Abstract
Purpose
To evaluate the use of coronary wall MRI as a measure of atherosclerotic disease burden in an asymptomatic population free of clinical cardiovascular disease.
Background
Coronary wall magnetic resonance imaging (MRI) is a noninvasive method for evaluation of arterial wall remodeling associated with atherosclerosis.
Materials and Methods
Asymptomatic participants of the Multi-Ethnic Study of Atherosclerosis (MESA) study were studied using black blood MRI. MRI assessed coronary wall thickness was compared to computed tomography calcium score, carotid intimal-medial thickness and risk factors for coronary artery disease.
Results
Eighty eight arterial segments were evaluated in 38 MESA participants (mean age, 61.3 ± 8.7 years). The maximum coronary wall thickness was greater for participants with 2 or more cardiovascular risk factors than for those with 1 or no risk factors (2.59 ± 0.33 mm versus 2.36 ± 0.30 mm, respectively, p=0.05.) For participants with zero calcium score, the mean and maximum coronary wall thickness for subjects with 2 or more risk factors for coronary artery disease were greater than the wall thickness for subjects with 1 or no risk factors (mean thickness: 1.95 ± 0.17 mm versus 1.7 ± 0.19 mm; maximum thickness: 2.67 ± 0.24 mm versus 2.32 ± 0.27 mm, respectively, p <0.05). Subjects with increased carotid intimal-medial thickness also had increased coronary artery wall thickness (p< 0.05).
Conclusions
Coronary artery wall MRI detects increased coronary wall thickness in asymptomatic individuals with subclinical markers of atherosclerotic disease and in individuals with zero calcium score.
Keywords: coronary artery disease, atherosclerosis, MRI, plaque
INTRODUCTION
Coronary atherosclerosis is the leading cause of death in industrialized western countries. (1) Atherosclerosis may be clinically silent, and plaque that causes only mild to moderate luminal narrowing may result in acute coronary syndromes.(2,3) Initially, atherosclerosis of the coronary artery results in outward remodeling of the arterial wall while the lumen diameter is preserved. Traditional risk factors alone do not accurately identify individuals with symptomatic or asymptomatic coronary artery disease (CAD).(4–7) Noninvasive approaches have therefore been proposed to identify plaque burden and coronary calcification assessed by computed tomography (CT) has been used for this purpose. Calcification may not be present in early atherosclerosis however, and calcium deposition is present in only a small fraction of the total atherosclerotic burden.(8,9)
Black blood coronary arterial wall magnetic resonance imaging (MRI) has been developed to directly assess coronary artery wall thickness as a measure of plaque burden.(10,11) The technique is noninvasive and without radiation exposure or contrast agent injection. There is a good correlation between MRI-measured coronary wall thickness and matched histopathology sections for human coronary artery specimens and in vivo animal models(r=0.94).(12) Good reproducibility has also been demonstrated.(13–15) MRI of coronary arteries in patients with more than 40% stenosis as assessed by x-ray angiography showed local increases in wall thickness compared to normal subjects(16) as did patients with 10–50% coronary diameter reduction.(10) A recent study showed that in asymptomatic type 1 diabetics, coronary wall MRI reveals greater coronary plaque burden in subjects with nephropathy compared with those with normoalbuminuria.(17)
The purpose of this study was to describe the application of coronary wall MRI as a measure of atherosclerotic disease burden in an asymptomatic population of the Multi-Ethnic Study of Atherosclerosis (MESA). We describe the associations between coronary artery wall thickness and other measures of atherosclerotic burden in the MESA population in order to assess the potential use of the MRI technique in individuals without known coronary heart disease.
MATERIALS AND METHODS
Study Design And Subject Selection
Study participants were recruited from the Multi-Ethnic Study of Atherosclerosis (MESA).(18) In brief, participants were 45 to 84 years old and free of clinically apparent cardiovascular disease at the time of their initial enrollment into the MESA study. Participation in the MRI examination was voluntary. The MESA is a multi-center study, but the present study was based on the Baltimore and Chicago cohort participants only.
Exclusion criteria were absence of sinus cardiac rhythm, heart rate above 85 beats per minute, claustrophobia and contraindications to MRI such as the presence of ferromagnetic implants. Fifty MESA participants were enrolled in the study. The study was approved by our institutional review board and informed consent was obtained from all subjects.
Coronary Artery Wall MRI
Of the 50 enrolled subjects, 48 individuals completed the cardiac MRI scan. Two participants did not complete the protocol: one due to sinus tachycardia (heart rate of 105 beats per minute) and the other due to inability to perform ECG gating during the MRI scan. The first 11 subjects were evaluated using a 1.5 T whole-body MRI system (Signa CVi, General Electric Medical Systems, Waukesha, WI) with 40 mT/m gradients using a four-element phased-array coil for signal reception. Subsequently, 39 subjects were imaged on a 1.5T whole-body clinical scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany) operating at a maximum gradient strength of 45 mT/m and slew rate of 200 T/m/sec using six anterior channels and six posterior channels for data acquisition.
