Abstract
Background
Molecular pathways of proliferation, angiogenesis, neuroendocrine differentiation, apoptosis and alterations in nuclear structure of cancer epithelial cells are important in the pathogenesis of prostate cancer (PCa). Therefore, we evaluated the prognostic value of these parameters multivariately in 105 clinically localized PCa tumors with long-term follow-up after radical prostatectomy for progression-free survival (PFS).
Method
Nuclear roundness variance (NRV) was calculated for tumor nuclei using the graphic tracing DynaCELL system. Immunohistochemistry assessed expression of Ki67, PCNA (proliferation), Chromogranin A (neuroendocrine differentiation), CD31 (angiogenesis), BCL2 (apoptosis), and Her-2/neu (oncogene) in the tumors. Cox proportional hazards regression, Spearman’s rank correlation, and Kaplan-Meier plots were employed to analyze the data.
Results
Gleason score, focal vs. non-focal extra-prostatic extension, organ confined status, NRV, Her-2/neu, CD-31 and Ki67 were univariately significant predictors of PFS. NRV was the most significant prognostic indicator with the highest concordance index (0.7) for PFS. Gleason score, NRV and Her-2/neu were multivariately significant and yielded a concordance index of 0.77.
Conclusion
Her-2/neu oncogene and NRV were shown to be significant in the prediction of PFS. The assessment of alterations in nuclear structure using NRV proved to be the most significant factor in the prediction of PFS. Integration of image analysis-based NRV and molecular biomarkers with pathologic parameters should be considered for validation in the prediction of PFS.
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy among men in the United States, with an anticipated 186,320 newly diagnosed cases and 28,660 deaths in 2008 (1). Nearly ~30–40% of men undergoing definitive treatment for clinically localized PCa report an isolated increase in prostate specific antigen (PSA) levels with long term follow-up (2–5). In a series of nearly 2,000 patients treated with radical prostatectomy (RP) at the Johns Hopkins Hospital, Pound et al. (6) identified 304 men that developed PSA recurrence (15%) and were monitored, without introduction of hormone adjuvant therapy or local radiation treatment, until demonstration of distant metastasis. Of these 304 men, 34% developed distant metastases over a median period of 8 years from the time of the first postoperative PSA elevation. Pound et al. (6) developed an algorithm to predict actuarial metastasis-free survival that combined Gleason score, time to biochemical progression (years) and PSA doubling time (months). Subsequently, Han et al. (7) updated this study cohort and reported 360 recurrences (17%) out of 2,091 men with PCa. They used three preoperative or post-operative parameters to create nomograms for assessment of biochemical recurrence free survival and demonstrated actuarial PSA-free survival probabilities of 5, 10, and 15 years were 84%, 72% and 61%, respectively.
The accumulation of repeated epigenetic and genetic insults to the prostate genome over time through diet, infection, inflammation and aging result in a cascade of biological and molecular events which can result in malignancy (8–11). Many of these alterations are permanent and therefore generate cellular, tissue architecture and molecular signatures that reflect the transition to malignancy and its subsequent progression to metastasis (8,11,12).
In the search for biomarkers that can predict the prognosis of men with PCa, numerous serologic and histologic biomarkers have been evaluated, and more are in various phases of development (8,11–15). Pathologically, Gleason grade and extent of disease (stage) are significant predictors of risk for progression and metastasis (16,17). Likewise, DNA alterations measured using semi-automated, computer-assisted image cytometry (18,19) detect abnormal DNA content representing large scale chromosomal alterations (i.e., tetraploidy, aneuploidy, hyperploidy, etc.) and reflect later stage changes of genetic instability in tumor cells (20). Additionally, quantitative measurements of nuclear structure alterations by digital image analysis have also been applied to assess PCa progression and metastasis (19,21–25).
In the current study, we evaluated the prognostic significance of nuclear roundness variance (NRV) and various molecular biomarkers of key PCa pathogenic pathways including cell proliferation (Ki67, PCNA), apoptosis (BCL2), neuroendocrine differentiation (Chromogranin A), angiogenesis (CD31), and Her-2/neu oncogene in a cohort of 105 men with mean follow-up of 17.3 (range: 2–26 years, median: 19) years.
MATERIALS AND METHODS
Patient Sample and Histologic Sectioning
124 PCa cases were selected from the original Pound series (6) as the most representative tissue blocks from the radical prostatectomy (RP) specimens for evaluation. Notably, a single pathologist (J.I.E.) performed routine pathologic assessment of the 124 tumors that were surgically removed by a single surgeon (P.C.W.) from 1975 to 1991. The selected blocks were re-embedded in fresh paraffin and sequential 5 micron sections were cut at UroCor Inc. (Oklahoma City, OK). The first section from the RP tissue was used to prepare H&E slides which were re-examined to ensure that cancer areas could be marked and a Gleason score reassigned. These H&E slides were used to conduct the nuclear roundness variance (NRV) measurements and then returned to UroCor Labs where they served as a “tumor template” for the subsequent consecutive sections that were immunohistochemically stained with the various molecular biomarkers. PCa Progression in this cohort was defined as a rise in postoperative PSA level of >0.2ng/ml and/or needle biopsy confirmed local or distant failure and/or metastatic lesions detected by bone scan. No patient received neoadjuvant therapy or adjuvant hormonal therapy prior to documentation of distant metastasis. All patients were evaluated every 3 months for the first year, semiannually for the second year and annually thereafter as described previously (6). Of these 124 patients, one patient who did not have a NRV measurement and 14 patients who did not have CD31, Ki67, BCL2, PCNA and Chromogranin A staining performed were excluded from our analyses. The numbers of tumors (n=109) with clinical stage were T1b (2), T1c (1), T2a (58), T2b (42), T2c (5) and T3a (1) respectively. As the number of patients with T1b (n=2), T1c (n=1) and T3a (n=1) were few, they were not considered in the data analysis and the T2b and T2c were merged and considered as T2b/c. Table 1 shows a summary of the pathologic information available for the cohort of 105 patients used in the final analyses. As the number of cases with Gleason score >7 were few, they were combined with the Gleason score 7 patients for the analyses.
