Abstract
This study applies a non-invasive and multi-system measurement approach (using salivary analytes) to examine associations between the psychobiology of the stress response and affective behavior in toddlers. Eighty-seven two-year-olds (48 females) participated in laboratory tasks designed to elicit emotions and behavior ranging from pleasure/approach to fear/withdrawal. Saliva samples were collected pre-task and immediately post-task, and assayed for markers of sympathetic nervous system (alpha-amylase or sAA) and hypothalamic-pituitary-adrenal axis (cortisol) activity. Individual differences in sAA were positively associated with approach behavior and positive affect; whereas, cortisol was positively associated with negative affect and withdrawal behavior. The findings suggest that individual differences in sAA may covary specifically with positive affect and approach behaviors or the predominant emotional state across a series of tasks. The results are discussed with respect to advancing biosocial models of the concomitants and correlates of young children’s affective behaviors.
Introduction
In the developmental literature, there is increasing evidence that specific aspects of temperamental reactivity are associated with individual differences in the psychobiology of the stress response during childhood (e.g., Buss, Goldsmith, & Davidson, 2005; Gunnar & Quevedo, 2006; Kagan, Reznick, & Snidman, 1987, 1999; Lupien, King, Meaney, & McEwen, 2000). Historically, research attention has focused on the physiological correlates and concomitants of behavioral inhibition (e.g., Garcia-Coll, Kagan, & Reznick, 1984; Rubin, Nelson, Hastings, & Asendorpf, 1999) and, its corollary, exuberance/uninhibited behavior (e.g., Pfeifer, Goldsmith, Davidson, & Rickman, 2002; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Over the past decade, studies have begun to narrow the scope of this focus to examine relationships between the specific, affective behaviors associated with inhibition (e.g., withdrawal behaviors, shyness, and fearful or anxious responses to the unfamiliar) and exuberance (e.g., intense positive affectivity, high approach behaviors to novel stimuli) and physiological arousal/regulation (e.g., Buss, Davidson, Kalin, & Goldsmith, 2004; Buss, Goldsmith, & Davidson, 2003; Davidson, 2001).
In parallel, within developmental science more generally, a consensus opinion has emerged that challenges scientists to create conceptual models that integrate measures of multiple biological systems, and to assess the co-regulation of affective behaviors and physiology in social contexts (e.g., Bauer, Quas, & Boyce, 2002; Granger & Fortunato, 2008). Investigators have responded to this challenge by non-invasively measuring individual differences in the activity of the two main components of stress psychobiology, sympathetic nervous system (salivary alpha-amylase; sAA) and hypothalamus-pituitary-adrenal (cortisol) axis activity in saliva. So far this approach has yielded intriguing individual differences in the relationships among sAA and cortisol, and characteristics of the immediate social environment, including infant-mother attachment (Hill-Soderlund et al., 2008), maternal insensitivity (Kivlighan, Granger, & Blair, 2005), maltreatment (Gordis, Granger, Susman, & Trickett, 2007), and social exclusion (Stroud et al., in press) as well as internalizing and externalizing problem behavior (El-Sheikh et al., 2008; Gordis et al, 2006). In the present study, we extend this endeavor by employing this “new” multi-system approach to investigate the links among individual differences in sAA activity, cortisol activity, and specific affective behaviors during the toddler period
Sympathetic Nervous System and Affective Behaviors
When examining associations between temperament and sympathetic nervous system (SNS) activity, researchers have focused on two dimensions of affective behavior-- approach and withdraw (e.g., Nigg, 2006; Gray, 1982). Approach behaviors involve a willingness to move toward stimuli perceived as being either an incentive or rewarding, and are strongly associated with extraversion and positive affect, and in extremes, externalizing behavior problems. Conversely, withdrawal behaviors involve a preparedness to move away when stimuli are viewed as unrewarding or uncertain and are associated with negative affect (fear, anxiety, sadness), neuroticism, and in excess, internalizing behavior problems. The neurological mechanisms underlying the approach and withdrawal systems suggest they are separate yet related systems with different developmental trajectories; the approach system develops sooner than the withdrawal system (Putnam & Stifter, 2002). Research exploring EEG asymmetry highlights the uniqueness within each system. Withdrawal behaviors have been associated with greater relative right frontal EEG asymmetry (Buss, Malmamstadt, Dolski, Kalin, Goldsmith, & Davidson, 2003) while approach behaviors have been linked to greater relative left frontal EEG asymmetry (Potegal, Goldsmith, Chapman, Senulis, & Davidson, 1998). Relationships between physiological indices and approach/withdrawal behaviors reflect how interconnected the systems are. Specifically, while increases in HPA activity are related to behavioral inhibition (Kagan, Resnick, & Snidman, 1988; Kalin, Shelton, Rickman, & Davidson, 1998; Buss et al., 2004), decreases in HPA activity have also been associated with anger, an approach behavior (Buss & Goldsmith, 2007). However, both approach and withdrawal behaviors have been associated with SNS influenced heart rate activity (Beauchaine, Gatzke-Kopp, & Mead, 2007; Buss et al., 2004).
Recently, there has been renewed interest in the integration of sAA into behavioral, developmental, and health-oriented research as a surrogate marker of SNS activation. A series of studies causally linked levels of sAA to the SNS component of the stress response (e.g., Chatterton, Vogelsong, Lu, Ellman, & Hudgens, 1996; Chatterton, Vogelsong, Lu, & Hudgens 1997; Skosnik, Chatterton, Swisher, & Park, 2000). That is, levels of sAA increase under both physically (e.g., exercise, heat and cold stress) and psychologically (e.g., written examinations) stressful conditions known to increase plasma catecholamines (Chatterton et al., 1996). Stress-related increases in sAA can be inhibited by adrenergic blockers (e.g., propranolol) and adrenergic agonists (e.g., yohimbine) are capable of stimulating sAA release (Gallacher & Petersen, 1983; Speirs, Herring, Cooper, Hardy & Hind, 1974). To the best of our knowledge, only a few studies have assessed the effects of stress or challenge on sAA in young children (see Granger et al., 2006) and no studies have yet employed sAA to explore more fine grained and specific relationships between individual differences in sAA activity and child emotionality or approach and withdrawal behaviors.
