Abstract
Rationale: The effectiveness and safety of aztreonam lysine for inhalation (AZLI) in patients with cystic fibrosis (CF) on maintenance treatment for Pseudomonas aeruginosa (PA) airway infection was evaluated in this randomized, double-blind, placebo-controlled study.
Objectives: To evaluate the safety and efficacy of inhaled aztreonam lysine in controlling PA infection in patients with CF.
Methods: After randomization and a 28-day course of tobramycin inhalation solution (TIS), patients (n = 211; ⩾6 yr; ⩾3 TIS courses within previous year; FEV1 ⩾ 25% and ⩽75% predicted values) were treated with 75 mg AZLI or placebo, twice or three times daily for 28 days, then monitored for 56 days. The primary efficacy endpoint was time to need for additional inhaled or intravenous antipseudomonal antibiotics. Secondary endpoints included changes in respiratory symptoms (CF Questionnaire-Revised [CFQ-R] Respiratory Scale), pulmonary function (FEV1), and sputum PA density. Adverse events and minimum inhibitory concentrations of aztreonam for PA were monitored.
Measurements and Main Results: AZLI treatment increased median time to need for additional antipseudomonal antibiotics for symptoms of pulmonary exacerbation by 21 days, compared with placebo (AZLI, 92 d; placebo, 71 d; P = 0.007). AZLI improved mean CFQ-R respiratory scores (5.01 points, P = 0.02), FEV1 (6.3%, P = 0.001), and sputum PA density (−0.66 log10 cfu/g, P = 0.006) compared with placebo; no AZLI dose–response was observed. Adverse events reported for AZLI and placebo were comparable and consistent with CF lung disease. Susceptibility of PA to aztreonam at baseline and end of therapy were similar.
Conclusions: AZLI was effective in patients with CF using frequent TIS therapy. AZLI delayed time to need for inhaled or intravenous antipseudomonal antibiotics, improved respiratory symptoms and pulmonary function, and was well tolerated.
Clinical trial registered with www.clinicaltrials.gov (NCT 00104520).
Keywords: cystic fibrosis, Pseudomonas, aztreonam, inhaled antibiotics, patient-reported outcomes, respiratory symptoms
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Cystic fibrosis is a chronic disease often involving endobronchial infection with Pseudomonas aeruginosa, which is difficult to treat.
What This Study Adds to the Field
Safety and efficacy data on inhaled aztreonam show that this new formulation may be an alternative treatment option for patients with cystic fibrosis and chronic P. aeruginosa infection.
Cystic fibrosis (CF) is an autosomal recessive disease characterized by pancreatic insufficiency and thick tenacious pulmonary secretions, which often lead to airway infection (1). Among older patients, the most common pathogen in CF airway infections is Pseudomonas aeruginosa (PA); these infections are associated with an accelerated decline in pulmonary function and increased mortality (2–4).
Over the past 15 years, management of patients with CF has improved (1, 2, 5–8). However, antimicrobial treatment options for chronic PA airway infections remain limited and additional therapies are needed to augment improvements in clinical outcomes.
Aztreonam lysine for inhalation (AZLI) is an aerosolized formulation of the monobactam antibiotic aztreonam and lysine (9). The intravenous aztreonam formulation contains arginine, which can cause airway inflammation after chronic inhalation therapy in patients with CF (10, 11). The study described herein included patients with CF who frequently used antibiotics for PA airway infections and were generally compliant with the recommended standard of care (12). The study assessed the effectiveness of AZLI in this intensively treated patient population after pretreatment with tobramycin inhalation solution (TIS), and compared patient responses to AZLI dosed twice or three times daily. Early treatment of pulmonary exacerbations has resulted in fewer hospitalizations; thus, need for hospitalization has become less clinically relevant as a study endpoint (13–15). Therefore, clinical deterioration was assessed with a new outcome measure: time to need for additional antipseudomonal antibiotics to treat symptoms indicative of pulmonary exacerbation (16). Patient-reported improvements in clinical symptoms were measured with the Cystic Fibrosis Questionnaire–Revised (CFQ-R), a validated, health-related, quality-of-life measure (17–19). An established efficacy measure, change in FEV1, was also included. This combination of endpoints provided a broad view of patient responses to AZLI therapy. The results of this study have been previously published in abstract form (20).
