Abstract
Rationale: By creating artificial communications through bronchial walls into the parenchyma of explanted lungs (airway bypass), we expect to decrease the amount of gas trapped and to increase the rate and volume of air expelled during forced expirations.
Objectives: To describe the mechanism by which airway bypass improves the mechanical properties of the emphysematous lung.
Methods: Lung compartments and mechanics were measured before and after airway bypass, which was created by placement of three or four stent-suppported fenestrations in 10 emphysematous lungs removed at transplantation surgery.
Measurements and Main Results: Minimal volume after passive deflation decreased by a mean of 1.54 L (range, 0.7–2.5 L) or 60% (range, 37–86%). Explanted VC increased by 1.30 L or 132% (range, 78–318%). Maximal expiratory flows and volumes increased. Flow resistance decreased.
Conclusions: Because these data show that airway bypass improves the mechanics of breathing in severely emphysematous lungs in vitro, there is now strong empirical support that this procedure can improve ventilatory function in patients by reducing gas trapping and flow resistance.
Keywords: transbronchial fenestrations, airway bypass, collateral ventilation
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Airway bypass has been proposed as a therapy for treatment of emphysema, but there is only limited information available on its physiologic effects.
What This Study Adds to the Field
Increased collateral ventilation achieved by stent-supported transairway fenestrations results in enhanced vital capacity and airflow as well as decreased airway resistance in lung removed from patients receiving transplantation for emphysema. These results suggest that airway bypass may improve ventilatory function in severely emphysematous lung.
Airway bypass is a therapy proposed for the treatment of emphysema (1, 2). The physiologic rationale is to tap into a region of trapped gas that cannot be expelled via the native bronchial tree and allow gas to escape. The procedure entails constructing artificial pathways or fenestrations through the wall of a bronchus into the surrounding parenchyma. Once the fenestration is made, drug-eluting stents are placed to maintain patency (3, 4). We report the mechanical properties of explanted emphysematous lungs before and shortly after the creation of three or four stent-supported fenestrations.
Lung volumes and capacities are defined by the structure of the lungs in concert with the function of the thorax; therefore, standard physiologic terms are not appropriate for use with explanted emphysematous lungs. This situation is acknowledged by the modified designation of E (for explant) to all the standard terms. We hypothesize that airway bypass should decrease the minimal lung volume, theoretically at zero transpulmonary pressure, which we call explanted residual volume (ERV), increase the explanted vital capacity (EVC), and thereby improve the mechanics of breathing. Measurements in the present study include explanted total lung capacity (ETLC), its components (EVC and ERV), explanted maximal expiratory flow volume (EMEFV) curves, static pressure–volume diagrams, and measurements of flow resistance. The purposes of this study were to determine how and why airway bypass improves mechanical function and whether the result might have potential therapeutic benefit for patients with advanced emphysema. Some of the results of these studies have been previously reported in the form of an abstract (5).
METHODS
We studied 10 severely emphysematous lungs explanted at lung transplantation. The Washington University institutional review board for human studies approved the protocol, and informed written consent was obtained from each patient. Preoperative clinical details are presented in the online supplement. The main bronchus was anastomosed to an appropriately sized polytetrafluoroethylene vascular prosthesis as described in previous reports (1, 6) and in the online supplement. The lung was inflated to ETLC as judged by visual inspection with a series of small-volume respiratory bag compressions delivered manually.
Minimal volume (ERV) was measured after inflation to ETLC and opening of the bronchus to room air for 5 minutes. The lung was then completely immersed in isotonic saline solution to measure volume by liquid displacement as described previously (7) and detailed in the online supplement. However, the volumes reported could be lower than ERV because the main bronchus was not occluded before complete immersion of the lung. Although the magnitude of any error is not known, it would be larger after than before airway bypass. Additional details are presented in the online supplement.
The lung was suspended by the bronchus to the central port atop a 25.2-L chamber as described previously (1, 7). The pneumotachograph was placed on a port to connect the chamber to room air. This allowed the lung to be inflated and deflated by changing pressure at the airway opening, and the chamber thus became a variable flow plethysmograph.