As the depth of knowledge about such relationships so limited, we review the literature utilizing cardiovascular indices of SNS activity, preejection period (PEP), and affective behaviors in an attempt to justify the nature of questions asked in the present study. PEP is the time (in milliseconds) between the onset of the ventricular contraction and the opening of the semilunar valve in the heart, and is considered a relatively “pure” measure of SNS influence on heart rate (e.g., Bernston, Cacioppo, Binkley, Uchino, Quigley & Fieldstone, 1994). Shorter PEP is associated with more SNS activity. At least one study with adults confirms the expected relationship that shorter PEP in response to stress correlated with increased sAA reactivity (e.g., West, Granger, Kivlighan, Psota, & Hurston, 2006). Some research and theory suggests that SNS activation plays a key role in withdrawal behaviors (e.g., Cannon, 1928; Kagan et al., 1987; Kagan, 1994). Withdrawal behaviors, characteristic of a fearful temperament profile, were associated with faster resting PEP measured during a subsequent laboratory visit (Buss et al., 2004). Decreases in PEP from a resting period were associated with withdrawal behaviors (fear and sadness) in 3 to 8-year old children during emotional challenges (e.g., Alkon et al., 2003); yet, other work fails to find associations between PEP and affect during emotion tasks (e.g., Buss et al., 2005; Quas, Hong, Alkon, & Boyce, 2000; Oosterman & Schuengel, 2007). Recently, Beauchaine and colleagues (2007) presented evidence that faster PEP is associated with reward seeking, approach behaviors. Taken together these findings suggest that SNS activation, as measured by PEP, underlies both approach and withdrawal behaviors. Beauchaine (2001) proposed that during environmental stressors, SNS activation is necessary for a person to modulate energy resources that facilitate approaching as well as withdrawing from stimuli. In addition, Nigg (2006) speculated that SNS activity may relate to overall arousal in the form of joint activation of both approach and withdrawal behaviors. Thus, it appears possible that individual differences in sAA may be associated with approach behaviors, withdrawal behaviors, or both. Clearly, it would be an advance to more specifically delineate when, if, and for whom, SNS activity (e.g., sAA) is associated with approach behaviors, withdrawal behaviors, or overall arousal.
Multi-System Measurement of the Psychobiology of the Stress Response
Nigg’s (2006) model, like many others (e.g., Bauer et al., 2002; Granger & Fortunato, 2008), calls for a multi-operational and multi-system measurement of biological processes in behavioral research. The assessment of biological analytes in saliva has enabled researchers to do so non-invasively, in ecologically valid social contexts, and in special populations (i.e., young children). This study assesses two salivary analytes that mark the activity of the main components of the psychobiology of stress-- the HPA axis and SNS. Cortisol is the primary product of the HPA axis (e.g., Kirschbaum & Helhammer, 1994). Although not entirely consistent, literature reveals that individual differences in cortisol levels and stress-related reactivity are associated with behaviors related to psychological distress, social inhibition, and negative emotionality (e.g., Stansbury & Gunnar, 1994; Uchino, et al., 1996; Granger, Stanbury, & Henker, 1994; Buss et al., 2004). However, a few studies have linked individual differences in HPA axis activation to uninhibited and exuberant behaviors (de Haan, Gunnar, Tout, Hart, & Stansbury, 1998; Donzella, Gunnar, Krueger, & Alwin, 2000; Tout, de Haan, Kipp-Campbell, & Gunnar, 1999).
In addition, there is mounting evidence that sAA activity is positively correlated with the acute SNS stress response in children and adults (e.g., Gordis et al., 2007; Hill-Soderlund et al., 2008; Nater et al., 2006; Rohleder, Wolf, Mablonada, & Kirschbaum, 2006). Unlike most salivary analytes that are actively transported or passively diffused into saliva from plasma (e.g., cortisol, testosterone), sAA is an enzyme that is locally produced in the oral mucosa of salivary glands. The salivary glands are innervated by sympathetic and parasympathetic nerves and salivary secretions from glands (e.g., parotid, submandibular, and sublingual) arise in response to neurotransmitter activation. This suggests that sAA is a prime candidate to specify autonomic activity (Garrett, 1999; Nater et al., 2005). Moreover, studies by Chatterton and colleagues have linked sAA to the SNS component of stress response (e.g., Chatterton, Vogelsong, Lu, Elman, & Hudgens, 1996; Chatterton, Vogelsong, Lu, & Hudgens, 1997). Specifically, these studies suggested that plasma norepinephrine concentrations associated with the stress response of the locus ceruleus/autonomic nervous system in humans can be estimated via concentration of sAA. There remains large gaps in our knowledge regarding which particular affective behaviors are associated with sAA activity in young children.
Further, Bauer, Quas and Boyce (2002) propose that the coordination between HPA and SNS activity in response to stress has implications for predicting individual differences in social behavior. In Bauer and colleagues’ additive model, the HPA and SNS contribute independently. It is assumed that adaptation to stress is optimal when there is a medium level of arousal, when both systems show moderate activity, or when one system has high activity and the other system has low activity. Problem behavior resultes when SNS and HPA activity are both either low or high. Conversely, in the interactive model, adaptation is optimal when arousal across systems is balanced, such that both systems have either high or low activity. Aberrant behavior results when these systems are dissociated, with either high HPA and low SNS activity, or low SNS and high HPS activity, in response to stress.
Three independent studies employing sAA and salivary cortisol support Bauer and colleagues assumptions (El-Sheikh et al., 2008; Gordis et al., 2006, 2007; Stroud et al., in press). For example, Gordis, Granger, Susman and Trickett (2006) reported that interactions between cortisol and sAA predicted externalizing behavior problems. The highest levels of aggression among adolescents were associated with low cortisol reactivity and low sAA reactivity (low-low, supporting the additive model), and early adolescents showing the lowest levels of agreession had high cortisol reactivity and low sAA reactivity (low-high, again supporting the additive model). Also, El-Sheikh and colleagues (2008) reported a similar pattern of results -- the high (cortisol)-high (sAA) pattern was linked to internalizing symptoms. Moreover, the lowest levels of internalizing and externalizing problems were found among children with lower cortisol and higher sAA activity, (or higher cortisol and lower SNS activity). Collectively, these findings imply that our understanding of the links between affective behavior and HPA axis activation in early childhood may be incomplete because these associations may be moderated by concurrent activity of the SNS.