METHODS
Study Design
This randomized, double-blind, placebo-controlled, study was conducted at 56 CF centers in the United States (February 2005–September 2006). After screening (Day −42; Figure 1), eligible patients were enrolled (Day −28), randomly assigned to 75 mg AZLI (twice or three times daily) or placebo (1:1:1), and began treatment with open-label TIS (Day −28). At baseline (Day 0), patients completed the course of TIS and began the randomized AZLI/placebo treatment. Patients were monitored midtreatment (Day 14), at end of treatment (Day 28), and during follow-up (Days 42, 56, 70, 84).
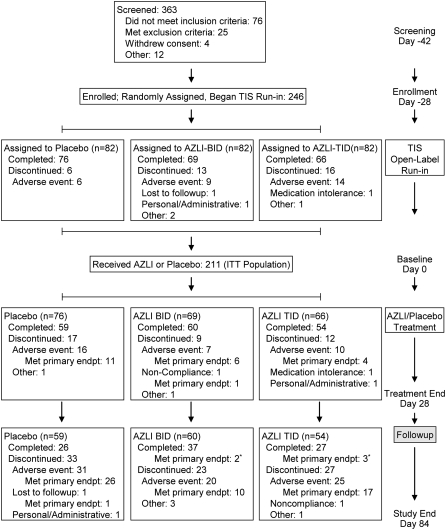
Figure 1.
Study design and patient disposition. Patients were randomly assigned to treatment with aztreonam lysine for inhalation (AZLI)/placebo before they all began the open-label tobramycin inhalation solution (TIS) run-in; their reasons for discontinuing during the TIS run-in are displayed by randomization group. The 211 patients remaining in the study at baseline (Day 0) received at least one dose of AZLI/placebo and comprise the intent-to-treat (ITT) population. The category labeled “Met primary endpt” includes patients who met the primary efficacy endpoint, need for additional inhaled, or intravenous antibiotics. * Met primary endpoint in analyses of Day 84–100 data.
A complete physical examination was performed at screening. Spirometry (American Thoracic Society standards) was performed at every study visit (before and 30 min after any treatment) (21). FEV1% predicted values were calculated using the Knudson equation (22).
TIS (300 mg, twice daily) was administered with the PARI LC PLUS jet nebulizer and AZLI (75 mg aztreonam, 52.5 mg lysine monohydrate) or placebo (5 mg lactose), diluted in 1 ml of 0.17% NaCl (twice or three times daily), with the eFlow (Altera) electronic nebulizer (PARI Innovative Manufacturers, Midlothian, VA) (23). Patients self-administered a bronchodilator at home before study medication and a short-acting β2-agonist 15 minutes before the first spirometry measurements at study visits. Patients continued any prescribed bronchodilator use, excluding the 6 hours before study visits.
TIS was dispensed on Day −28 and AZLI/placebo on Day 0; used and unused vials were subsequently collected to assess treatment compliance. Compliance was defined as missing no more than 1 dose per day, or receiving ⩾50% doses for TIS (twice daily dosing) and receiving ⩾66% doses for AZLI/placebo (compliance threshold set for three-times-daily dosing).
This study was conducted in compliance with the Declaration of Helsinki. Institutional review boards approved the study for each site and all patients or their guardians provided written informed consent before any study procedures.
Study Population
Eligible patients (⩾6 yr, documented diagnosis of CF) had current PA airway infections (PA present in expectorated sputum or throat swab culture at screening), three or more TIS courses within previous year, the ability to perform reproducible pulmonary function tests, and, at screening, FEV1 ⩾25% and ⩽75% predicted values and arterial oxygen saturation ⩾90% on room air. Chronic azithromycin use was allowed if the regimen was unchanged in the previous 3 months and if additional antipseudomonal therapy had been used since initiating azithromycin.