Table 1.
Prostate Cancer Patients Demographics (N=105)
| Variable | Progression
|
|
|---|---|---|
| No (N = 43) | Yes (N = 62) | |
| Age | 59.62 ± 5.97 | 59.95 ± 6.62 |
|
| ||
| Gleason Score (%) | ||
| <7 | 34 (79.1) | 30 (48.4) |
| ≥7 | 9 (20.9) | 32 (51.6) |
|
| ||
| TNM Stage (%) | ||
| T2a | 22 (51.2) | 36 (58.1) |
| T2b/c | 21 (48.8) | 26 (41.9) |
|
| ||
| Organ Confined (%) | ||
| Yes | 19 (44.2) | 11 (17.7) |
| No | 24 (55.8) | 51 (82.3) |
|
| ||
| Extra Capsular Penetration (%) | ||
| Absent | 32 (74.4) | 30 (48.4) |
| Present | 11 (25.6) | 32 (51.6) |
|
| ||
| Focal Capsular Penetration (%) | ||
| Absent | 22 (51.2) | 13 (21.0) |
| Present | 21 (48.8) | 49 (79.0) |
|
| ||
| Surgical Margin (%) | ||
| Negative | 33 (76.7) | 37 (59.7) |
| Positive | 10 (23.3) | 25 (40.3) |
|
| ||
| Seminal Vesicle (%) | ||
| Negative | 43 (100.0) | 61 (98.4) |
| Positive | 0 (0) | 1 (1.6) |
|
| ||
| Lymph Node (%) | ||
| Negative | 43 (100.0) | 62 (100.0) |
|
| ||
| Her-2/neu (%) | ||
| Negative | 15 (34.9) | 6 (9.7) |
| Positive | 28 (65.1) | 56 (90.3) |
|
| ||
| Nuclear Roundness Variance | 2.60 ± 1.96 | 5.14 ± 4.02 |
|
| ||
| CD31 | 5.81 ± 8.0 | 11.26 ± 18.6 |
|
| ||
| Ki67 | 2.41 ± 5.04 | 4.35 ± 8.54 |
|
| ||
| PCNA | 1.06 ± 1.58 | 1.04 ± 1.26 |
|
| ||
| BCL2 | 8.83 ± 10.92 | 9.92 ± 11.35 |
|
| ||
| Chromogranin A | 23.28 ± 50.3 | 20.88 ± 45.31 |
Immunohistochemistry (IHC)
To determine Her-2/neu expression in tissue sections, the monoclonal antibody (Ab-3, Clone OP-15) provided by Oncogene Science, Inc. (Uniondale, NY) was used (19). Anti-human Chromogranin A, a purified mouse monoclonal antibody, was obtained from Boehringer-Mannheim (Mannheim, Germany). PCNA (PC-10 clone, M-879), BCL-2 (Clone 124), and Ki67 (Clone MIB-1) mouse IgGs monoclonal antibodies were purchased from DAKO (Carpentaria, CA). The supersensitive MultiLink™ kit (BioGenex Inc., San Ramon, CA), which employs the streptavidin-biotin complex alkaline phosphatase labeling method, was used for monoclonal antibody detection. All staining was performed with the MicroProbe™ manual staining system (Fisher Scientific, Pittsburgh, PA). Incubation temperature for the monoclonal antibodies was 4°C overnight and remaining staining methods followed the recommended procedure of the BioGenex MultiLink™ kit.
IHC Scoring
Scoring was performed in the pathologist-confirmed cancerous areas (J.I.E.) of each archival specimen by two experienced readers, with a third reader used when there was a >10% discrepancy between the results of the first two readers. Interpretation of results for Her-2/neu expression utilized a scoring method developed by J.I.E. and categorized patients as Her-2/neu negative if there was staining in <5% of the marked tumor area, as focal Her-2/neu staining if 5–30% of the marked tumor area showed staining, and diffuse Her-2/neu staining if >30% of the marked tumor area showed staining. PCNA and BCL2 scoring utilized the number of positive staining nuclei per 1000 nuclei scored in a 40X field within the area designated by the expert pathologist. Ki67 scoring utilized the number of positive cells identified in 10 contiguous 40X fields divided by the tissue area evaluated in square mm. Chromogranin A scoring utilized the number of positive cells identified in 10X field divided by the tissue area evaluated in square mm. CD31 angiogenesis scoring was determined from the tumor areas with highest number of positive staining vessels at 10X by counting the number of positive staining vessels in four contiguous 40X fields.
Nuclear Roundness Variance
A total of ~150 intact cancerous nuclei from the primary tumor were analyzed with a Zeiss inverted IM microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a Zeiss Planochromatic 100X oil emersion objective at a total magnification of 2440X. The images were analyzed with the DynaCELL Motility Morphometry Measurement workstation (JAW Associates, Inc., Annapolis, MD). Nuclear roundness factor represents a dimensionless, size-invariant shape descriptor based on the calculated radius for the measured perimeter divided by the calculated radius for the measured area of the nucleus. A value 1.0 is a perfect circle and deviation from circularity is reflected by values > 1.0. For each nucleus analyzed, the computer recorded the nuclear area, perimeter and nuclear roundness factor. Nuclear roundness was defined as the degree to which the nucleus in cross section approximated a perfect circle (21).
Circumference (R) is obtained as follows: the distance around a nuclear perimeter is measured directly, and the computer then calculates R by assuming that the measured perimeter has been obtained from a perfect circle (P = 2πR). The area (r) is obtained as follows: the computer, by integration, calculates the actual area of the nucleus and then calculates “r” for an equivalent circle by assuming that the nuclear area measured was contained within a perfect circle (A = πr2).