To measure two or three EMEFV curves, the lung was inflated with a syringe to a pressure of approximately 10 cm H2O and held for a few seconds before opening the bronchial connection with a solenoid valve to a 55-gallon pressure reservoir set at −10 cm H2O. This technique avoided the gas compression artifact that can distort MEFV curves (8). Additional details are presented in the online supplement.
Static pressure–volume diagrams were measured in duplicate with the chamber open to room air while the lung was inflated and deflated stepwise by 100- or 200-ml increments using a calibrated syringe and with a 3- to 5-second pause at each step for measurement of pressure. EVC was determined by averaging the mean data obtained from pressure–volume diagrams with the mean results for MEFV curves.
After the physiologic measurements were made, the lung was suspended in the chamber for bronchoscopic placement of three or four stent-supported fenestrations as previously described (3, 4), with at least two in the upper lobe. After the stents were in place, all physiologic measurements were repeated.
Explanted maximal expiratory flow static recoil curves were constructed by matching flow with lung elastic recoil pressures at identical volumes. This was done at four separate levels of lung inflation. Dividing the maximal flow into the pressure measured the resistance upstream of dynamically compressed airways (9).
Analysis of Data
Mean values are presented with their SD. Differences in mean values were analyzed with the Student's two-tailed t test (10).
RESULTS
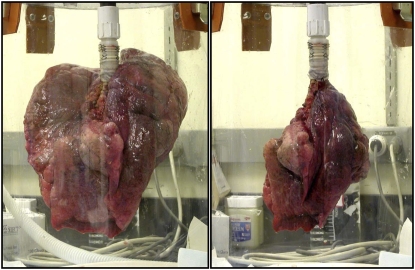
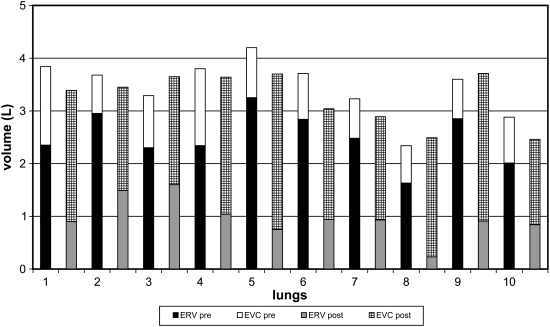
Figure 1 shows photographs of an emphysematous lung before and after airway bypass to illustrate the change in ERV. The results of ERV may be erroneously low after bypass, but the decompression of emphysematous lungs is unequivocal. The subdivisions of ETLC are shown before and after bypass for each lung in Figure 2. The numerical data are presented in Table E2 in the online supplement.
Figure 1.
Two photographs of the same emphysematous lung after inflation to total lung capacity and passive emptying with the bronchus open to room air for 5 minutes. The left panel shows the lung before airway bypass. The right panel shows the lung after placement of three transbronchial stents. The minimal lung volume (explanted residual volume) decreased markedly after airway bypass.
Figure 2.
Bar graph illustrating the change in lung compartments after airway bypass with three or four transbronchial stents. For each lung number on the abscissa, the vertical bar directly above the numeral depicts explanted residual volume (ERV) and explanted vital capacity (EVC) before bypass, and the bar on the right shows the same parameters in each lung after bypass. The reduction in ERV was uniformly accompanied by an increase in EVC.