In order to localize the coronary arteries, a coronary magnetic resonance angiogram (MRA) was acquired using either breath-hold technique (n=23) or free breathing whole heart technique (n=25).(19) Images were acquired during the longer of the systolic or diastolic rest period based on visual inspection of coronary wall motion observed on a transaxial steady state free precession cine MRI acquisition obtained at the mid-ventricular level. On the General Electric scanner, a double oblique breath-hold coronary MRA was obtained using a T2 prepared fat suppressed three-dimensional steady state free precession acquisition with relaxation time/echo time (TR/ TE) = 4.7/1.9 ms, flip angle = 65°, readout bandwidth = 970 Hz/pixel, field of view = 280×224 mm, matrix = 256×192, slice thickness = 2 mm with interpolation to 1 mm, number of partitions = 12. On the Siemens scanner, axial whole heart free breathing coronary MRA parameters were TR/TE = 3.7/1.7 mm, flip angle = 90°, readout bandwidth = 870 Hz/pixel, parallel imaging factor=2, field of view = 320 mm, matrix = 288 × 288, slice thickness = 3.0 mm with sinc-interpolation to 1.5 mm, number of partitions = 40.
For black blood coronary MRI, seven cross-sectional slices at 5 mm intervals were obtained from the proximal portions of the coronary arteries (left main coronary artery (LM), left anterior descending coronary artery (LAD) and the right coronary artery (RCA)). Each cross-sectional image was individually prescribed based on double oblique multiplanar reformations to be orthogonal to the local longitudinal axis of the coronary wall. None of the patient studies had high grade (more than 50% coronary stenosis) and cross-sectional slices were positioned based on a fixed distance from coronary landmarks (the coronary ostia for the RCA and LM, and the bifurcation of the LM to the LAD and circumflex arteries for the LAD) rather than through areas of coronary artery narrowing. Coronary wall images were acquired using a breath-hold, ECG-gated, turbo spin echo (TSE) sequence with double inversion pulse preparation for nulling the blood for the General Electric scanner and a free-breathing navigator technique for the Siemens scanner. TSE pulse sequence settings were optimized for comparability based on ex vivo scanning of coronary arteries of human hearts. A spectrally selective fat suppression pulse was also used to increase the contrast between the vessel wall and the epicardial fat. For the General Electric scanner, imaging parameters were: TR = 2R–R, TE = 5.1 ms, echo train length = 16, bandwidth = 244 Hz/pixel, matrix = 256 × 192; field of view = 280 × 224 mm; 1.1× 1.1 mm pixel size with zero-filling to 0.55×0.55; slice thickness = 4 mm. For the Siemens scanner, imaging parameters were TR = 2 R-R intervals, TE = 33 ms, echo train length = 11–13, bandwidth = 305 Hz/pixel, matrix = 416×416, field of view = 420 × 420 mm; 1.0×1.0 pixel size with zero filling to 0.50×0.50; slice thickness = 5 mm. All acquisitions were performed during the same rest period as determined for the coronary MRA. The acquisition window duration was 70–100 msec. Acquisition times per image were 1–3 minutes.
Positioning of the MRI cross-sections of the coronary wall were done blinded to the results of CT performed for calcification and all other imaging and serologic data.
MRI Analysis
Coronary wall images were analyzed using VesselMASS software (Leiden University Medical Center) (Figure 1 and Figure 2) (20). The images were zoomed to 500%. The outer and inner boundaries of the vessel wall were traced manually by a single observer blinded to other MESA parameters (discussed below) using a region of interest tool. The software then reported the mean and maximum vessel wall thickness. The coefficient of repeatability (COR) for interobserver variation was 0.28 mm; the COR for inter-study reproducibility was 0.39 mm.(13)
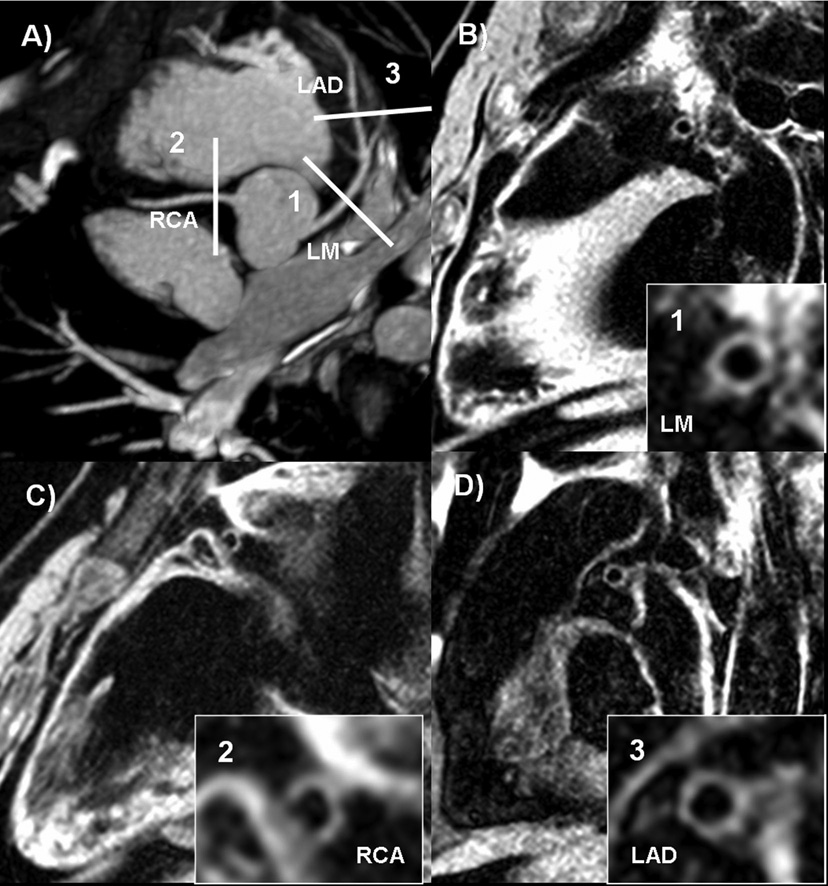
Figure 1.