Next, NRV was calculated for each case using the variance of the nuclear roundness factors from ~150 nuclei captured for each case. The resulting variance values were multiplied by 10,000 to determine the NRV factor for each case.
Statistical Analysis
All data was analyzed using Stata™ v10.0 statistical analyses software (Stata Corporation, College Station, TX). Correlations of evaluated pathologic parameters with evaluated biomarkers were determined using Spearman’s rank correlation coefficients. Optimal thresholds for the biomarkers in the differentiation of the various binary outcomes were determined using the classification and regression tree (CART) method. Univariate and multivariate Cox proportional hazards regression was used to identify significant prognostic factors for prediction of progression-free survival (PFS). All ties were handled by the Breslow method and the proportional hazards assumption was verified by examination of residual plots. Kaplan-Meier survival plots were created to demonstrate the ability of the pathologic variables and evaluated biomarkers to predict PFS. Univariately significant variables both as continuous and dichotomized inputs were further assessed using in multivariate Cox regression. Statistical significance in this study was set as p ≤ 0.050.
RESULTS
The demographic, clinical and pathologic information of the cohort evaluated in this study are shown in Table 1. Mean follow-up time after RP for all 105 patients was 17.3 years (range: 2–26 years, median: 19 years), with 92.4% of the patients (97/105) having ≥10 years of follow-up. The median time for biochemical PFS for the cohort was 13 years.
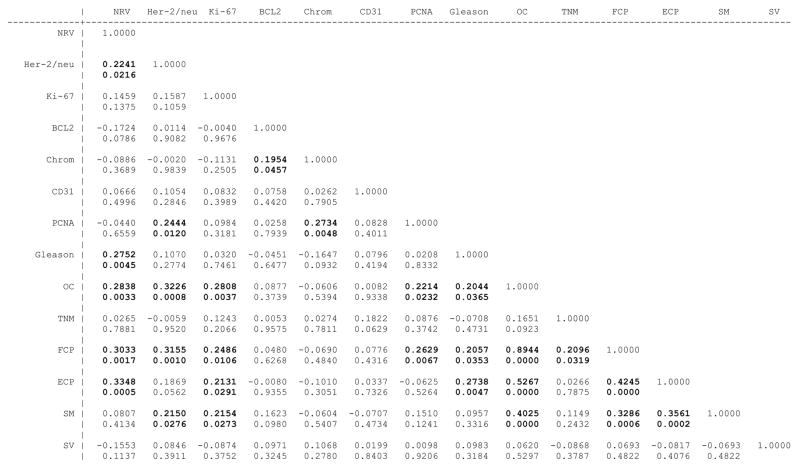
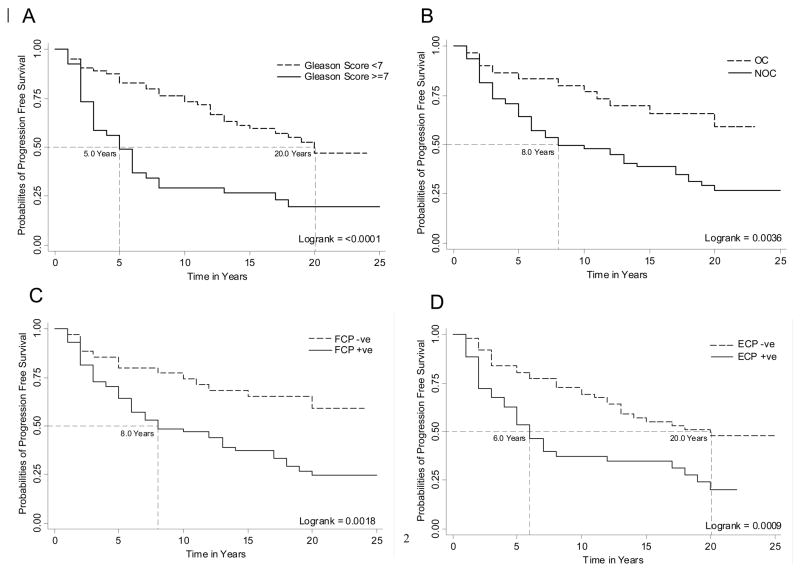
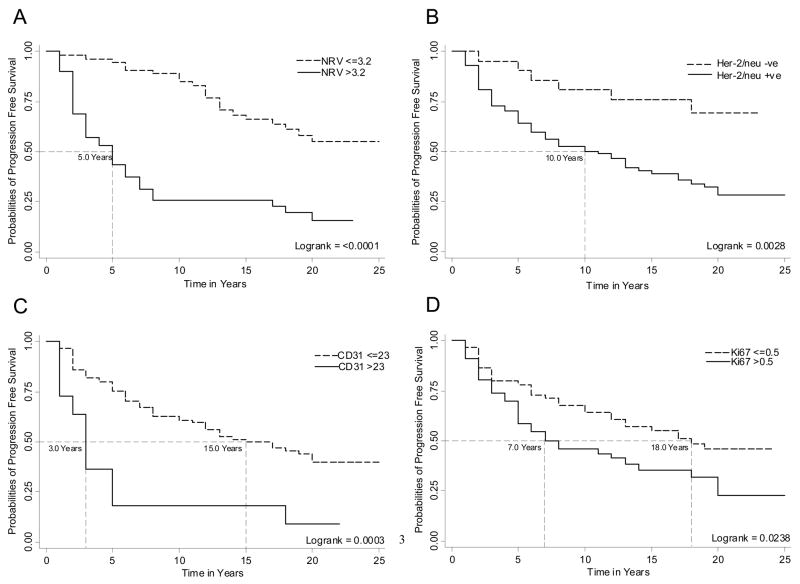
Figure 1 shows the Spearman’s rank correlation coefficients (top number) of evaluated pathologic parameters (i.e. Gleason score, organ confined status, non-focal extra-prostatic extension and focal extra-prostatic extension) with evaluated biomarkers (i.e. NRV, Her-2/neu expression, CD31, Ki-67, Chromogranin A, PCNA and BCL2) and their significance level (bottom number). Those variables with significant correlation coefficients have been bolded, and notably although several of the molecular biomarkers showed significant correlation with the pathologic staging variables, only NRV showed significant correlation with the Gleason score. In univariate Cox regression analyses, Gleason score, organ confined status, non-focal extra-prostatic extension, and focal extra-prostatic extension were significant predictors of PFS among the pathological parameters evaluated (Table 2). The Harrell’s concordance indices for the pathology parameters of Gleason score, organ confined status, non-focal extra-prostatic extension and focal extra-prostatic extension were 0.64, 0.59, 0.61 and 0.59 respectively. Among the biomarkers evaluated using univariate Cox regression analysis, both as continuous/categorical and optimized dichotomized variables, NRV, Her-2/neu expression, CD31 and Ki-67 were univariately significant for PFS prediction (Table 2). The Harrell’s concordance indices for NRV, Her-2/neu expression, CD31 and Ki67 were 0.70, 0.58, 0.54 and 0.57 respectively. Notably, the BCL2 and Chromogranin A biomarkers, either as continuous or optimized dichotomized variables, were not significant, and PCNA as continuous variable was not univariately significant for prediction of PFS (Table 2). Therefore, these three biomarkers i.e. BCL2, Chromogranin and PCNA, were not considered in our multivariate Cox Regression analyses. Kaplan-Meier survival curves for the univariately significant pathologic parameters and biomarkers are presented in Figures 2 A–D and 3 A–D.