The Present Study
The present study is unique in that it tested the joint and independent relationships between SNS and HPA activity with affective behavior during the toddler period. Toddlers were observed in the lab during challenge tasks designed to elicit behaviors and emotions ranging from approach behaviors and positive affect to withdrawal behavior and negative affect. Saliva, later assayed for sAA and cortisol, was collected before and immediately after the challenge tasks. Specifically, we speculated that the relationship among SNS activity, HPA activity, and affective behaviors in toddlers would reveal three potential patterns. One possibility was that individual differences in SNS and HPA activity would show parallel relationships with affective behavior. In this case, we speculated that pre- and/or post-challenge sAA and cortisol activity would be associated with negative affect and withdrawal behavior. Alternatively, it is possible that HPA activity co-varies with specific affective behavior, but SNS arousal parallels the frequency or intensity of the predominant affective behavior in this context. Thus, in contrast to cortisol, it may be that sAA pre- and/or post-challenge series sAA activity covaries with affective behaviors that are most frequent or intense, regardless of valence (i.e., “differential fractionation,” Stern et al., 1980). A third possibility is that sAA activity reflects overall physiological arousal, and therefore, sAA levels will not be associated with any particular affective behavior, but with the total amount of affective behaviors. Finally, following Bauer and colleagues (2003) theoretical model, and the recent findings by our colleagues (Gordis et al., 2006; Stroud et al, in press; El-Sheikh et al., 2008), we hypothesized that individual differences in sAA levels would moderate the relationship between cortisol and the affective behavior.
Method
Participants
One-hundred and eleven toddlers (M = 24.05 months, SD = 1.52) and their primary caregiver participated in a laboratory visit embedded within a larger study of emotional development. Typically developing, healthy, toddlers and their families were recruited, via letter, from local, published, birth announcements. Participants were primarily Caucasian (93% Caucasian, 3% African American, 1% Hispanic, 2% Asian American, and 1% American Indian) and middle class (mean Hollingshead index = 48.65, range = 17 to 66), with a relatively equal number of boys (n = 62) and girls (n = 49). The 87 children included in these analyses (46 girls) were a subset who donated sufficient saliva sample volumes (at least 50 ul) for cortisol assays pre-task and at two post-task times. After the cortisol assays were complete, 54 toddlers had sufficient residual sample volumes (at least 10 ul) to be assayed for sAA (n = 26 girls). Comparisons of toddlers who did and did not provide pre-task and post-task saliva samples showed no statistical differences (p < .05) between groups in positive affect, negative affect, approach, or withdrawal.
Procedure
Families were contacted and consented by letter, filled out a packet of questionnaires (not used in the current analyses), and invited to a child development laboratory in a small city in the Midwestern United States for a 90 minute assessment session. Upon arrival the parent completed a short interview with the primary female experimenter about expectations of their toddler’s behavior and provided information about their toddler’s health and sleep patterns for that day. The primary female experimenters conducted all sessions and were graduate students and research staff trained by the fourth author on all procedures. The experimenter then collected a saliva sample from the toddler (see below). Next, toddlers participated in a series of tasks modified from the Toddler and Preschool Laboratory Temperament Assessment Battery (Lab-TAB: Goldsmith et al., 1994; Buss & Goldsmith, 2000) and provided a second saliva sample at the conclusion of the task series. A third saliva sample was collected 20 minutes after the conclusion of the laboratory visit. Mothers were present for all activities but the experimenter instructed her not to initiate interactions with her toddler and to remain affectively neutral. Activities were ended early if either the mother or the experimenter felt the child was becoming too distressed (e.g., hard crying for 30 sec or more); however children rarely became extremely distressed.
Challenging tasks
Toddlers participated in a series of 12 tasks.1 Each of the tasks lasted between 2 and 5 minutes and were completed in one of two laboratory rooms with dimensions approximately 4 × 3.75 meters. The first task was the modified Risk Room paradigm designed to assess behavioral inhibition when given opportunities to play with novel toys (see Buss et al., 2004). The room contained a tunnel (3 meters), a balance beam (1 meter), a large black box (approximately 1×1 meter) with painted eyes and a mouth opening, and a rubber Gorilla mask placed on a wooden stand (approximately 1 meter high). Three of the tasks involved the toddlers interacting with unfamiliar adults (trained undergraduate psychology students); Stranger Approach (a short conversation with a male stranger while the child played with toys), Stranger Working (an unfamiliar female stranger completing paperwork in the corner and did not initiate interaction with the toddler), and a Clown (an energetic female clown wearing a wig and nose who invited the toddler to play catch with beach balls, blow bubbles, and play with musical instruments). In these three interactions with unfamiliar adults, the toddler was in the room with his/her mother and the unfamiliar adult.
Three other episodes involved interactions with novel objects; a Robot (an 8in tall, unpredictable robot moving in a corner for 30 seconds before an experimenter enters and asked the toddler to touch the robot), a Spider (a .5m., black spider attached to a remote controlled car approached the toddler twice before an experimenter invites the child to touch the spider, e.g. Buss & Goldsmith, 1998), and a Puppet Show ( stuffed elephant and lion puppets invited the toddler to play catch, go fishing, and take a sticker). During these interactions with novel objects, the toddler was alone in the room with his/her mother and the objects.
Toddlers also participated in two joy/exuberance episodes with a familiar female experimenter; Balls in Baskets (a game of quickly throwing six brightly colored playground balls into a laundry basket) and Cascade Tower (cars going down a wooden ramp). A Free Play episode in which the toddler was alone in the room with his/her mother and the toys (3 minutes) was also included to assess children’s activity level and low-intensity pleasure. The additional two episodes assessed inhibitory control through games with the primary female experimenter; a Snack Delay task (children waited varying amounts of time before taking a snack that their mother selected – M&Ms, goldfish, or raisins) and Tower of Patience (turn taking game using 15 large blocks).
Behavioral Assessment
Raters viewed each videotaped episode and separately evaluated negative affect, positive affect, withdrawal, and approach using a 5-point Likert scale [1 = no affect/behavior shown, 2 = one or two displays of low intensity affect/behavior, 3 = many long displays of low intensity affect/behavior, 4 = a few intense displays of affect/behavior, or consistent display of low intensity affect/behavior, 5 = display of affect that lasts the whole episode or long displays of intense affect]. Positive affect scores were determined by the presence and intensity of the following behaviors: facial pleasure/smiling, laughter, and positive vocalizations or statements. Negative affect scores were determined by the presence and intensity of the following behaviors: facial fear, facial sadness, negative vocalizations or statements, and crying. A negative affect code of “5” was given for any episode which was ended early due to toddler distress. Approach scores were determined by the presence and intensity of the following behaviors: approaching stimuli, boldness/surgency, exuberance, and controlling the situation (i.e., changing the activity in some way). Withdrawal scores were determined by the presence and intensity of the following behaviors: shyness, hesitancy, withdrawal from stimuli, escape/leaving room, and avoidance of activity/interaction. For each task, 15% to 20% of cases were double coded, and discrepancies of more than 1 point were resolved by a master coder. Inter-coder reliability was calculated using intra-class correlations for each behavior and ranged from 0.62 to 0.89 for negative affect, 0.66 to 0.82 for positive affect, 0.77 to 0.86 for withdrawal, and 0.66 to 0.89 for approach. To reflect each child’s expression and experience across all 12 tasks, behavior ratings were aggregated for each target behavior. The positive affect and approach behavior aggregated ratings were significantly correlated, (r = .72, p < .001); while, negative affect was significantly associated with withdrawal behaviors, (r = .52, p < .001).