Normal coronary MRA and normal coronary wall thickness. 55 year-old female participant, with one risk factor for CAD and a calcium score of zero.
a) Coronary MRA demonstrating slice positioning for representative cross-sectional slices for coronary wall imaging.
b), c) and d) Coronary wall images of the LM, RCA and LAD, respectively.
Figure 2.
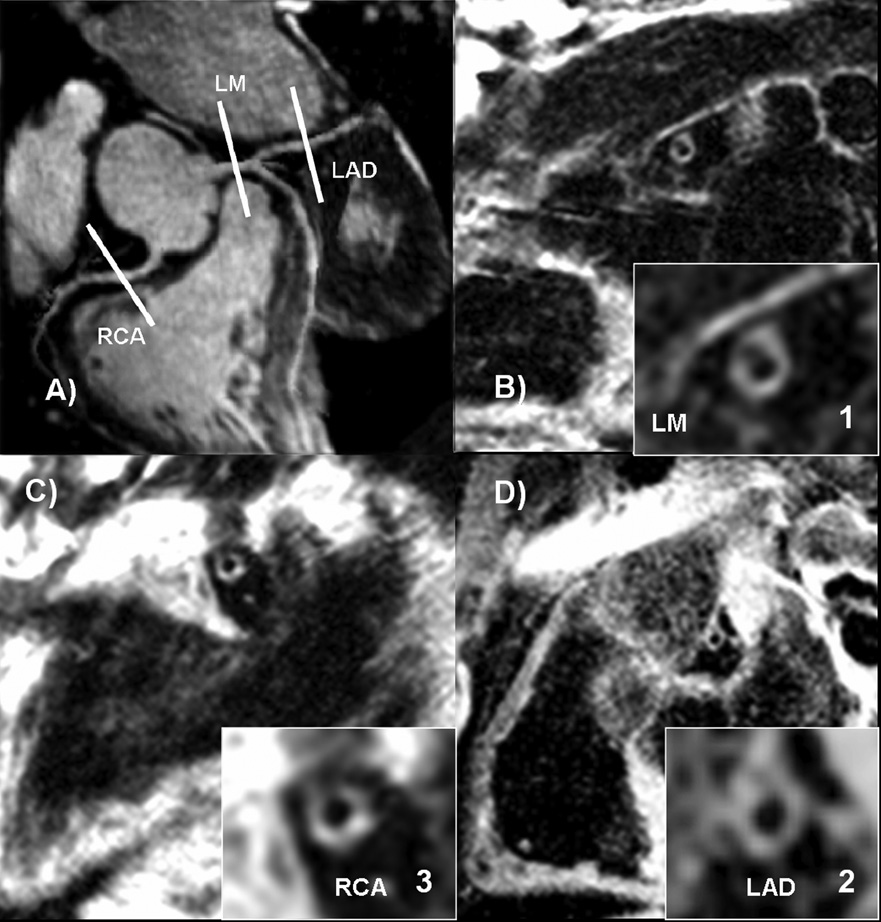
Normal coronary MR angiogram with increased coronary artery wall thickness. 73 year-old male participant with 2 risk factors for CAD and Agatston score of 200.
a) Coronary MRA demonstrating slice positioning of representative slices for coronary wall imaging.
b), c) and d) Coronary wall images of the LM, RCA and LAD, respectively, showing increased wall thickness.
CAD Risk Factors
Hypertension was defined as diastolic blood pressure (DBP) ≥ 90 mm Hg, systolic blood pressure (SBP) ≥ 140 mm Hg, or treatment for hypertension. Total cholesterol ≥ 240 mg/dl (6.2 mmol/l) was considered as elevated. HDL < 40 mg/dl (1.0 mmol/l) was defined as low. Diabetic subjects were defined as either having fasting plasma glucose ≥ 126 mg/dl (7.0 mmol/l) or treatment for diabetes. Smoking status was defined by history of smoking (ever or never smoked). Age greater than or equal to 65 years and male gender were also classified as coronary artery disease risk factors.
Coronary Artery Calcium
Computed tomography scanning of the chest was performed with a prospectively ECG-triggered scan acquisition at 50% of the RR interval with a multidetector computed tomography system (Siemens, Volume Zoom) with 2.5-mm slices in a sequential scan mode. Each participant was scanned twice. Scans were read centrally at the MESA CT reading center at the University of California Los Angeles.(21) The average Agatston score was used in all analyses. The coronary artery calcium (CAC) scores between participants were adjusted with a standard calcium phantom scanned simultaneously with each participant.