Exclusion criteria included current oral corticosteroid use (equivalent to >10 mg prednisone daily); airway cultures yielding Burkholderia cepacia complex in the previous 2 years; oxygen supplementation, daily continuous or more than 2 L/minute at night; monobactam-antibiotic hypersensitivity; inhaled short-acting β2-agonist intolerance; recent changes in antimicrobial, bronchodilator, antiinflammatory, corticosteroid medications, or physiotherapy technique/schedule; lung transplantation; significant acute finding on chest radiograph (e.g., lobar infiltrate and atelectasis, pneumothorax, or pleural effusion) at screening or in previous 90 days; aspartate transaminase (AST) or alanine transaminase (ALT) more than five times or serum creatinine more than two times the upper limit of normal (at screening); pregnancy; lactation; or, in opinion of investigator, medical or psychiatric illness interfering with study participation.
Efficacy Measures
The primary efficacy endpoint was time to need for additional inhaled or intravenous antipseudomonal antibiotics to treat symptoms indicative of pulmonary exacerbation. The predefined list of symptoms included decreased exercise tolerance, increased cough, increased sputum production/chest congestion, or decreased appetite (16). Major secondary efficacy endpoints included changes in clinical symptoms (CFQ-R respiratory symptoms scale), pulmonary function, PA density (colony-forming units [cfu]/g sputum, log10 transformed), time to hospitalization, hospitalizations, and weight. Scores for CFQ-R scales ranged from 0 to 100; increasing scores indicated improvement (19). The CFQ-R was administered at the beginning of study visits to minimize any influence of physiologic data or study personnel on patient responses. The minimal clinically important difference (MCID) score is the smallest change that a patient can detect on a patient-reported outcome measure such as the CFQ-R (24, 26). Using responses during the TIS phase to the respiratory domain of a Global Rating of Change Questionnaire, an MCID score of 5 was determined for the CFQ-R respiratory scale (25). Thus, 5-point changes on the CFQ-R respiratory symptom scale indicated improving or worsening respiratory symptoms detectable by the patient.
Microbiological endpoints included the minimum inhibitory concentration (MIC) of aztreonam for PA, and the prevalence of other pathogens.
Safety Measures
Safety was assessed by monitoring adverse events and changes in clinical laboratory values, vital signs, and airway reactivity. Worsening CF symptoms were treated as adverse events and patients were withdrawn from the study if they exhibited any of the predefined symptoms of pulmonary exacerbation (16). Patients who withdrew for the combination of predefined symptoms and need for additional inhaled or intravenous antipseudomonal antibiotics met the primary efficacy endpoint, completing their study participation.
Statistical Analyses
Efficacy and safety analyses included all randomly assigned patients receiving one or more 1 doses of AZLI/placebo. As specified in the study protocol, responses of twice-daily placebo and three-times-daily placebo groups were pooled for the primary efficacy analyses and were compared with the AZLI-pooled group as a gatekeeper approach. If the null hypotheses based on pooled data were rejected, then analyses were conducted on the three-times-daily AZLI and on the twice-daily AZLI groups (AZLI-TID and AZLI-BID) versus pooled placebo. Safety analyses were conducted on the pooled placebo, AZLI-BID, and AZLI-TID groups. All statistical tests were conducted at the two-sided 0.05 level.
A sample size of 210 patients for AZLI/placebo treatment was estimated as providing more than 90% power to detect a difference in time to antibiotic need, with α = 0.05. The sample size estimate was based on the Lakatos normal approximation method with exponential rates of loss to follow-up assumed as 10% for both treatment groups. Under these distributions, the Day 84 rates were hypothesized to be 55% placebo (pooled) and 32% AZLI pooled. Therefore, by the end of Day 84, 39 placebo patients and 45 AZLI patients were predicted to require additional inhaled or intravenous antibiotics (the predicted absolute number of events is higher for AZLI than placebo because there are twice as many patients in the pooled AZLI group).