Although we were primarily interested in overall levels of positive affect, approach, negative affect, and withdrawal behaviors across the entire visit, we confirmed that by design, the episodes elicited the expected behaviors from toddlers. Examining the pattern of means for each behavioral code across the episodes revealed that negative affect (M range 1.23 to 2.74) and withdrawal (M range 1.27 to 2.96) were highest for the Spider, Robot, Risk Room, and the Stranger episodes. In contrast, positive affect (M range 1.85 to 3.22) and approach (M range 2.05 to 3.17) were highest for Puppet Show, Free Play, Balls in Basket, and Snack Delay.
Saliva Collection and Preparation
Parents were asked to restrict their toddlers’ consumption of food or drink for at least thirty minutes prior to donating saliva samples. Saliva samples were collected using the 1 × 4 cm cotton swab from the Salivette device (Sartstedt, Newton, NC). To encourage participation, toddlers were allowed to mouth the cotton roll after dipping it into sugar crystals. After collection samples were kept cold until the end of the visit when they were frozen at −50°. On average, the pre-task sample was collected at 11:38 am (SD = 3:11) and immediate post-task sample at 12:35 pm (SD = 3:41). Sample collection time was statistically controlled in all analyses.
Determination of Salivary Analytes
All samples were transported on ice to the Behavioral Endocrinology Laboratory at Penn State University and stored frozen at −80°C until assayed for cortisol and sAA. On the day of testing, all samples were centrifuged at 3000 rpm for 15 minutes to remove mucins.
Alpha-Amylase (sAA)
Samples were assayed by a kinetic reaction assay (Salimetrics, State College, PA) employing a chromagenic substrate linked to maltotriose (Granger et al., 2007). The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2 minute period) in absorbance at 405 nm. The test used 10 µL of saliva, results are computed in U/mL of sAA, and average intra-and inter-assay coefficients of variation (CV) are less than 10%.
Cortisol
All samples were assayed for salivary cortisol using an enzyme immunoassay US FDA (510k) cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, PA). The test used 25 µL of saliva, had a range of sensitivity from .007 to 3.0 µg/dL, with average intra-and inter-assay CVs less than 5% and 10%, respectively.
Data Transformation
As in previous studies (e.g., Gordis et al., 2006), salivary analyte distributions were strongly (cortisol) and modestly (sAA) skewed in a positive direction. Cortisol distributions were subject to natural log transformation; and sAA distributions to square-root transformations (Tabachnick & Fidell, 2001). The transformations normalized the data, and resulted in skewness statistics of less than .35 for all the cortisol and sAA distributions. After transformation, statistical outliers (< or > 3 STDEVs) were identified and recoded to the next highest value in the respective distribution. Transformed values were utilized in the statistical analyses but ug/dl (cortisol) and U/mL (sAA) units are presented in the text, table, and figures for descriptive purposes.
Results
Preliminary and Descriptive Analyses
T-tests and bivariate correlations (p < .05) were computed to determine demographic, procedural, behavioral, or health-related factors to include as covariates in the main analyses in which sAA and cortisol were dependent measures.2 These analyses revealed pre-task sAA scores were positively associated with time since waking, r (39) = .47, p < .01, and toddlers whose mothers reported they were “Warm or flushed” had higher sAA levels (M = 8.06; SD=.63) than toddlers who were not feeling this way (M =5.60; SD = 1.62) at the time of the assessment session, t (37) = 2.60, p < .05. All subsequent analyses involving pre-task sAA covaried these factors.
Next, for each salivary analyte a 2 (gender) by 2 (pre-task, post-task) mixed model ANOVA was computed with sampling time included as a repeated measure. Consistent with the diverse nature of the episodes, there were no main or interactive effects for either sAA or cortisol. However, an individual difference approach revealed that 56.9% of the toddlers’ sAA levels increased, and 31.4% decreased, by at least 10% from pre- to the immediate post task samplings. By comparison, 39.2% of the toddlers’ cortisol levels increased, and 51.0% decreased, by at least 10% from pre- to the immediate post task samplings. Table 1 presents means and standard deviations for sAA and cortisol by sample collection time. Within each sample collection time, sAA, and cortisol were not correlated.
Table 1.
Cortisol (ug/dl) and Salivary Alpha-Amylase (U/mL) means and (standard deviations) for toddlers before and immediate post-task and 20-minute post-task.
| Pre-task | Immediate Post-task | 20-min Post-task | |
|---|---|---|---|
| Alpha Amylase | 43.61 (35.05) | 65.75 (73.06) | 40.43 (59.21) |
| Cortisol | .23 (.24) | .17 (.13) | .15 (.11) |
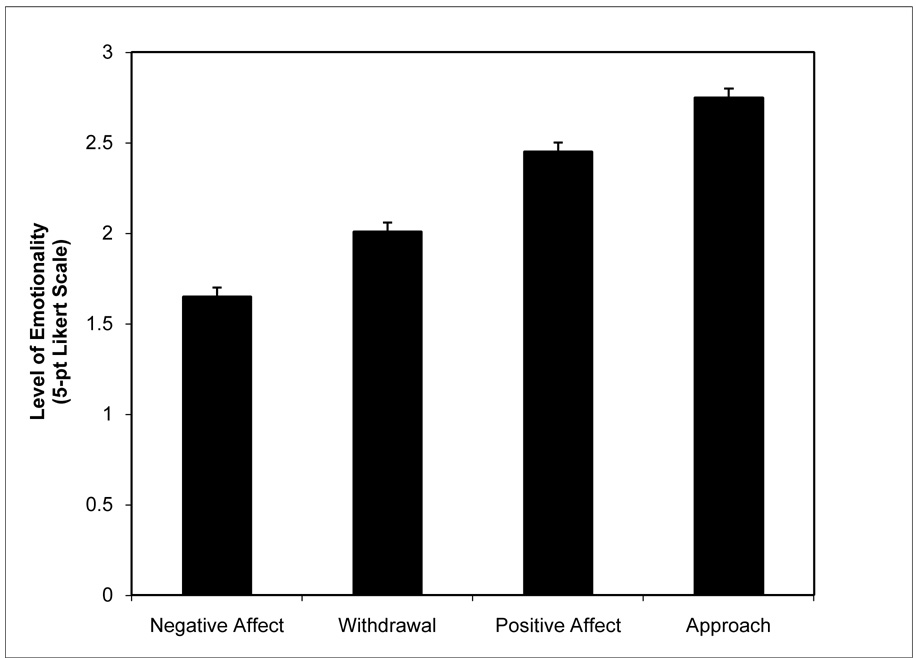
Finally, we computed descriptive statistics to characterize the overall behavioral landscape across all of the tasks. As can be seen in Figure 1, the most prevalent behaviors were approach (M = 2.78, SD = .11) and positive affect (M = 2.41, SD = .10) with negative affect (M = 1.78, SD = .10) and withdrawal (M = 2.14, SD = .11) being relatively less common across the entire visit.