Carotid Ultrasound
For carotid ultrasonography, images of the common carotid arteries were captured, including images of the near and far wall, using high-resolution B-mode ultrasound with the Logiq 700 ultrasound machine (General Electric Medical Systems). Readings were performed centrally at the MESA ultrasound reading center, New England Medical Center.(18,22)
Statistical Analysis
Continuous data are expressed as mean values with standard deviations. Comparisons were made based on each artery rather than arterial segment. Unpaired t-tests were performed to evaluate differences between groups. Associations of coronary wall thickness with calcium score and common carotid intimal-medial thickness were evaluated using linear regression. Because the distribution of calcium scores was not normal, the logs of the (Agatston) scores were used for assessing the correlation with coronary wall thickness. The Student t-test was performed for univariate analysis and was used only for normally distributed data. We considered p-values of less than or equal to 0.05 to be statistically significant.
RESULTS
Study Population
Demographic and risk factor profiles for the participants in this study are presented in Table 1. The study population was 46% male, 54% African American and 46% Non-Hispanic White. The mean age was 61.3 ± 8.7 years. The average BMI was fairly high (29.5), 42% had hypertension, and 27% were being treated for hypertension. Mean total cholesterol was 198 mg/dL and almost one-half of the participants reported ever smoking. Average fasting glucose was 101 mg/dl. Only 4 individuals reported a history of diabetes. Prior to statistical analysis, participants were dichotomized into two groups based on their number of risk factors: four participants had no cardiovascular risk factors and 13 had 1 risk factor (age ≥ 65 years, male gender, hypertension, abnormal lipid levels, diabetes or smoking); these participants were considered a low risk group. Thirty one other participants had 2 or more risk factors. Non-zero calcium scores were present in 44% of study participants, and among these the average Agatston score was 78. Average carotid intimal-medial thickness was 0.91 mm.
Table 1.
Characteristics of MESA participants evaluated by coronary wall MRI
| n (%) | Range | |
|---|---|---|
| Demographic data | ||
| Men | 22 (45.8) | |
| Women | 26 (54.2) | |
| Non-Hispanic white | 22 (45.8) | |
| African American | 26 (54.2) | |
| Age (yrs) | 61.3 ± 8.7 | 46–76 |
| BMI (kg/m2) | 29.5 ± 5.7 | 23–45.7 |
| Blood pressure | ||
| SBP (mm Hg) | 127 ± 21 | 89–193 |
| DBP (mm Hg) | 72 ± 11 | 46–99 |
| Self-reported hypertension | 11 (23.4) | |
| Treatment for hypertension | 13 (27.1) | |
| Hypertension(DBP>=90 or SBP>=140 or treatment for Hypertension) | 20 (41.7) | |
| Lipids | ||
| Total cholesterol, mg/dl | 197.9 ± 40.5 | 118–290 |
| LDL cholesterol, mg/dl | 122.0 ± 38.4 | 23–211 |
| HDL cholesterol, mg/dl | 52.5 ± 14.0 | 32–94 |
| Triglycerides, mg/dl | 116.6 ± 58.0 | 31–294 |
| Treatment for hyperlipidemia | 7 (14.6) | |
| Elevated lipid level | 17 (35.4) | |
| Smoking* | ||
| Never smoked | 25 (53.2) | |
| Former smoking | 18 (38.3) | |
| Current smoking | 4 (8.5) | |
| Glucose | ||
| Plasma glucose, mg/dl | 101.4 ± 25.6 | 73–202 |
| History of diabetes mellitus | 4 (8.5) | |
| Insulin-treated | 0 (0) | |
| Positive calcium score | 21(43.8%) | |
| Calcium score | 78.2 ± 227.0 | 0–1447.5 |
| Common carotid intimal-medial thickness (mm) | 0.91 ± 0.18 | 0.59–1.37 |
| Mean coronary wall thickness (mm) | 1.83 ± 0.25 | 1.32–2.33 |
| Maximum coronary wall thickness (mm) | 2.50 ± 0.34 | 1.78–3.35 |
Data are presented as n, mean ± SD, or range. Percentages are in parentheses.
BMI = body mass index; DBP = diastolic blood pressure; ECG = electrocardiogram; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SBP = systolic blood pressure.
(One participant’s smoking status was unknown, thus only 47 were considered.)
Overall, 38/48 (79%) subjects had diagnostic scans for coronary wall imaging. Eleven participants had breath-hold imaging and 27 participants were studied with a free breathing navigator technique. 8/48 (17 %) participants with nondiagnostic scans had significantly higher (p<0.05) heart rate and shorter cardiac rest periods that resulted in more cardiac motion artifacts compared to participants with diagnostic scans. The average length of the MRI examination was 45 minutes.