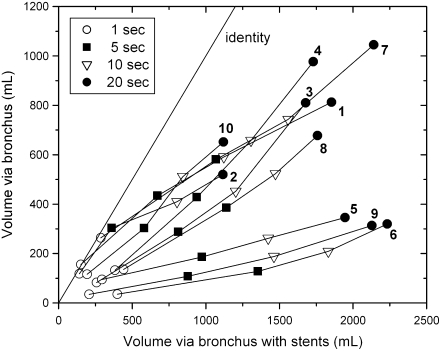
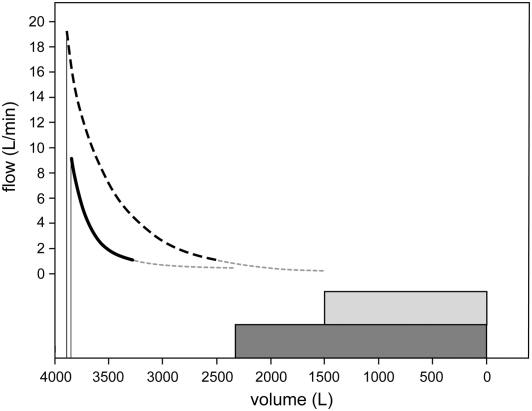
Figure 3 shows mean results of EMEFV curves in all 10 lungs as an isotime identity plot of volumes expelled at identical times during expiration before (ordinate) and after (abscissa) bypass. The diagonal is the line of identity, and each point indicates the time after onset of expiration with specific symbols. More volume escaped from the lung after bypass. In addition, the slope of any line joining two time points gives the ratio of the mean expiratory flow before bypass to that after bypass during the time range indicated by the symbols. Half of these ratios were less than 0.5, and only one exceeded 1.0. The numerical data are presented in Table E3. Individual but representative EMEFV curves before and after bypass are plotted in Figure 4, with ERV results in showing the differences in flow and volume after stent placement.
Figure 3.
Isotime identity plot of volume–time curves before and after airway bypass for each of the 10 lungs studied. Pre-bypass volumes are plotted on the ordinate at four points in time, and post-bypass data at these same time points are plotted on the abscissa. The diagonal line marks the line of identity. Additional considerations are presented in the text, and the data are presented in Table E3.
Figure 4.
Individual but representative explanted maximal expiratory flow volume curves from lung 2, plotted together with the explanted residual volume measurements determined by liquid volume displacement and depicted by the shaded panels before (the smaller curve) and after airway bypass (the larger curve). Peak flow occurs roughly 0.2 seconds after the onset of each curve. The thick solid lines (before bypass) and dashed lines (after bypass) depict recorded data. The light lines connect these to the previously measured explanted residual volume values. After airway bypass, the rate and volume of flow increased significantly. Additional details are presented and discussed in the text and the data are presented in Table E3.
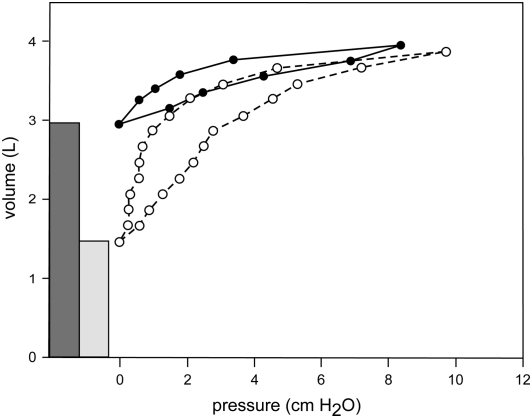
Static pressure–volume curves are shown before and after airway bypass in Figure 5. This emphasizes the marked decrease in ERV and the increase in EVC. The effects of bypass on these curves together with values of static compliance estimated as the slope of a tangent to the curve at 40% EVC of the deflation limb are discussed in detail in the online supplement, and the data are shown in Table E4. The compliance results are variable, but the pattern shows statistically significant increases after airway bypass. Individual measurements are provided in Table E5.
Figure 5.
Individual but representative static pressure volume diagrams from lung 2, plotted with explanted residual volume determined from liquid displacement and depicted by the shaded bars before and after airway bypass. The solid lines (before bypass) and dashed lines (after bypass) depict recorded data. Additional details are discussed in the text, and the data are presented in Table E4.
Upstream resistances calculated from the explanted maximal expiratory flow static recoil data (9) averaged 53 cm H2O/L/second prebypass. In sharp contrast, after stent placement, the mean value was 10 cm H2O/L/second. These data confirm a significant reduction of airflow resistance after airway bypass (P = 0.003). Individual measurements are provided in the online supplement.