Figure 1.
Correlation matrix of pathologic parameters and evaluated biomarkers. Spearman’s correlation coefficient is provided as the top number and the significance of the correlation is provided as the bottom number for each comparison.
Table 2.
Univariate Cox Regression Analysis of Evaluated Pathologic Parameters and Biomarkers
| Variable | Continuous | Dichotomized† | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Evaluated Pathologic parameters | ||||
| Prostatectomy Gleason Score | 2.7 (1.63–4.48) | <0.0001 | ||
| Clinical TNM Stage | 0.77 (0.47–1.27) | 0.308 | ||
| Organ Confined | 2.49 (1.30–4.80) | 0.006 | ||
| Focal Extra-Prostatic Extension | 2.51 (1.36–4.64) | 0.003 | ||
| Non-Focal Extra-Prostatic Extension | 2.24 (1.36–3.70) | 0.002 | ||
| Surgical Margin | 1.61 (0.96–2.68) | 0.066 | ||
| Evaluated Biomarkers | ||||
| Nuclear Roundness Variance | 1.17 (1.1 – 1.24) | <0.0001 | 4.0 (2.34 – 6.84) | <0.0001 |
| Her-2/neu‡ | 3.26 (1.40 – 7.59) | 0.006 | ||
| CD31 | 1.02 (1.01 – 1.03) | 0.009 | 3.17 (1.60 – 6.29) | 0.001 |
| Ki67 | 38.74 (3.53 – 424.93) | 0.003 | 1.74 (1.06 – 2.87) | 0.030 |
| PCNA | 1.03 (0.86 – 1.23) | 0.771 | 1.76 (1.06 – 2.92) | 0.029 |
| BCL2 | 1.00 (0.97 – 1.02) | 0.662 | 1.1 (0.57 – 2.10) | 0.789 |
| Chromogranin A | 0.89 (0.50 – 1.56) | 0.674 | 0.45 (0.16 – 1.24) | 0.122 |
Dichotomized variables:
Gleason Score <7 (n = 64) vs. Gleason Score ≥7 (n = 41)
TNM Stage T2a (n = 58) vs. T2b/c (n = 47)
Organ Confined (n = 30) vs. Non Organ Confined (n = 75)
No Non-Focal Extra-Prostatic Extension (n = 62) vs. Non-Focal Extra-Prostatic Extension (n = 43)
No Focal Extra-Prostatic Extension (n = 30) vs. Focal Extra-Prostatic Extension (n = 75)
Negative Surgical Margin (n = 70) vs. Positive Surgical Margin (n = 35)
Nuclear Roundness Variance ≤3.2 (n = 54) vs. Nuclear Roundness Variance >3.2 (n = 51)
Negative (<5%) Her-2/neu (n = 21) vs. Focal (5–30%) or Diffuse (>30%) Her-2/neu (n = 84)
CD31 ≤23 (n = 94) vs. CD31 >23 (n = 11)
Ki67 ≤0.5 (n = 59) vs. Ki67 >0.5 (n = 46)
PCNA ≤0.5 (n = 54) vs. PCNA >0.5 (n = 51)
BCL2 ≤19.25 (n = 90) vs. BCL2 >19.25 (n = 15)
Chromogranin A ≤38 (n = 93) vs. Chromogranin A >38 (n = 12)
Her-2/neu had three categories i.e. Negative (<5%), Focal (5–30%) and Diffuse (>30%) expression. However, Focal and Diffuse Her-2/neu expression had similar Kaplan-Meir curves and hazard ratio i.e. 3.3 (1.32–8.27) and 3.25 (1.37– 7.71), so these two were pooled together and considered as Her-2/neu positive for analysis.
Figure 2.
A, B, C and D shows Kaplan-Meier curves of Gleason score, organ confined status, focal extra-prostatic extension and non-focal extra-prostatic extension for prediction of PCa progression-free survival in patients treated with RP. The Logrank test was used to test the equality of survivor function across the two groups.
Figure 3.
A, B, C and D shows Kaplan-Meier curves of nuclear roundness variance, Her-2/neu, CD31 and Ki67 biomarkers for prediction of PCa progression-free survival in patients treated with RP. The Logrank test was used to test the equality of survivor function across the two groups.