Figure 1.
Mean affective behavior across the series of tasks demonstrate that approach and positive affect were the predominant behaviors.
Pre-task sAA, Cortisol, and Affective Behavior
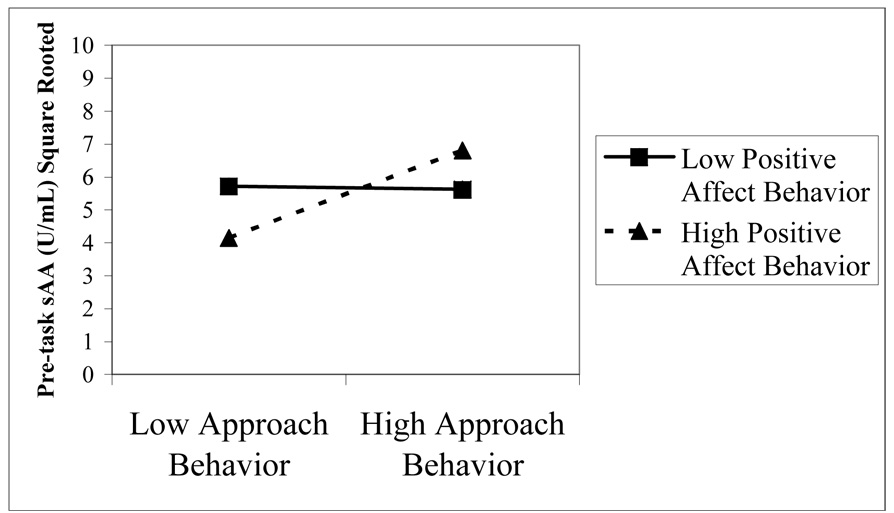
Regression equations were constructed to test the association between pre-task sAA and cortisol levels (separately) with each of the four behavior ratings scales as predictors. Covariates were entered in a first step and the behavior ratings were entered stepwise (p entry = .05). The analyses revealed that pre-task sAA levels was associated with positive affect (β=.28, t(47)=2.66, R2=.08, p<.05) and approach (β=.40, t(47)=3.00, R2=.15, p<.01). As expected, positive affect and approach behaviors were highly correlated r(71)=.52 , p < .001. Therefore, in follow-up analyses, we tested the main and interactive effects of these behavioral variables (both standardized) on pre-task sAA levels using a multiple regression approach. After adding the covariates, only approach was associated with sAA levels, β = .30, t (43) = 2.05, ΔR2=.06, p < .05. Interestingly, the interaction between approach and positive affect was associated with pre-task sAA levels, β = .35, t (43) = 2.76, p < .01, accounting for 11% of the variance. As recommended by Aiken and West (1991), the interaction was plotted (see Figure 2). When positive affect was low, approach behavior was not related to sAA pre-task levels. When positive affect was high and approach behavior was low toddlers had the lowest pre-task sAA levels. In contrast, when positive affect and approach were both high toddlers had the highest pre-task sAA levels. By contrast, pre-task cortisol levels were related only to the intensity of negative affect (β = .31, t (76) = 2.87, R2 = .10, p < .01).
Figure 2.
The interaction effect of approach and positive affect behavioral scores across the entire task series were significantly associated with pre-task sAA levels such that increased frequency/intensity of approach and positive affect behaviors were related to higher pre-task sAA levels; while, decreased frequency/intensity of approach and positive affect were related to lower pre-task sAA.
Post-task sAA, Cortisol, and Affective Behavior
Next, parallel regression equations were computed predicting post-task sAA and cortisol from the four behavior ratings. As expected, these equations revealed that negative affect (β = .27, t (83) = 2.53, R 2 = .08, p < .01), and withdrawal behaviors (β = .20, t (47) = 1.88, R2 = .05, p < .06) were associated with post-task cortisol levels. Negative affect and withdrawal were highly correlated r (73) = .72, p < .001, and, as above, we tested the main and interactive effects of these behavioral variables (both standardized) on post-task cortisol. There were no main or interactive effects of affective behavior ratings on post-task sAA. With both negative affect and withdrawal in the model, only negative affect had a main effect on cortisol levels, β = .65, t (61) = 3.45, ΔR2=.09, p < .001.
sAA and Cortisol Symmetry or Asymmetry: Relation to Affective Behavior
Following the logic of Bauer and colleagues (2002), each of the affective behaviors including positive affect, approach, negative affect, and withdrawal were regressed separately on baseline sAA and cortisol (both standardized), and on their interaction. There was a trend that the interaction between pre-task sAA and cortisol was associated with toddler’s approach, β= −.28, t (43) = −2.00, p =.05, accounting for 8% of the variance. When levels of pre-task sAA were high and cortisol levels were low, toddlers' displayed (slightly) less frequent and intense approach behaviors than if both pre-task sAA and cortisol levels were low.
Specific Affective Behavior or Simply Overall Affective Arousal?
A series of regression analyses tested the alternative hypothesis that overall arousal may be related to sAA activity. An overall, mean, behavioral arousal variable was computed by aggregating the global, affective behavior scores during each task and computing the means across the entire task series for each child. Also area under the curve (AUC) of the aggregated global, affective behaviors scores across the task series for each child was computed using the trapezoid formula with respect to ground (Pruessner et al., 2003). The formula is Σ [ti*(mi+mi+1)/2], where t is the interval between task i and task i+1, m is the aggregated level of the global behavior scores during task i for the overall AUC arousal variable, or m is the behavior score for each affective behavior. Regressions were computed using mean levels of behavioral arousal and AUC behavioral arousal as predictors, and sAA and cortisol as the dependent variable. Equations were constructed by first entering the order of the task series and then the mean or AUC levels for each of the different affective behaviors were entered stepwise. These regressions revealed that overall arousal across the task series measured by mean behavior or AUC did not significantly predict pre- or immediate post-task sAA or cortisol levels.