CFQ-R and FEV1 efficacy analyses used the last observation carried forward convention. Analyses of continuous variables used analysis of covariance models with treatment as the fixed-effect and baseline (Day 0) values as covariates. The highest aztreonam MIC at baseline was the covariant for analyzing log10 PA colony-forming units. Changes in FEV1 (L) and changes in FEV1% predicted were analyzed using relative values; increases or decreases were calculated as percentages of the baseline FEV1 or FEV1% predicted values. Time to antibiotic need and to hospitalization were analyzed using Kaplan-Meier estimates and treatment groups compared using the log-rank test. Cox proportional hazards regression was used to estimate hazard ratios. Hospitalizations were analyzed using Wilcoxon rank sum test (days) and Fisher's exact test (proportion of patients). Aztreonam concentrations (Alta Analytical Laboratory, El Dorado Hills, CA) in plasma and sputum were summarized, as were aztreonam or tobramycin MIC values inhibiting 50% (MIC50) or 90% (MIC90) of PA isolates, proportion of patients with aztreonam or tobramycin MIC values above parenteral breakpoints, and the prevalence of other pathogenic bacteria (Covance Central Laboratory Services, Indianapolis, IN) (1, 9, 27). SAS versions 8.02 and 9.1 were used (SAS Institute, Inc., Cary, NC).
If patients needed oral antibiotics without need for additional inhaled or intravenous antibiotics, they were discontinued from the study and were not considered as having met the primary endpoint. If patients needed oral antibiotics and also needed additional inhaled or intravenous antibiotics, they were discontinued from the study. If they also had one of the four predefined symptoms indicative of pulmonary exacerbation, they were considered as having met the primary endpoint. Patients who prematurely discontinued or completed the study without meeting the criteria for the primary endpoint were to be censored in the analysis of time to need for inhaled or intravenous antibiotics due to predefined symptoms. However, some of these patients were experiencing the precursor of a pulmonary exacerbation. To minimize any potential bias due to this possibly informative censoring, patients who were experiencing respiratory symptoms at the time of early termination or study completion were monitored to determine if inhaled or intravenous antibiotics were prescribed during the subsequent 2 weeks. The study protocol specified that patients were to be followed for safety purposes until adverse events resolved, but did not specify the inclusion of events after Day 84 in the primary efficacy endpoint. Analyses of the data collected during this 2-week period of time were completed before unblinding the study.
RESULTS
Of 363 patients screened, 211 completed the 28-day TIS run-in and began the 28-day AZLI/placebo treatment; 173 (82%) completed the AZLI/placebo treatment period, and 90 (43%) completed the study (Figure 1). Dosing compliance was 99.5% during TIS run-in and 95.3% during AZLI/placebo treatment.
Patient Characteristics
Patient characteristics appeared generally well balanced between treatment groups (Table 1). Mean age was 26.2 years; 165 (78%) patients were 18 years or older. The proportion of patients younger than 18 years in the placebo group (15.8%) was smaller than that in the AZLI-pooled group (25.2%). Mean FEV1% predicted was 55.1%; FEV1 was ⩽50% predicted value for 76 (36%) patients. Concomitant medications used at screening for more than 50% of patients were pancreatic enzymes (92%), salbutamol (89%), dornase alfa (85%), vitamins (84%), azithromycin (70%), and fluticasone propionate with salmeterol (56%). Average TIS use was 5.3 courses in the year before the study; 6.5 courses/year is the maximum number approved (6).
TABLE 1.