DISCUSSION
Airway bypass produces a striking reduction in the minimal volume of explanted emphysematous lungs, as illustrated in Figure 1, and measured after prolonged passive deflation from full lung inflation. The values reported could be erroneously low. The magnitude of any error is not known but is greater after than before airway bypass. The immersion was brief, and we believe the errors are unlikely to be large. Even with allowance for moderate error in reported ERV, the decompression of explanted emphysematous lungs remains unequivocal and impressive. The data from 10 lungs are shown in Figure 2. This topic is discussed in more detail together with numerical data in Table E2.
The creation of new pathways for the flow of air into and out of the distal lung units allows trapped gas to escape, decreases resistance to the flow of air, and thereby provides improved lung mechanics. Expiratory flows and volumes regularly increase (Figure 3). The data after bypass are displaced from the line of identity toward increased volume. Individual but representative EMEFV curves emphasize this point in Figure 4. The uniform occurrence of increased expiratory flow suggests the artificial airways created by stent-supported fenestrations also decrease airway resistance. This was confirmed by the reduced upstream resistance, which dropped from 53 to 10 cm H2O/L/second after bypass. The numerical data are presented in Table E5 with additional details about the calculations in the online supplement.
Lung volume reduction surgery (LVRS) prompted the search for pulmonary function studies to predict successful surgical outcomes. The RV/TLC ratio was identified as a reliable index of the mismatch in size between hyperinflated lungs and the thorax in patients with advanced emphysema by Fessler and colleagues (11–13). By stepwise multiple regression analysis, an RV/TLC ratio above 0.65 was identified as the only independent preoperative predictor of an improved VC after LVRS. RV/TLC does not predict an increase in FEV1 because its two determinants, FVC and FEV1/FVC, correlate in opposite directions. As RV/TLC increases, the postoperative prediction of FEV1/FVC decreases, whereas the postoperative prediction of FVC increases. The percentage of change in predicted FVC and FEV1/FVC intersect, with values around 25% at the RV/TLC ratio of 0.7. FEV1 does not increase postoperatively in the absence of an increase in FVC.
The present study included measurements of EFEV1 (Figure 3); results follow the principles described by Fessler and colleagues (13). Airway bypass, like LVRS, improves lung mechanics in the emphysematous lung by reducing the volume of trapped gas. By doing so, the EVC uniformly increases, and the ERV/ETLC ratio decreases. However, the ratio of EFEV1/EFVC did not change in this study after airway bypass. Bypass produced an unexpectedly large decrease in airway resistance, but the fenestrations had no effect on the changes in elastic structural abnormalities of the emphysematous lung, and the elastic properties of the lung remain unchanged. The increased static compliance resulted from the increased volume of participating airspaces. This differs from the result of LVRS, where the removal of large cystic airspaces may lead to reexpansion and ventilation of previously atelectatic parenchyma with more nearly normal structure and increased elastic recoil as compared with the excised lung.
Lausberg and colleagues reported that three stent-supported fenestrations led to an increase in EFEV1 approaching 100% in explanted emphysematous lungs, with even greater increases after the addition of two more stents (1). Choong and colleagues substantially reduced trapped gas through transpleural communications in explanted emphysematous lungs; in addition, using a hyperpolarized helium technique, they measured a greater volume of lung parenchyma ventilated transpleurally than through the tracheobronchial tree (7). This was evidently the case with airway bypass.
Airway bypass with drug-eluting stents is being investigated in patients with severe emphysema in a large, multicenter, phase 3 clinical trial. Phase 2 studies have shown that 1 day postprocedure, patients had reductions in RV, increases in VC and FEV1, and improvements in dyspnea. At 6 months, patients with markedly hyperinflated lungs (as assessed by the RV/TLC ratio) before the procedure showed a 1.1-L mean reduction in RV and a 43% reduction in dyspnea as assessed by the modified Medical Research Council dyspnea scale and a modest but significant increase in FVC (5).
This further suggests that the plausible therapeutic benefit of bypass could result from trapped gas escaping via artificial airways and decreased flow resistance. Subsequent partial occlusion of the bronchial–parenchymal communications reversed the latter so that the initial improvement in FEV1 was transient, whereas the remaining slow leak allowed the RV to remain decreased. Because we breathe about 25,000 times per day, a small leak allowing expiration of only 0.1 ml/breath greater than inspiration would in 24 hours result in the loss of 2.5 L of trapped gas.