Discussion
To the best of our knowledge, this is the first demonstration of associative relationships between individual differences in SNS activity, as measured by sAA, and affective behaviors in toddlers. The findings suggest that individual differences in sAA levels were positively correlated with positive affect and approach, across this series of tasks and not simply overall levels of expressed emotion and affective behavior. We demonstrated that these behaviors also occurred with the greatest frequency and intensity. This is in contrast to the observation that salivary cortisol levels were associated specifically with individual differences in expression of negative affect and withdrawal behaviors. These findings underscore the idea that the affective, behavioral and contextual processes in early childhood that are correlated with SNS activation may be different than those processes correlated with HPA axis activation, and have several noteworthy implications.
The integration of sAA into developmental science is a relatively recent phenomenon. In fact, the first studies to do so were reported within the past few years (Granger et al., 2006). Not surprisingly, knowledge regarding the behavioral concomitants and correlates of individual differences in toddlers’ sAA activity is just now starting to accumulate. Thus, there are a few possible explanations for the main findings. First, individual differences in sAA activity are associated with positive affect and in particular those associated with approach behaviors and boldness. Second, these individual differences in sAA activity are not associated with these positive behavioral attributes specifically, but these associations are revealed because positive affective and approach behaviors were the predominant behaviors observed in the tasks. If this were the case, different patterns of sAA-behavior associations would be expected as shifting contextual demands across tasks influence the prevalence of other behavior patterns. A couple recent studies suggest this latter interpretation is veridical. In the only other study conducted with preschoolers to date, when sAA was measured in response to anger-inducing tasks (i.e., not-sharing task), higher levels of sAA were associated with higher levels of expressed anger (Spinrad, Eisenberg & Granger, 2007) associated with approach motivation. Also, when sAA was measured in response to a social exclusion task, adolescents with higher levels of internalizing problem behaviors showed the highest sAA reactivity (Stroud et al., in press). It is possible, that in contrast to cortisol, sAA-behavior relationships may be more situation-specific and depend on a confluence of contextual and person factors. Therefore, sAA reactivity can be associated with both approach and withdrawal behaviors. This notion fits in theory with the seminal work of Stanley Schachter (1966) revealing that an individual’s interpretation of autonomic nervous system arousal experimentally induced by injecting epinephrine depends on feedback from the immediate social context. Future work to explore this “predominant behavior hypothesis” seems well justified.
A third interpretation of our main findings involves possible temperamental differences in the baseline sAA levels of children. In the present study, pre-task saliva samples were collected approximately 10 minutes after children entered the laboratory for the first time. As sAA reactivity is found to peak approximately 10 minutes post stressor (Gordis et al., 2006; Stroud et al., 2006), it is possible that children who are temperamentally bold or exuberant showed pre-task sAA levels that actually reflected the novelty of entering the laboratory rather than a true baseline measure. So, the children who showed positive affect/approach behaviors during the tasks may have SNS systems that respond to novel situations. Using maternal report of temperament, we examined this possibility in a set of post-hoc correlations. Consistent with this interpretation, toddlers who were rated by mothers as high in positive emotionality and activity level and low in fear showed high positive affect across the tasks (rs = .19, .20, −.25 and ps < .05, respectively). In addition, toddlers rated low in fear exhibited more boldness across the tasks (rs = −.25, −.24 and ps < .05, respectively).
In addition, these findings corroborate what appears to be an emergent pattern linking individual differences in sAA levels/reactivity to social affiliation, positive emotions, active coping, and appropriate social behavior in toddlers and adults. In support of this interpretation, Kivlighan and Granger (2006) reported that higher sAA reactivity during competition was associated with higher levels of performance, dominance, and interest in team bonding in young adult collegiate athletes. Parallels between Kivlighan’s findings and the association between sAA and approach behaviors in the present study may be more than coincidental because performance, dominance, and team bonding are the predominant and appropriate behaviors expressed in competitive environments. Moreover, using a daily diary approach to capture momentary emotions in combination salivary samplings sAA, Adam and colleagues report positive/engaged adjectives in the diary (e.g., interested, concentrating, happy, strong, active, excited, cheerful, caring, low sleepy, low tired) as well as a positive mood and active/engaged factors that emerged from a factor analysis were positively associated with sAA levels (personnel communication, November 15, 2007). Using a similar approach with adults, Nater and colleagues (2007) report momentarily high levels of positive emotion were related to higher sAA levels (at least at the trend level). Likewise, in a study of 6-month olds, Kivlighan and colleagues (2005) informal observations revealed that infants with the highest sAA reactivity to an arm restraint task showed more appropriate emotion regulation strategies and exhibited the least distress.
Our observations support Bauer and colleagues’ general assumptions (Bauer et al., 2002) regarding the importance of SNS activity as a moderator of the links between HPA activity and behavior. That is, lower levels of cortisol in combination with higher levels of sAA, were associated with lower levels of positive affect and approach behavior. Recent reviews (e.g., Boyce & Ellis, 2005) hypothesized intricate effects of context on the coordination of individual differences in SNS and HPA activity with potential to impact differences in children’s developmental trajectories. Although these theories are well conceived and articulated, few empirical studies have specifically tested the core assumptions of these models. In contrast to Gordis et al., (2006; 2007) and El-Shiekh and colleagues (2008), the pattern observed here (lower levels of cortisol in combination with higher levels of sAA were associated with lower levels of approach) fits neither with Bauer’s Additive nor Interactive models. Clearly, studies are needed that investigate the intrinsic and contextual determinants of variability in the nature of the interactive relationship between affective behaviors and the coordination of the SNS and HPA axis. At the very least, our findings suggest that the generalizability of the additive and interactive models may be limited, and perhaps these concepts will require some fine tuning once sufficient empirical attention has focused to actually put the core assumptions to the test.