Coronary Wall Thickness And CAD Risk Factors
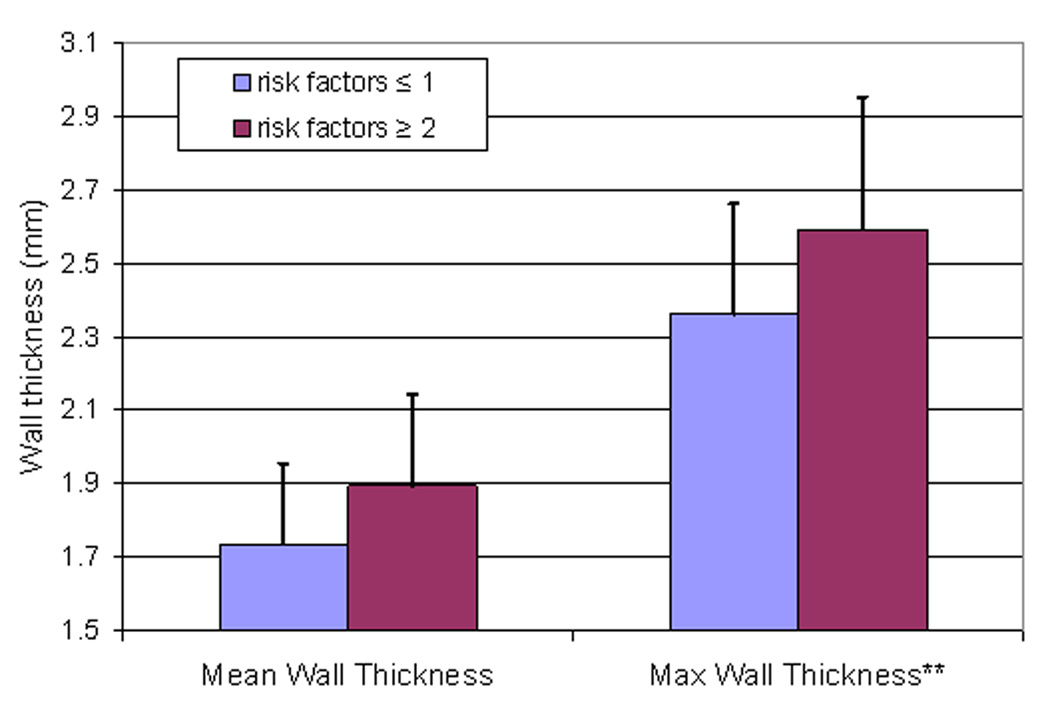
Participants with two or more risk factors for CAD had higher mean and maximum coronary wall thickness compared to participants with one or no risk factors (maximum wall thickness: 2.59 ± 0.33 mm versus 2.36 ± 0.30 mm, respectively, p=0.05, Figure 3). The maximum coronary wall thickness of the left main coronary artery in participants with HDL < 40 mg/dl was significantly higher compared to participants with HDL ≥ 40mg/dl (2.71 ± 0.0.44 mm versus 2.46 ± 0.33 mm, respectively, p=0.05). The mean coronary wall thickness of the left main coronary artery with a history of smoking was significantly higher compared to participants who never smoked (2.10 ± 0.39 mm versus 1.78 ± 0.29 mm, respectively, p=0.02). No significant differences in coronary wall thickness by age (<65 or ≥65 years-old), gender, total cholesterol (<240 or ≥240mg/dl), diabetes (yes or no) or hypertension (yes or no) were detected.
Figure 3.
Mean and maximum coronary wall thickness as a function of coronary risk factor group (all subjects).
Considering all participants, those with any calcium present in the coronary arteries had higher mean and maximum coronary wall thickness compared to those with no calcium, except in the LAD subgroup, but these differences were not statistically significant. The overall linear correlation between mean and maximum coronary wall thickness and calcium score (or log transformed calcium score) was not significant. Inspection of the CAC score versus wall thickness distribution showed that some participants with zero calcium scores had increased coronary wall thickness substantially above the mean values, potentially indicating areas of noncalcified atherosclerosis.
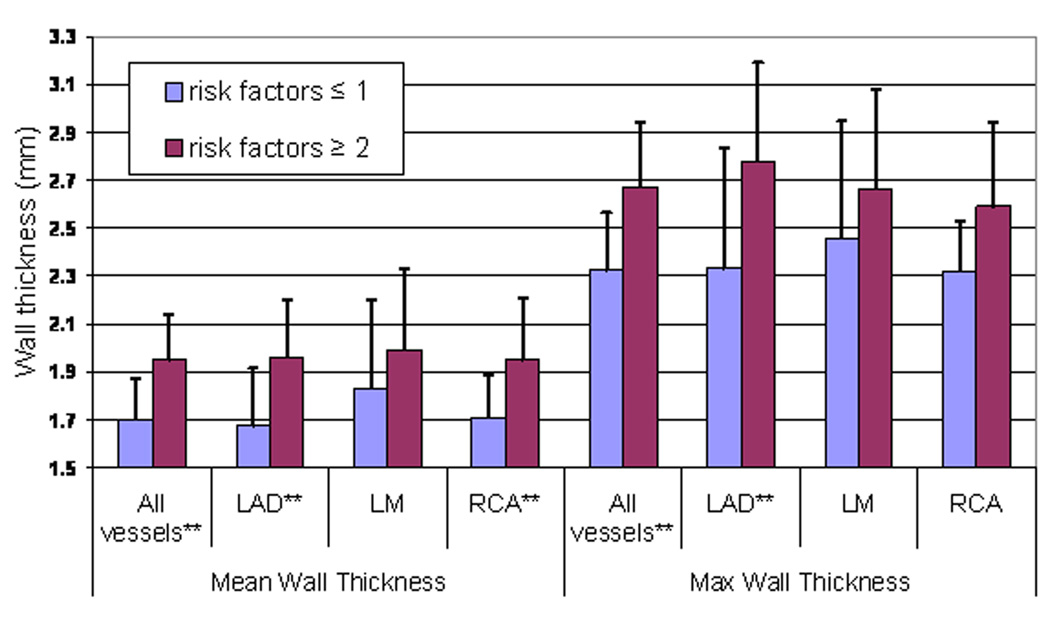
A separate statistical analysis of coronary artery wall thickness was then performed for subjects with zero calcium score (n=23) in order to determine if atherosclerosis would be detected by MRI in this subgroup. For participants with zero calcium score and two or more risk factors for CAD, the coronary wall thickness was significantly greater for those with 1 or no risk factors (Figure 4, mean thickness: 1.95 ± 0.17 mm versus 1.70 ± 0.19 mm; maximum thickness: 2.67 ± 0.24 mm versus 2.32 ± 0.27 mm, respectively, p <0.05). Similar patterns were also present for the left anterior descending coronary artery (mean thickness: 1.96 ± 0.24 mm versus 1.68 ± 0.24 mm; maximum thickness: 2.78 ± 0.5 mm versus 2.33 ± 0.4 mm, respectively, p<0.05) and for the right coronary artery (1.95 ± 0.18 mm versus 1.71 ± 0.26 mm, respectively).