These experiments demonstrate an improvement of lung mechanical properties unobtainable by other therapeutic interventions in emphysema except lung transplantation. The results reported here represent the potential for improvement in patients with severe emphysema.
Supplementary Material
Acknowledgments
The authors thank Drs. G. Alexander Patterson and Bryan F. Meyers, Diane Toeniskoetter for technical assistance, Dawn Schuessler and Nai-Lun Chang for editorial assistance, and Drs. Peter G. Tuteur and Stephen S. Lefrak for editorial suggestions.
Supported by National Institutes of Health grant R01 HL62194.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200712-1832OC on July 31, 2008
Conflict of Interest Statement: C.K.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.T.M. is a paid consultant and chair of the Scientific Advisory Board for Broncus Technologies. For these activities, P.T.M. received Can $17,007 in 2005 and Can $23,100 in 2006. J.A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.A.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.O.M does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.D.C. receives a consulting fee from Broncus, Inc. This is considered by Broncus as an advance against potential royalties due to J.D.C. for his development of the lung bypass procedure.
References
- 1.Lausberg HF, Chino K, Patterson GA, Meyers BF, Toeniskoetter PD, Cooper JD. Bronchial fenestration improves expiratory flow in emphysematous human lungs. Ann Thorac Surg 2003;75:393–397. [DOI] [PubMed] [Google Scholar]
- 2.Rendina EA, De Giacomo T, Venuta F, Coloni GF, Meyers BF, Patterson GA, Cooper JD. Feasibility and safety of the airway bypass procedure for patients with emphysema. J Thorac Cardiovasc Surg 2003;125:1294–1299. [DOI] [PubMed] [Google Scholar]
- 3.Choong CK, Haddad FJ, Gee EY, Cooper JD. Feasibility and safety of airway bypass stent placement and influence of topical mitomycin C on stent patency. J Thorac Cardiovasc Surg 2005;129:632–638. [DOI] [PubMed] [Google Scholar]
- 4.Choong CK, Phan L, Massetti P, Haddad FJ, Martinez C, Roschak E, Cooper JD. Prolongation of patency of airway bypass stents with use of drug-eluting stents. J Thorac Cardiovasc Surg 2006;131:60–64. [DOI] [PubMed] [Google Scholar]
- 5.Macklem PT, Cardoso P, Snell G, Hopkins P, Sybrecht GW, Pierce J, Cooper J. Airway bypass: a new treatment for emphysema. Proc Am Thorac Soc 2006;3:A726. [Google Scholar]
- 6.Choong CK, Haddad FJ, Martinez C, Hu DZ, Pierce JA, Meyers BF, Patterson GA, Cooper JD. A simple, reproducible, and inexpensive technique in the preparation of explanted emphysematous lungs for ex vivo studies. J Thorac Cardiovasc Surg 2005;130:922–923. [DOI] [PubMed] [Google Scholar]
- 7.Choong CK, Macklem PT, Pierce JA, Lefrak SS, Woods JC, Conradi MS, Yablonskiy DA, Hogg JC, Chino K, Cooper JD. Transpleural ventilation of explanted human lungs. Thorax 2007;62:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingram RH Jr, Schilder DP. Effect of gas compression on pulmonary pressure, flow, and volume relationship. J Appl Physiol 1966;21:1821–1826. [DOI] [PubMed] [Google Scholar]
- 9.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximal expiratory flow. J Appl Physiol 1967;22:95–108. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy S, Weardon S. Statistics for research. New York: John Wiley & Sons; 1983.
- 11.Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med 1998;156:715–722. [DOI] [PubMed] [Google Scholar]
- 12.Fessler HE, Wise RA. Lung volume reduction surgery. Am J Respir Crit Care Med 1999;159:1031–1035. [DOI] [PubMed] [Google Scholar]
- 13.Fessler HE, Scharf SM, Permutt S. Improvement in spirometry following lung volume reduction surgery. Am J Respir Crit Care Med 2002;165:34–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.