Limitations
In the shadow of this studies’ innovative approach and unique set of questions are several design limitations. First, due to the saliva sampling design, wherein post-task samples were taken approximately one hour (on average) after pre-task saliva sample, we were unable to assess the relationship between SNS and HPA reactivity to task-specific affective behaviors. Our sampling schema did not allow us to determine the reactivity pattern of sAA or cortisol because of the variation in time to peak levels following novelty or stress: approximately 10 minutes for sAA and approximately 20 minutes for cortisol (Gordis et al., 2006; Stroud et al., 2006; Dickerso & Kemeny, 2004).. Second, although there is preliminary evidence suggesting that overall sAA levels and not sAA reactivity is differentially correlated with approach and withdrawal behaviors encompassed in attachment status during the Strange Situation Paradigm (Hill-Sodurland et al., 2008), further research that utilizes a saliva sampling schema specific to both sAA and cortisol reactivity patterns is still warranted to determine associations with affective behaviors during different challenge tasks. Third, future studies with the correct saliva sampling schema could utilize designs in which children are randomly assigned to one of two tasks series: task series that primarily elicit negative affect/shyness or task series that mainly educe positive affect/boldness in toddlers. This would allow researchers to determine whether the predominant emotion over the whole series of tasks is associated with sAA levels or if positive behavioral affect is specifically linked to sAA levels in toddlers. Fourth, since some of the tasks in the series are designed to produce high stress reactivity in toddlers, selective attrition in toddlers with the highest stress reactivity could have occurred as a result of low saliva volume, overall non-compliance, and high behavioral reactivity (Granger et al., 2007). However, this is likely not the case in the present study as we found no significant differences in the affective behaviors of toddlers who did and did not provide pre and post-task saliva samples. Lastly, the sample size limits the power to test and detect associations between the affective behavior and the interaction between SNS and HPA activity. Moreover, the homogeneous nature of the sample of toddlers (primarily Caucasian and middle class) limits the generalizability of results. Overall, these limitations highlight that future studies should aim to incorporate physiological sampling designs that are specific to the multiple markers when examining affective behavioral correlates of SNS and HPA activity in diverse populations from varying levels of SES.
Conclusions
Despite these constraints, this study contributes to the literature by describing a novel pattern of bio-behavioral relationships in early childhood. The findings suggest that the physiological systems associated with stress response, SNS and HPA axis, may act differentially depending on the context-specific behaviors that are elicited. In particular, SNS activity, as measured by sAA levels, may be associated with the predominant, affective behaviors in a given situation or the specific behaviors or traits of positive affect and approach in toddlers; while, HPA activity is associated with the specific behaviors of negative affect and withdrawal. To determine the “value” of this phenomenon in relation to child development more generally, future work needs to test continuity and discontinuity in SNS and HPA-behavior associations across situations within individuals and developmental periods, and (more importantly) within individuals across development stages (Granger & Fortunato, 2008). It may be of particular importance to include sAA in conjunction with cortisol in studies that investigate children of varying higher-order, temperamental characteristics (e.g., exuberance and approach versus behavioral inhibition and withdrawal) across different contexts in order to determine if the predominant, affective behaviors elicited by children with different temperaments are associated with certain patterns of sAA, as well as sAA and cortisol interactions.
Table 2.
Regression analyses predicting mean approach across all tasks from pre-task sAA and cortisol levels.
| β | t | ΔR2 | FΔ | df | |
|---|---|---|---|---|---|
| Approach | |||||
| Step 1: Main Effects | |||||
| Pre-task sAA | .33 | .2.26* | .07 | 3.39 | 1, 46 |
| Pre-task Cortisol | −.07 | −.47 | .01 | .29 | 1, 45 |
| Step 2: Pre-task sAA × Pre-task Cortisol | −2.84 | −2.00 | .08 | 3.93* | 1, 44 |
p = .05
Acknowledgments
We thank the students and staff in the Emotion Development Lab for assistance with data collection and behavioral coding, and Mary Curran and Becky Hamilton at the Behavioral Endocrinology Laboratory for biotechnical support with salivary assays. This study was supported by a grant from NIH (MH67797) to Dr. Kristin A. Buss. In the interest of full disclosure, Dr. Granger is the founder and CEO of Salimetrics LLC.
Footnotes
The larger project from which the current study data are drawn was designed to examine both individual differences in emotional reactivity as well as contextual difference across the tasks. Given specific hypotheses about context-effects in the larger project, a counterbalancing of particular episodes was necessary. Toddlers were randomly assigned to 1 of 4 episode orders. Only the novel or higher threat tasks were counterbalanced (i.e., spider, robot, stranger interactions, puppet show, and clown). Risk room was the first episode during the visit for all toddlers because we wished to maximize the novelty of the lab room for that task. There were no significant effects of episode order on any of the variables used in the current study.
Sample time of day and order of task series were not associated with cortisol or sAA levels. Sample time of day and order of task series were included in all regression analyses, but were removed from all models because they revealed non-significant associations.