PATIENT DEMOGRAPHICS AND CHARACTERISTICS
| Placebo (n = 76) | AZLI-BID (n = 69) | AZLI-TID (n = 66) | AZLI-Pooled (n = 135) | |
|---|---|---|---|---|
| Age, mean yr (range) | 27.9 (10–65) | 26.5 (10–50) | 24.1 (7–50) | 25.3 (7–50) |
| Age < 18 yr, n (%) | 12 (15.8) | 17 (24.6) | 17 (25.8) | 34 (25.2) |
| Male, n (%) | 45 (59.2) | 38 (55.1) | 38 (57.6) | 76 (56.3) |
| Weight, mean kg (SD) | 61.6 (13.0) | 57.1 (12.9) | 57.7 (16.9) | 57.4 (14.9) |
| BMI, mean kg/m2 (SD) | 21.7 (3.0) | 20.9 (3.3) | 21.0 (4.5) | 20.9 (3.9) |
| TIS courses in previous year, mean | 5.26 | 5.46 | 5.26 | 5.36 |
| CFTR genotype, n (%) | ||||
| Homozygous for ΔF508 | 34 (45) | 25 (36) | 31 (47) | 56 (41) |
| Heterozygous for ΔF508 | 20 (26) | 15 (22) | 12 (18) | 27 (20) |
| Unidentified or other | 22 (29) | 29 (42) | 23 (35) | 52 (39) |
| Dornase alfa use, % patients | 89.5 | 81.2 | 84.8 | 83.0 |
| Azithromycin use, % patients | 65.8 | 69.6 | 74.2 | 71.9 |
| Hypertonic saline use, % patients | 11.8 | 8.7 | 12.1 | 10.4 |
| MIC of tobramycin for all PA isolates, μg/ml | ||||
| MIC50 | 2 | 1 | 2 | 1 |
| MIC90 | 256 | 16 | 64 | 32 |
| No. of isolates tested | 137 | 104 | 107 | 211 |
| FEV1% predicted, mean (SD) | 53.9 (15.3) | 56.3 (14.8) | 55.4 (16.3) | 55.8 (15.5) |
| Patients with FEV1 ⩽ 50% predicted value, n (%) | 30 (39.5) | 24 (35.3) | 22 (33.3) | 46 (34.3) |
| CFQ-R respiratory score, mean (SD) | 62.1 (19.7) | 63.1 (16.7) | 64.2 (18.1) | 63.7 (17.4) |
| MIC of aztreonam for all PA isolates, μg/ml | ||||
| MIC50 | ⩽1 | 2 | 2 | 2 |
| MIC90 | 64 | 64 | 32 | 32 |
| Minimum MIC | ⩽1 | ⩽1 | ⩽1 | ⩽1 |
| Maximum MIC | 1,024 | >2,048 | 1,024 | >2,048 |
| No. of isolates tested | 125 | 105 | 111 | 216 |
Definition of abbreviations: AZLI-BID = twice-daily dosage of aztreonam lysine for inhalation; AZLI-TID = three-times-daily dosage of aztreonam lysine for inhalation; BMI = body mass index; CFQ-R = Cystic Fibrosis Questionnaire–Revised; MIC = minimum inhibitory concentration; PA = Pseudomonas aeruginosa; TIS = tobramycin inhalation solution.
Age and concomitant medications were measured at screening; FEV1% predicted and MIC of tobramycin for PA were measured at Day −28; CFQ-R respiratory scores and MIC of aztreonam for PA were measured at Day 0. There were no statistically significant differences in demographic or baseline characteristics between the AZLI and placebo groups.
Patient Disposition during AZLI/Placebo Treatment Period and Follow-up Period
Of the 121 patients discontinuing the study during the AZLI/placebo treatment or follow-up periods (Days 0–84), 45 patients (21% of 211 patients beginning the AZLI/placebo treatment period) discontinued without meeting the primary study endpoint. The majority of discontinuing patients (76 of 121) met the primary study endpoint, need for intravenous or inhaled antipseudomonal antibiotics (Days 0–84; AZLI-BID: 17 of 32 withdrawing; AZLI-TID: 21 of 39; placebo: 38 of 50; Figure 1). This group included 11 patients (AZLI-BID: 3 patients; AZLI-TID: 3 patients; placebo: 5 patients) whose need for inhaled or intravenous antipseudomonal antibiotics to treat symptoms indicative of a pulmonary exacerbation (an event) occurred after discontinuing from the study. An additional five patients had an event on the day they completed the study (AZLI-BID: 2 patients; AZLI-TID: 3 patients); they are included in the 90 patients listed as completing the study (Figure 1). Among these 16 patients, 5 had events reported after study end (Day 84); thus, the time to antibiotic need analysis was extended beyond study end (Day 84) so that data from these patients could be included (Figure 2). Of the 81 patients who met the primary endpoint, 17 (21%) received treatment with intravenous antibiotics (initial treatment or subsequent to inhaled antibiotics).