Figure 4.
Mean and maximum coronary wall thickness for participants with zero calcium score as a function of coronary risk factor group.
Coronary Wall Thickness And Common Carotid Intimal-medial Thickness (IMT)
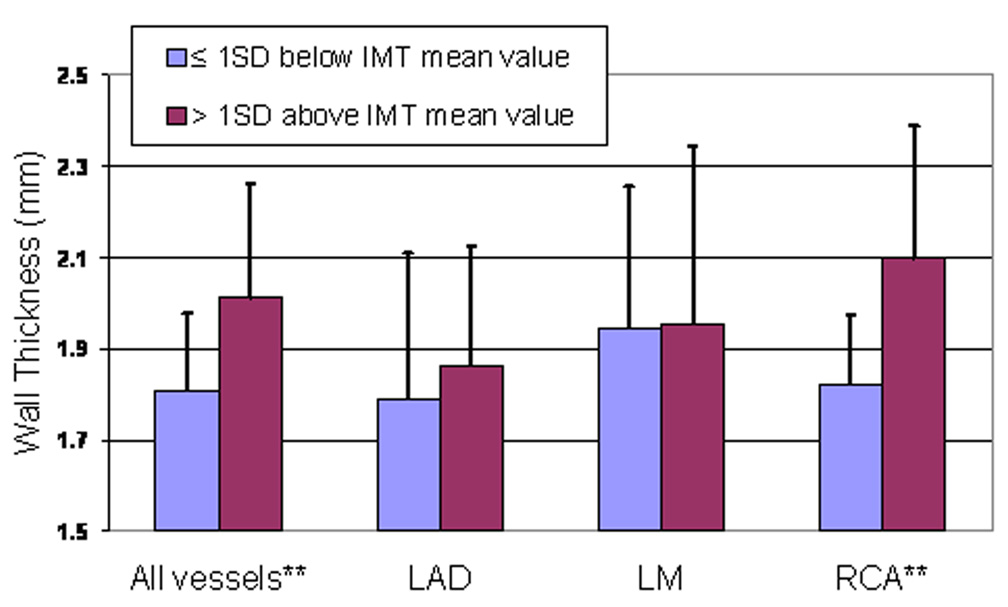
Participants with carotid IMT thickness more than one standard deviation above the mean (1.09 mm) had higher mean and maximum coronary wall thickness compared to participants with carotid IMT less than or equal to one standard deviation above the mean (e.g. mean coronary thickness 1.99 ± 0.14 mm versus 1.76 ± 0.21 mm, respectively, p< 0.05) (Figure 5). Linear regression analysis showed that increased mean coronary wall thickness was positively associated with increased carotid IMT for all vessels combined (r2, 0.48 ± 0.22 (p<0.05).
Figure 5.
Mean coronary wall thickness by carotid artery intimal-medial thickness (IMT) (all subjects).
DISCUSSION
In this study we report the use of MRI to directly and noninvasively measure coronary artery wall thickness as a measure of atherosclerotic disease burden. Our objective in this study was to assess the potential utility of coronary artery wall thickness as a marker of atherosclerotic burden in a population free of clinical cardiovascular disease. In this study, participants with 2 or more cardiovascular risk factors had significantly greater maximum coronary wall thickness than those with 1 or no risk factors. This relationship was also present even in subjects without coronary calcification by CT scan. Coronary artery wall thickness overall showed better correlation with carotid intimal-medial thickness than coronary calcium score.
The extent of coronary calcium correlates with overall plaque burden.(8,23) Calcium is a minor component of total plaque and is not present in early plaque on autopsy specimens.(8,9) Despite a strong correlation between coronary calcium and coronary atherosclerosis, there is substantial interindividual variation in calcium deposition that is apparently due to differences in the tendency for plaque to calcify. (24,25) For example, a “zero” calcium score is associated with mild to moderate coronary narrowing in 15% of patients.(26) The role of coronary calcium scoring seems to be greatest for detection of advanced atherosclerosis in individuals at intermediate risk.(14,26–28)
We found that there was a trend toward greater coronary wall thickness for increasing levels of calcium but overall this relationship was not statistically significant. Reasons for lack of correlation between calcium score and MRI wall thickness may be related to the threshold method for calcium scoring used for computed tomography, low spatial resolution of the MRI, or both. The calcium scoring method defines a threshold level of x-ray density (130 Hounsfield units), below which calcium (and therefore atherosclerosis) is defined to be absent. We noted that some individuals with a calcium score of zero had increased coronary wall thickness above the mean value. When we evaluated only participants with zero calcium score, we found that participants with 2 or more risk factors had significantly increased coronary wall thickness compared to those with 1 or no risk factors. The ability of MRI to identify coronary artery wall abnormalities in subjects without clinical cardiovascular disease and with zero calcium scores suggests that MRI could be used as an early marker of subclinical coronary atherosclerosis.