Table 3 shows the multivariate Cox regression analyses using the univariately significant predictors (i.e. Gleason score, organ confined status, non-focal extra-prostatic extension extra capsular penetration, focal extra-prostatic extension, nuclear roundness variance, Her-2/neu expression, CD31 and Ki-67). Gleason score, NRV and Her-2/neu expression remained significant in both multivariate models (Table 3). To avoid overfitting, a bootstrap resampling procedure with 200 replications was used to perform Cox regression analysis with backward elimination (26). A significance level of p>0.05 was used for removal from the model and a significance level of p <0.01 was required for reentry into the model. The goal was to identify the most important predictor of PFS. NRV turned out to be the most important predictor of PFS (Table 4). Parameters having greater than 50% inclusion frequency (i.e. Gleason score, NRV, and Her-2/neu) were selected for development of a multivariate model, which resulted a concordance index of 0.77 for prediction of PFS.
Table 3.
Multivariate Cox Regression Analysis of Univariately Significant Pathologic Parameters and Biomarkers
| Variable | Continuous† | Dichotomized‡ | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Prostatectomy Gleason Score | 2.15 (1.26 – 3.67) | 0.005 | 2.09 (1.21 – 3.62) | 0.008 |
| Organ Confined | 0.73 (0.14 – 3.70) | 0.703 | 0.71 (0.14 – 3.60) | 0.682 |
| Focal Extra-Prostatic Extension | 2.04 (0.48 – 8.57) | 0.333 | 1.94 (0.46 – 8.23) | 0.370 |
| Non-Focal Extra-Prostatic Extension | 1.18 (0.62 – 2.23) | 0.622 | 1.27 (0.70 – 2.31) | 0.429 |
| Nuclear Roundness Variance | 1.10 (1.03 – 1.19) | 0.007 | 2.98 (1.68 – 5.30) | <0.0001 |
| Her-2/neu | 2.73 (1.13 – 6.59) | 0.025 | 2.41 (1.00 – 5.80) | 0.049 |
| CD31 | 1.01 (1.00 – 1.03) | 0.085 | 2.25 (1.1 – 4.64) | 0.028 |
| Ki67 | 1.03 (1.00 – 1.05) | 0.027 | 1.40 (0.83 – 2.38) | 0.203 |
Nuclear Roundness Variance, CD31 and Ki67 were continuous
All variables were dichotomized
Table 4.
Inclusion Frequencies of Significant Pathologic Parameters and Biomarkers in Bootstrap Resampling with 200 Iterations
| Variable | Continuous† | Dichotomized‡ | ||
|---|---|---|---|---|
| Out of 200 | % | Out of 200 | % | |
| Prostatectomy Gleason Score | 169 | 84.5 | 155 | 77.5 |
| Organ Confined | 27 | 13.5 | 31 | 15.5 |
| Focal Extra-Prostatic Extension | 63 | 31.5 | 58 | 29 |
| Non-Focal Extra-Prostatic Extension | 21 | 10.5 | 45 | 22.5 |
| Nuclear Roundness Variance | 169 | 84.5 | 190 | 95 |
| Her-2/neu | 157 | 78.5 | 133 | 66.5 |
| CD31 | 77 | 38.5 | 124 | 62 |
| Ki67 | 110 | 55 | 56 | 28 |
Nuclear Roundness Variance, CD31 and Ki67 were continuous
All variables were dichotomized
DISCUSSION
The prospect of recurrence determined by PSA monitoring produces a constant level of anxiety among men diagnosed and definitively treated for PCa by either surgery or radiation. The definition of recurrence is often based on a PSA nadir for which the kinetics definitely differs for men that undergo RP versus radiation (6,7). Because PSA levels can be tracked fairly reliably over time, the finding of increased serum PSA concentrations is considered evidence of biochemical recurrence and hence is an intervention determinant for a PCa patient (2–7).
The accurate quantitative measurement of nuclear shape alterations has been thoroughly assessed using computer-assisted image analysis and has been found to be a strong predictor of PCa outcome (19,21–25). In the present long-term follow-up study of PCa, NRV proved to be extremely predictive of PFS (Table 3 and 4). NRV had the highest concordance index (0.7) for PFS in univariate analysis and when combined with Gleason score and Her-2/neu yielded a concordance index of 0.77. Others investigators, also using computer-assisted image analysis, have clearly shown that nuclear shape variables are important predictors of PFS and metastasis (19, 24–25).
Tumors stimulate angiogenesis by directly secreting angiogenic substances or by activating and releasing angiogenic substances stored in the extracellular matrix (27). Bigler et al. (28) demonstrated increased angiogenesis in PCa compared with benign prostate tissue. In our cohort, tumor angiogenesis was shown to be a significant predictor of PCa progression (Table 2). However, in multivariate analyses, it didn’t have independent prognostic value for PCa progression which may be due to tissue heterogeneity, small sample size and due to correlation with some of the other biomarkers evaluated (Figure 1 & Table 3). Silberman et al. (29) showed that tumor microvessel density correlated with PCa progression after RP, specifically in tumors with Gleason score 5 to 7. However, other investigators (30,31) reported no association of tumor angiogenesis with PCa progression.
Cohen et al. (32) suggested that neuroendocrine (NE) cells take part in tumor progression and should be considered as an independent prognostic variable. They showed a worse outcome in PCa patients exhibiting greater NE differentiation than in those with a low percentage of NE differentiation. Weinstein et al. (33) showed that chromogranin A has independent prognostic value for PCa progression in clinically localized PCa patients treated with RP. These investigators found NE differentiation is particularly useful in predicting prognosis for intermediate Gleason sum (5 to 6) tumors. Also, increased circulating chromogranin A has demonstrated as having independent prognostic value in patients with hormone refractory disease (34). Berruti et al. (35) recently showed that chromogranin A expression in PCa biopsies is an independent predictive factor of hormone refractory disease in patients with newly diagnosed PCa who are on early androgen deprivation therapy. In our cohort, however, chromogranin A expression was not a significant predictor of PCa progression (Table 2), which has also been reported by other investigators (36).
