimulated macrophages. Biochem Biophys Res Commun. 2007 doi: 10.1016/j.bbrc.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HY, Chu LC, Hua KF, Chao LK. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. J Cell Physiol. 2008;215:603–612. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 19.Anderson MT, Staal FJ, Gitler C, Herzenberg LA, Herzenberg LA. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc Natl Acad Sci U S A. 1994;91:11527–11531. doi: 10.1073/pnas.91.24.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YQ, Sengchanthalangsy LL, Hackett A, Ghosh G. NF-kappaB p65 (RelA) homodimer uses distinct mechanisms to recognize DNA targets. Structure. 2000;8:419–428. doi: 10.1016/s0969-2126(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhu M, Zhang Y, Cooper S, Sikorski E, Rohwer J, Bowden GT. Phase II enzyme inducer, sulforaphane, inhibits UVB-induced AP-1 activation in human keratinocytes by a novel mechanism. Mol Carcinog. 2004;41:179–186. doi: 10.1002/mc.20052. [DOI] [PubMed] [Google Scholar]