Indeed, carotid IMT overall showed better correlation with coronary wall thickness than did calcium score. Carotid IMT is abnormal in individuals with coronary heart disease;(29,30) and the relationship between IMT and CHD severity is constant but not strong.(14,31) In the Cardiovascular Health Study, carotid IMT greater than 80th percentile had a sensitivity of 47% for predicting coronary artery calcium (CAC) score equal to or greater than 400.(32) In the present study, participants with IMT values higher than one standard deviation above the mean (greater than the 68th percentile) had thicker coronary artery walls compared to those with lower IMT values. Since atherosclerosis is a systemic disease, these results confirm the expected co-development of atherosclerosis in multiple vascular territories.
The results of this study show that asymptomatic individuals with increased risk factors for cardiovascular disease have thicker coronary walls than those one or no risk factors. Our results support prior studies using echocardiography, which can interrogate the left anterior descending coronary artery.(33,34) Risk factor reduction, including statin therapy,(35–38) can reverse the adverse cardiovascular effects of atherosclerosis, but currently there is no noninvasive method that can reliably quantify the anatomic correlate of risk factor reduction in the coronary arteries. Most studies demonstrating the benefit of medical intervention have targeted a population with advanced stages of atherosclerosis because of the inability to detect earlier disease, but a greater reduction in risk is expected if an earlier stage of vascular disease can be identified and quantitatively monitored for response to preventive interventions. Modest reductions in coronary plaque burden after intervention have been reported with intravascular ultrasound,(39) but the technique is invasive and not easily repeated nor performed on asymptomatic individuals.
There were several limitations of this study. The coronary MRI technique is technically challenging, and only approximately 80% of participants who completed the protocol had interpretable images. As indicated, the sample size was small, limiting the ability to detect graded responses of wall thickness over multiple categories. The spatial resolution of MRI is limited and overestimates the wall thickness of normal coronary arteries.(40) The spatial resolution used in this study was similar to other studies for MRI of coronary plaque(10) that have also noted correlation with measures of coronary wall thickness. Focal deposits of calcium may cause loss of signal on the coronary wall images. The MRI readers were blinded to the CT images for calcium, so the mean wall thickness in particular could be reduced if heavy deposits of calcium were present. Despite these issues, the multiple associations between coronary wall thickness by MRI and other subclinical measures of atherosclerosis that were detected in this and other studies thus relates to the identification of abnormal coronary walls by the method. Another limitation of this study is its cross-sectional nature, which prevented us from assessing the temporal sequence between MRI-defined coronary wall thickness and the other variables.
In conclusion, coronary artery wall thickness appears to be related to the coronary artery disease risk factors and carotid IMT, a general marker of atherosclerosis. Further studies are needed to determine the relationship of coronary artery wall thickness to the subsequent development of luminal narrowing and ultimately to the development of cardiovascular events.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
This research was supported by R01 HL78909 and contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Abbreviations
- MRI
magnetic resonance imaging
- MESA
Multi ethnic study of atherosclerosis
- CT
computed tomography
- ECG
electrocardiogram
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
- CAD
coronary artery disease
- HDL
high density lipoprotein
- CAC
coronary artery calcium
- IMT
intimal-medial thickness
Footnotes
CONFLICT-OF-INTEREST: none
FINANCIAL DISCLOSURE STATEMENTS: none.