PCNA and Ki-67 are two nuclear antigens that are related to cell proliferation. Ki67 is present throughout the cell cycle (G1, S, G2 and M) but not present when cells are at rest (G0) or in early G1 phase. PCNA is not cell cycle-specific and it functions as a cofactor for DNA polymerase-δ, which reaches its maximal synthesis during the S phase and during DNA synthesis associated with DNA damage-repair mechanisms. Several investigators (37–39) have shown that Ki-67 and PCNA nuclear staining relate to the biological aggressiveness and prognosis in PCa. As a marker, monoclonal antibodies to Ki67 and PCNA antigens have been shown to provide a reliable method of estimating cellular proliferation correlating well with uptake of bromodeoxyuridine and thymidine labelling (40). In our cohort, PCNA expression did not significantly predicted PFS (Table 2). Ki67 expression significantly predicted PFS in univariate analysis (Table 2), however, when Ki67 expression was combined with other evaluated biomarker and pathologic parameters, it did not show additional prognostic value for PFS (Table 3).
Rubio et al. (41) have shown that BCL2 expression is a significant prognosticator for PCa biochemical recurrence free survival. In our cohort, BCL2 expression was not significant predictive of PFS (Table 2), which could be due to the small sample size and tissue heterogeneity. Borre et al. (42), in a cohort of 221 PCa patients followed expectantly, showed that BCL2 expression was predictive of disease specific and overall survival. However, their multivariate analysis demonstrated an insignificant prognostic value for BCL2 expression. Stackhouse et al. (43) failed to show prognostic value of BCL2 to predict PCa recurrence in needle biopsies specimens.
Slamon et al. (44) were the first to show that Her-2/neu gene amplification and protein overexpression are associated with an increased risk of relapse and death in patients with breast cancer. Since then several investigators (45–48) have explored the role of the Her-2/neu oncogene in PCa progression and metastasis. Myers et al. (45) suggested that increased expression and changes in the subcellular distribution of Her-2/neu is an early event in the development and progression of PCa. Signoretti et al. (46) showed that PCa progression towards androgen independence is characterized by increased Her-2/neu expression in tumor cells. In our long-term PCa follow-up series, Her-2/neu protein over-expression proved to be a significant predictor of PFS in men with clinically localized PCa (Table 3 and 4). However, others have found no significant differences in the increase or decrease of Her-2/neu expression in androgen independent tumors (47,48). The differences in the Her-2/neu results obtained from other laboratories may be due to technical assay differences, including variability in tissue fixation protocols, antibodies to different Her-2/neu epitopes, antibody production species, lack of standard operating immunohistochemistry protocols, and different scoring methodologies. Studies on Her-2/neu amplification at the molecular level have shown that amplification of this gene in PCa is a rare event (46,47). However, Her-2/neu protein overexpression in PCa tissue sections has been shown to be associated with significantly decreased survival in androgen independent tumors (49). Similar to tissue levels, circulating levels of Her-2/neu have been associated with disease progression and aggressive clinical outcomes in PCa (50,51). Circulating levels of Her-2/neu have also been shown to correlate with gene amplification and tissue Her-2/neu protein overexpression (51).
Limitations of the study include small sample size and evaluation of parameters in clinical stage T2 only. The NRV marker though the best predictor of PFS is currently not available commercially and the image analysis-based assay would have to be re-engineered to more rapidly and in a semi-automated fashion measure nuclear roundness. Another cohort that includes more diverse clinical population with a larger representation of T1c clinical stage must be considered for repeating these studies. In the future once the best biomarkers (i.e. nuclear shape and Her-2/neu), for example, are validated using an automated imaging system and made cost-effective from a re-imbursement perspective, they will need to be performed and interpreted by an expert pathologist.
In conclusion, Her-2/neu oncogene, and NRV were found to be significant in the prediction of PFS in our cohort with a mean follow-up time 17.3 years (range: 2–26 years, median: 19 years) after RP. The assessment of alterations in nuclear shape using NRV proved to be the most important factor in the prediction of PFS. Therefore, integration of the image analysis-based NRV and molecular biomarkers with pathologic parameters should be considered in the prediction of PFS.