References
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;43:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology. 2004;40:583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Buss KA, Goldsmith HH. Fear and anger regulation in infancy: Effects on the temporal dynamics of affective expression. Child Development. 1998;69(2):359–374. [PubMed] [Google Scholar]
- Buss KA, Goldsmith HH. Manual and normative data for the Laboratory Temperament Assessment Battery – Toddler Version. University of Wisconsin-Madison: Psychology Department Technical Report; 2000. [Google Scholar]
- Buss KA, Goldsmith HH. Biobehavioral approaches to early socioemotional development. In: Brownell CA, Kopp CB, editors. Transitions in Early Socioemotional Development: The Toddler Years. New York: Guilford; 2007. [Google Scholar]
- Buss KA, Goldsmith HH, Davidson RJ. Cardiac reactivity is associated with changes in negative emotion in 24-month-olds. Psychobiology. 2005;46:118–132. doi: 10.1002/dev.20048. [DOI] [PubMed] [Google Scholar]
- Buss KA, Malmstadt-Schumacher J, Dolski I, Kalin NA, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdraw behavior in 6 month old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Neural organization for emotional expression. Feelings and motivations: the Wittenberg symposium. Worchester: Clark University Press; 1928. [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clinical Physiology. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Hudgens GA. Hormonal responses to psychological stress in men preparing for skydiving. Clinical Endocrinology and Metabolism. 1997;82:2503–2509. doi: 10.1210/jcem.82.8.4133. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Toward a biology of personality and emotion. Annals of the New York Academy of Sciences. 2001;935:191–207. doi: 10.1111/j.1749-6632.2001.tb03481.x. [DOI] [PubMed] [Google Scholar]
- de Haan M, Gunnar MR, Tout K, Hart J, Stansbury K. Familiar and novel contexts yield different associations between cortisol and behavior among 2-year-olds. Developmental Psychobiology. 1998;31:93–101. doi: 10.1002/(sici)1098-2302(199807)33:1<93::aid-dev8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Donzella B, Gunnar MR, Krueger WK. Cortisol and vagal tone responses to competitive challenge in preschoolers: Associations with temperament. Developmental Psychobiology. 2000;37:209–220. doi: 10.1002/1098-2302(2000)37:4<209::aid-dev1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Erath SA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36(4):601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Fox N, Henderson H, Rubin K, Calkins S, Schmidt L. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gallacher DV, Petersen OH. Stimulus-secretion coupling in mammalian salivary glands. International Review of Physiology. 1983;28:1–52. [PubMed] [Google Scholar]
- Garcia-Coll C, Kagan J, Reznik JS. Behavioral inhibition in young children. Child Development. 1984;55:1005–1019. [Google Scholar]
- Garrett JR. Effects of autonomic nerve stimulations on salivary parenchyma and protein secretion. In: Garrett JR, Ekstrfm J, Anderson LC, editors. Oral Biology. Neural Mechanisms of Salivary Gland Secretion, Front; 1999. pp. 59–79. [Google Scholar]
- Goldsmith HH, Reilly HH, Lemery KS, Longley S, Prescott A. Manual for Preschool Lab-TAB. 1994 Unpublished. [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2007;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Fortunato C. Integration of salivary biomarkers and analytes into developmental science: A critical review of theory, tactics, and strategies. Monographs of the Society for Research in Child Development. 2008 Manuscript under review. [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua G-L. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Buckhalt JA, Stroud LR, Handwerger K, Schwartz EB. Integrating the measurement of salivary a-amylase into studies of child health, development, and social relationships. Journal of Personal and Social Relationships. Special Issue: Physiology and Human Relationships. 2006;23:267–290. [Google Scholar]
- Granger DA, Stansbury K, Henker B. Preschooler's behavioral and neuroendocrine responses to social challenge. Merrill-Palmer Quarterly. 1994;40:190–211. [Google Scholar]
- Gray JA. The neuropsychology of anxiety; An enquiry into the functions of the speto-hippocampal system. New York: Oxford University Press; 1982. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review in Psychology. 2006;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins S, Granger DA, Moore GA, Gariep JL, Cox MJ. Parasympathetic and sympathetic responses to the Strange Situation in infants and mothers from avoidant and securely attached dyads. Developmental Psychobiology. 2008;50(4):361–376. doi: 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- Kagan J. Galen’s prophecy: Temperament in human nature. New York: Basic Books; 1994. (Originally published in 1998) [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in young children. Child Development. 1987;58:1359–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. In: Chess S, Thomas A, Hertzig ME, editors. Annual progress in child psychiatry and child development. Philadelphia: Brunner/Mazel; 1989. pp. 102–127. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortiosl in infant and mother rhesus monkeys. Behavioral Neuroscience. 1998;112(1):251–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA. Stress responsivity to competition: Gender and experiential differences in salivary alpha-amylase and cortisol activity. Psychoneuroendocrinology. 2006;31:703–714. doi: 10.1016/j.psyneuen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Blair C The Family Life Project Investigators. Salivary alpha-amylase and cortisol: Levels and stress reactivity in 6-month-old infants and their mothers. Paper presented at the biennial meeting of Society for Research in Child Development; Atlanta, GA. 2005. Apr, [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity- associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Gaab J, Berger S, Berger S, Jud A, Kirschbaum C, Ehlert U. Human salivary alpha-amylase reactivity in psychosocial stress paradigm. International Journal of Psychophysiology. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47(3):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Oosterman M, Schuengel C. Autonomic reactivity of children to separation and reunion with foster parents. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1196–1203. doi: 10.1097/chi.0b013e3180ca839f. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Development. 2002;73:1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- Potegal M, Goldsmith HH, Chapman R, Senulis J, Davidson R. Tantrums, temperament, and temporal lobes; Paper presented at the International Society for Research on Aggression; Ramapo, NJ. 1998. Jul, [Google Scholar]
- Pruessner JA, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-depednent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Stifter CA. Development of approach and inhibition in the first year: parallel findings from motor behavior, temperament ratings and directional cardiac response. Developmental Science. 2002;5(4):441–451. [Google Scholar]
- Rohleder N, Wolf JM, Maldonado EG, Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43:645–652. doi: 10.1111/j.1469-8986.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- Quas JA, Hong M, Alkon A, Boyce WT. Dissociations between psychobiologic reactivity and emotional expression in children. Developmental Psychobiology. 2000;37:153–175. doi: 10.1002/1098-2302(200011)37:3<153::aid-dev4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Nelson LJ, Hastings P, Asendorpf J. The transaction between parents’ perceptions of their children’s shyness and their parenting styles. International Journal of Behavioral Development. 1999;23:937–957. [Google Scholar]
- Schachter S. The interaction of cognitive and physiological determinants of emotional state. In: Berkowitz L, editor. Advances in experimental psychology. New York: Academic Press; 1964. [Google Scholar]
- Skosnik PD, Chatterton RT, Swisher T, Park S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. International Journal of Psychophysiology. 2000;39:59–68. doi: 10.1016/s0167-8760(99)00100-2. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Speirs RL, Herring J, Cooper WD, Hardy CC, Hind CR. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Archives of Oral Biology. 1974;19:747–752. doi: 10.1016/0003-9969(74)90161-7. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Granger DA, Eisenberg N. Individual differences in preschoolers' salivary cortisol and alpha-amylase reactivity; Presented at the Biennial Meeting of the Society for Research on Child Development; Boston, MA. 2007. Mar, [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monogragraphs of the Society for Research on Child Development. 1994;59 1-8-134. [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Davis CM. PsychophysiologicalRecording. Oxford University Press; 1980. Brain: Electroencephalograph; pp. 88–98. [Google Scholar]
- Stroud LR, Foster E, Papandonatos G, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus social rejection stress. Development and Psychopathology. doi: 10.1017/S0954579409000042. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th ed. New York: Allyn and Bacon; 2001. [Google Scholar]
- Tout K, de Haan M, Kipp Campbell E, Gunnar MR. Social behavior correlates of cortisol activity in child care: Gender differences and time-of-day effects. Child Development. 1998;69:1247–1262. [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- West SG, Granger DA, Kivlighan KT, Psota TL, Hurston KL. Salivary alpha-amylase response to the cold pressor is correlated with cardiac markers of sympathetic activation; Presented at the annual meeting of the American Psychosomatic Society; Denver, CO. 2006. [Google Scholar]