References
- 1.Association AH. Heart Disease and Stroke Statistics — 2004 Update. Dallas, TX: American Heart Association; 2003. [Google Scholar]
- 2.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 3.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 Pt 1):1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 4.Oliver MF. Strategies for preventing and screening for coronary heart disease. Br Heart J. 1985;54(1):1–5. doi: 10.1136/hrt.54.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langou RA, Huang EK, Kelley MJ, Cohen LS. Predictive accuracy of coronary artery calcification and abnormal exercise test for coronary artery disease in asymptomatic men. Circulation. 1980;62(6):1196–1203. doi: 10.1161/01.cir.62.6.1196. [DOI] [PubMed] [Google Scholar]
- 6.Olofsson BO, Bjerle P, Aberg T, Osterman G, Jacobsson KA. Prevalence of coronary artery disease in patients with valvular heart disease. Acta Med Scand. 1985;218(4):365–371. doi: 10.1111/j.0954-6820.1985.tb08860.x. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection E, And Treatment of High Blood Cholesterol In Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed]
- 8.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 9.Schmermund A, Schwartz RS, Adamzik M, et al. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with 'healthy' subjects dying out of hospital. Atherosclerosis. 2001;155(2):499–508. doi: 10.1016/s0021-9150(00)00598-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106(3):296–299. doi: 10.1161/01.cir.0000025629.85631.1e. [DOI] [PubMed] [Google Scholar]
- 11.Botnar RM, Kim WY, Bornert P, Stuber M, Spuentrup E, Manning WJ. 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magn Reson Med. 2001;46(5):848–854. doi: 10.1002/mrm.1268. [DOI] [PubMed] [Google Scholar]
- 12.Worthley SG, Helft G, Fuster V, et al. High resolution ex vivo magnetic resonance imaging of in situ coronary and aortic atherosclerotic plaque in a porcine model. Atherosclerosis. 2000;150(2):321–329. doi: 10.1016/s0021-9150(99)00386-x. [DOI] [PubMed] [Google Scholar]
- 13.Hazirolan T, Gupta SN, Mohamed MA, Bluemke DA. Reproducibility of black-blood coronary vessel wall MR imaging. J Cardiovasc Magn Reson. 2005;7(2):409–413. doi: 10.1081/jcmr-200053464. [DOI] [PubMed] [Google Scholar]
- 14.Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res. 2001;89(4):305–316. doi: 10.1161/hh1601.095596. [DOI] [PubMed] [Google Scholar]
- 15.Desai MY, Lai S, Barmet C, Weiss RG, Stuber M. Reproducibility of 3D free-breathing magnetic resonance coronary vessel wall imaging. Eur Heart J. 2005;26(21):2320–2324. doi: 10.1093/eurheartj/ehi357. [DOI] [PubMed] [Google Scholar]
- 16.Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102(5):506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 17.Kim WY, Astrup AS, Stuber M, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007;115(2):228–235. doi: 10.1161/CIRCULATIONAHA.106.633339. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman BA, Sharrett AR, Lai S, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA) Stroke. 2008;39(2):329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 21.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22(9):1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 23.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 24.Janowitz WR, Agatston AS, Kaplan G, Viamonte M., Jr Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am J Cardiol. 1993;72(3):247–254. doi: 10.1016/0002-9149(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Detrano RC, Secci A, et al. Detection of coronary calcification with electronbeam computed tomography: evaluation of interexamination reproducibility and comparison of three image-acquisition protocols. Am Heart J. 1996;132(3):550–558. doi: 10.1016/s0002-8703(96)90237-9. [DOI] [PubMed] [Google Scholar]
- 26.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 27.Fuseini M, Goodwin WJ, Ferris EJ, Mehta JL. Does electron beam computer tomography provide added value in the diagnosis of coronary artery disease? Curr Opin Cardiol. 2003;18(5):385–393. doi: 10.1097/00001573-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Greenland P, Smith Jr SC, Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104(15):1863–1867. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 29.Crouse JR, 3rd, Tang R, Espeland MA, Terry JG, Morgan T, Mercuri M. Associations of extracranial carotid atherosclerosis progression with coronary status and risk factors in patients with and without coronary artery disease. Circulation. 2002;106(16):2061–2066. doi: 10.1161/01.cir.0000033833.54884.34. [DOI] [PubMed] [Google Scholar]
- 30.Terry JG, Tang R, Espeland MA, et al. Carotid arterial structure in patients with documented coronary artery disease and disease-free control subjects. Circulation. 2003;107(8):1146–1151. doi: 10.1161/01.cir.0000051461.92839.f7. [DOI] [PubMed] [Google Scholar]
- 31.Crouse JR, 3rd, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92(5):1141–1147. doi: 10.1161/01.cir.92.5.1141. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler Thromb Vasc Biol. 2002;22(10):1674–1679. doi: 10.1161/01.atv.0000033540.89672.24. [DOI] [PubMed] [Google Scholar]
- 33.Gradus-Pizlo I, Sawada SG, Wright D, Segar DS, Feigenbaum H. Detection of subclinical coronary atherosclerosis using two-dimensional, high-resolution transthoracic echocardiography. J Am Coll Cardiol. 2001;37(5):1422–1429. doi: 10.1016/s0735-1097(01)01160-3. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi M, Yoshitani H, Miyazaki C, Yoshikawa J. Relationship between the number of coronary risk factors and coronary atherosclerosis assessed by high-frequency transthoracic echocardiography. J Am Soc Echocardiogr. 2006;19(8):1056–1062. doi: 10.1016/j.echo.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a) Jama. 1995;274(22):1771–1774. [PubMed] [Google Scholar]
- 36.Zhao XQ, Brown BG, Hillger L, et al. Effects of intensive lipid-lowering therapy on the coronary arteries of asymptomatic subjects with elevated apolipoprotein B. Circulation. 1993;88(6):2744–2753. doi: 10.1161/01.cir.88.6.2744. [DOI] [PubMed] [Google Scholar]
- 37.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87(6):1781–1791. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- 38.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Arteriographic view of treatment to achieve regression of coronary atherosclerosis and to prevent plaque disruption and clinical cardiovascular events. Br Heart J. 1993;69(1 Suppl):S48–S53. doi: 10.1136/hrt.69.1_suppl.s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. Jama. 2004;291(9):1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 40.Schar M, Kim WY, Stuber M, Boesiger P, Manning WJ, Botnar RM. The impact of spatial resolution and respiratory motion on MR imaging of atherosclerotic plaque. J Magn Reson Imaging. 2003;17(5):538–544. doi: 10.1002/jmri.10287. [DOI] [PubMed] [Google Scholar]