Acknowledgments
Funding for this project was provided by The Patana Fund, The Prostate Cancer Foundation, The Johns Hopkins University Prostate Cancer SPORE (Grant number: P50CA58236), and the Early Detection Research Network (EDRN) NCI/NIH (Grant number CA086323-06).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Smith DS. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. The Journal of urology. 1994;152(5 Pt 2):1837–1842. doi: 10.1016/s0022-5347(17)32397-2. [DOI] [PubMed] [Google Scholar]
- 3.Catalona WJ, Smith DS. Cancer recurrence and survival rates after anatomic radical retropubic prostatectomy for prostate cancer: intermediate-term results. The Journal of urology. 1998;160(6 Pt 2):2428–2434. doi: 10.1097/00005392-199812020-00012. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. Jama. 2005;294(4):433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Mangold LA, Walsh PC, Partin AW. The prostatic specific antigen era is alive and well: prostatic specific antigen and biochemical progression following radical prostatectomy. The Journal of urology. 2005;174(4 Pt 1):1276–1281. doi: 10.1097/01.ju.0000173907.84852.ec. discussion 1281; author reply 1281. [DOI] [PubMed] [Google Scholar]
- 6.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 7.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. The Journal of urology. 2003;169(2):517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 8.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. The New England journal of medicine. 2003;349(4):366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 9.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer metastasis reviews. 2002;21(1):3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. The Journal of urology. 2004;171(2 Pt 2):S36–40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 11.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361(9361):955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 12.Veltri RW. Molecular biology of serum biomarkers of prostate cancer. In: Kirby RS, Partin AW, Feneley MR, Parsons JK, editors. Prostate cancer: Principles and Practice. London & New York: Taylor & Francis; 2006. pp. 269–284. [Google Scholar]
- 13.Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res. 2004;10(12 Pt 1):3943–3953. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- 14.Stenman UH, Abrahamsson PA, Aus G, Lilja H, Bangma C, Hamdy FC, Boccon-Gibod L, Ekman P. Prognostic value of serum markers for prostate cancer. Scandinavian journal of urology and nephrology. 2005;(216):64–81. doi: 10.1080/03008880510030941. [DOI] [PubMed] [Google Scholar]
- 15.di Sant’Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer. 1992;70(1 Suppl):254–268. doi: 10.1002/1097-0142(19920701)70:1+<254::aid-cncr2820701312>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JI. Pathologic assessment of the surgical specimen. The Urologic clinics of North America. 2001;28(3):567–594. doi: 10.1016/s0094-0143(05)70164-6. [DOI] [PubMed] [Google Scholar]
- 17.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005;293(17):2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael MJ, Veltri RW, Partin AW, Miller MC, Walsh PC, Epstein JI. Deoxyribonucleic acid ploidy analysis as a predictor of recurrence following radical prostatectomy for stage T2 disease. The Journal of urology. 1995;153(3 Pt 2):1015–1019. [PubMed] [Google Scholar]
- 19.Veltri RW, Partin AW, Epstein JE, Marley GM, Miller CM, Singer DS, Patton KP, Criley SR, Coffey DS. Quantitative nuclear morphometry, Markovian texture descriptors, and DNA content captured on a CAS-200 Image analysis system, combined with PCNA and HER-2/neu immunohistochemistry for prediction of prostate cancer progression. Journal of cellular biochemistry. 1994;19:249–258. [PubMed] [Google Scholar]
- 20.Stein GS, Montecino M, van Wijnen AJ, Stein JL, Lian JB. Nuclear structure-gene expression interrelationships: implications for aberrant gene expression in cancer. Cancer research. 2000;60(8):2067–2076. [PubMed] [Google Scholar]
- 21.Diamond DA, Berry SJ, Umbricht C, Jewett HJ, Coffey DS. Computerized image analysis of nuclear shape as a prognostic factor for prostatic cancer. The Prostate. 1982;3(4):321–332. doi: 10.1002/pros.2990030402. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JI, Berry SJ, Eggleston JC. Nuclear roundness factor. A predictor of progression in untreated Stage A2 prostate cancer. Cancer. 1984;54(8):1666–1671. doi: 10.1002/1097-0142(19841015)54:8<1666::aid-cncr2820540830>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Partin AW, Walsh AC, Pitcock RV, Mohler JL, Epstein JI, Coffey DS. A comparison of nuclear morphometry and Gleason grade as a predictor of prognosis in stage A2 prostate cancer: a critical analysis. The Journal of urology. 1989;142(5):1254–1258. doi: 10.1016/s0022-5347(17)39049-3. [DOI] [PubMed] [Google Scholar]
- 24.Veltri RW, Khan MA, Miller MC, Epstein JI, Mangold LA, Walsh PC, Partin AW. Ability to predict metastasis based on pathology findings and alterations in nuclear structure of normal-appearing and cancer peripheral zone epithelium in the prostate. Clin Cancer Res. 2004;10(10):3465–3473. doi: 10.1158/1078-0432.CCR-03-0635. [DOI] [PubMed] [Google Scholar]
- 25.Veltri RW, Miller MC, Isharwal S, Marlow C, Makarov DV, Partin AW. Prediction of Prostate-Specific Antigen Recurrence in Men with Long-term Follow-up Postprostatectomy Using Quantitative Nuclear Morphometry. Cancer Epidemiol Biomarkers Prev. 2008;17(1):102–110. doi: 10.1158/1055-9965.EPI-07-0175. [DOI] [PubMed] [Google Scholar]
- 26.Sauerbrei W. The use of resampling methods to simplify regression models in medical statistics. Applied Statistics. 1999;48(3):313–329. [Google Scholar]
- 27.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 28.Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignant prostate tissue. Human pathology. 1993;24(2):220–226. doi: 10.1016/0046-8177(93)90304-y. [DOI] [PubMed] [Google Scholar]
- 29.Silberman MA, Partin AW, Veltri RW, Epstein JI. Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in Gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer. 1997;79(4):772–779. doi: 10.1002/(sici)1097-0142(19970215)79:4<772::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Rubin MA, Buyyounouski M, Bagiella E, Sharir S, Neugut A, Benson M, de la Taille A, Katz AE, Olsson CA, Ennis RD. Microvessel density in prostate cancer: lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology. 1999;53(3):542–547. doi: 10.1016/s0090-4295(98)00561-5. [DOI] [PubMed] [Google Scholar]
- 31.Gettman MT, Bergstralh EJ, Blute M, Zincke H, Bostwick DG. Prediction of patient outcome in pathologic stage T2 adenocarcinoma of the prostate: lack of significance for microvessel density analysis. Urology. 1998;51(1):79–85. doi: 10.1016/s0090-4295(97)00464-0. [DOI] [PubMed] [Google Scholar]
- 32.Cohen RJ, Glezerson G, Haffejee Z. Neuro-endocrine cells--a new prognostic parameter in prostate cancer. British journal of urology. 1991;68(3):258–262. doi: 10.1111/j.1464-410x.1991.tb15318.x. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein MH, Partin AW, Veltri RW, Epstein JI. Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Human pathology. 1996;27(7):683–687. doi: 10.1016/s0046-8177(96)90398-6. [DOI] [PubMed] [Google Scholar]
- 34.Isshiki S, Akakura K, Komiya A, Suzuki H, Kamiya N, Ito H. Chromogranin a concentration as a serum marker to predict prognosis after endocrine therapy for prostate cancer. The Journal of urology. 2002;167(2 Pt 1):512–515. doi: 10.1016/S0022-5347(01)69075-X. [DOI] [PubMed] [Google Scholar]
- 35.Berruti A, Mosca A, Porpiglia F, Bollito E, Tucci M, Vana F, Cracco C, Torta M, Russo L, Cappia S, Saini A, Angeli A, Papotti M, Scarpa RM, Dogliotti L. Chromogranin A expression in patients with hormone naive prostate cancer predicts the development of hormone refractory disease. The Journal of urology. 2007;178(3 Pt 1):838–843. doi: 10.1016/j.juro.2007.05.018. quiz 1129. [DOI] [PubMed] [Google Scholar]
- 36.Ahlegren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson P. Neuroendocrine differentiation is not prognostic of failure after radical prostatectomy but correlates with tumor volume. Urology. 2000;56(6):1011–1015. doi: 10.1016/s0090-4295(00)00838-4. [DOI] [PubMed] [Google Scholar]
- 37.Bubendorf L, Tapia C, Gasser TC, Casella R, Grunder B, Moch H, Mihatsch MJ, Sauter G. Ki67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Human pathology. 1998;29(9):949–954. doi: 10.1016/s0046-8177(98)90199-x. [DOI] [PubMed] [Google Scholar]
- 38.Khoo VS, Pollack A, Cowen D, Joon DL, Patel N, Terry NH, Zagars GK, von Eschenbach AC, Meistrich ML, Troncoso P. Relationship of Ki-67 labeling index to DNA-ploidy, S-phase fraction, and outcome in prostate cancer treated with radiotherapy. The Prostate. 1999;41(3):166–172. doi: 10.1002/(sici)1097-0045(19991101)41:3<166::aid-pros3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Bettencourt MC, Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Moul JW. Ki-67 expression is a prognostic marker of prostate cancer recurrence after radical prostatectomy. The Journal of urology. 1996;156(3):1064–1068. [PubMed] [Google Scholar]
- 40.Cher ML, Chew K, Rosenau W, Carroll PR. Cellular proliferation in prostatic adenocarcinoma as assessed by bromodeoxyuridine uptake and Ki-67 and PCNA expression. The Prostate. 1995;26(2):87–93. doi: 10.1002/pros.2990260205. [DOI] [PubMed] [Google Scholar]
- 41.Rubio J, Ramos D, Lopez-Guerrero JA, Iborra I, Collado A, Solsona E, Almenar S, Llombart-Bosch A. Immunohistochemical expression of Ki-67 antigen, cox-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. European urology. 2005;48(5):745–751. doi: 10.1016/j.eururo.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Borre M, Stausbol-Gron B, Nerstrom B, Overgaard J. Immunohistochemical BCL-2 and Ki-67 expression predict survival in prostate cancer patients followed expectantly. Prostate Cancer Prostatic Dis. 1998;1(5):268–275. doi: 10.1038/sj.pcan.4500252. [DOI] [PubMed] [Google Scholar]
- 43.Stackhouse GB, Sesterhenn IA, Bauer JJ, Mostofi FK, Connelly RR, Srivastava SK, Moul JW. p53 and bcl-2 immunohistochemistry in pretreatment prostate needle biopsies to predict recurrence of prostate cancer after radical prostatectomy. The Journal of urology. 1999;162(6):2040–2045. doi: 10.1016/S0022-5347(05)68095-0. [DOI] [PubMed] [Google Scholar]
- 44.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 45.Myers RB, Srivastava S, Oelschlager DK, Grizzle WE. Expression of p160erbB-3 and p185erbB-2 in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Journal of the National Cancer Institute. 1994;86(15):1140–1145. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- 46.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M. Her-2-neu expression and progression toward androgen independence in human prostate cancer. Journal of the National Cancer Institute. 2000;92(23):1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 47.Calvo BF, Levine AM, Marcos M, Collins QF, Iacocca MV, Caskey LS, Gregory CW, Lin Y, Whang YE, Earp HS, Mohler JL. Human epidermal receptor-2 expression in prostate cancer. Clin Cancer Res. 2003;9(3):1087–1097. [PubMed] [Google Scholar]
- 48.Savinainen KJ, Saramaki OR, Linja MJ, Bratt O, Tammela TL, Isola JJ, Visakorpi T. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. The American journal of pathology. 2002;160(1):339–345. doi: 10.1016/S0002-9440(10)64377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards J, Traynor P, Munro AF, Pirret CF, Dunne B, Bartlett JM. The role of HER1-HER4 and EGFRvIII in hormone-refractory prostate cancer. Clin Cancer Res. 2006;12(1):123–130. doi: 10.1158/1078-0432.CCR-05-1445. [DOI] [PubMed] [Google Scholar]
- 50.Osman I, Mikhail M, Shuch B, Clute M, Cheli CD, Ghani F, Thiel RP, Taneja SS. Serum levels of shed Her2/neu protein in men with prostate cancer correlate with disease progression. The Journal of urology. 2005;174(6):2174–2177. doi: 10.1097/01.ju.0000181205.23233.65. [DOI] [PubMed] [Google Scholar]
- 51.Domingo-Domenech J, Fernandez PL, Filella X, Martinez-Fernandez A, Molina R, Fernandez E, Alcaraz A, Codony J, Gascon P, Mellado B. Serum HER2 extracellular domain predicts an aggressive clinical outcome and biological PSA response in hormone-independent prostate cancer patients treated with docetaxel. Ann Oncol. 2008;19(2):269–275. doi: 10.1093/annonc/mdm490. [DOI] [PubMed] [Google Scholar]