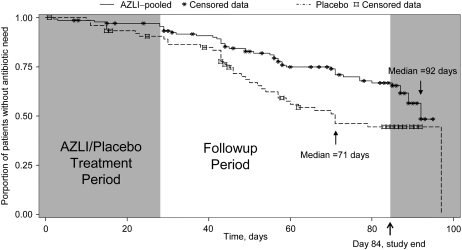
Figure 2.
Time to need for additional inhaled or intravenous antipseudomonal antibiotics to treat symptoms indicative of pulmonary exacerbation. The median time to antibiotic need is shown for both treatment groups (aztreonam lysine for inhalation [AZLI] vs. placebo, P = 0.007).
Efficacy
The median time to need for additional inhaled or intravenous antipseudomonal antibiotics to treat symptoms indicative of pulmonary exacerbation was 21 days longer for the AZLI-pooled group than for the placebo group (92 vs. 71 d, measured from baseline; P = 0.007; Figure 2). Median time to antibiotic need was also longer in the AZLI-BID (>92 d, P = 0.002) and AZLI-TID (87 d, P = 0.182) groups, compared with placebo (71 d). This corresponded to a 45% reduction in risk for needing additional intravenous or inhaled antibiotics. To determine if these results were overly influenced by including the additional patient data described above, we also conducted the time to need analysis using data collected only during the 84-day study period (Days 0–84). Comparable results were obtained; time to antibiotic need was significantly longer for AZLI-treated patients than for placebo-treated patients (P = 0.002; Figure 2).
We also examined the effects of age. In both age subgroups, the proportion of patients requiring inhaled or intravenous antibiotics was smaller in the AZLI-treated group than that in the placebo-treated group (<18 yr of age: [ALZI pooled: 9 of 34, 26%], [placebo: 5 of 12, 42%]; ⩾18 yr of age: [AZLI pooled: 34 of 101, 34%], [placebo: 33 of 64, 52%]). For patients 18 years or older, time to need for additional antibiotics was significantly prolonged for AZLI-treated patients compared with placebo-treated patients (P = 0.021). The difference was not significant for the younger patients; however, the group sizes were small.
Adjusted mean CFQ-R respiratory scores increased 5.01 points in the AZLI-pooled group compared with placebo (Day 28; 95% confidence interval [CI], 0.81–9.21; P = 0.020). Significant improvements were observed for both AZLI-BID and AZLI-TID groups compared with placebo (Figure 3). Responses of the AZLI-BID and AZLI-TID groups were comparable. Scores decreased during the follow-up period (Day 84; AZLI-pooled: 0.71 points; placebo: −0.78 points; change from Day 0). During AZLI/placebo treatment (Days 0–28), CFQ-R respiratory scores improved for more AZLI-treated than placebo-treated patients (⩾5-point increase; AZLI, 52%; placebo, 37%) and worsened for fewer AZLI-treated patients (⩾5-point decrease; AZLI, 28%; placebo, 38%; overall categorical comparison, P = 0.029).
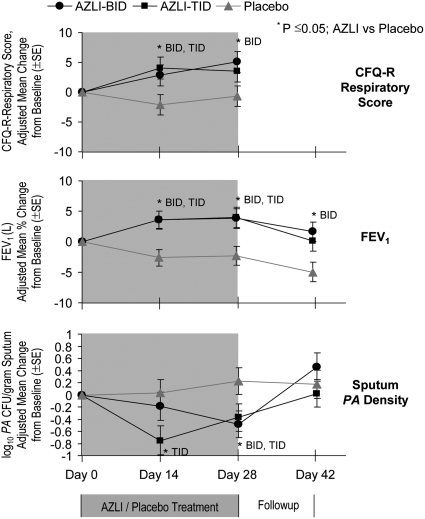
Figure 3.
Changes in Mean Cystic Fibrosis Questionnaire–Revised (CFQ-R) respiratory scores, FEV1, and Pseudomonas aeruginosa (PA) density in sputum. Child, teen, and adult CFQ-R respiratory scores were combined. The CFQ-R was not administered on Day 42. For CFQ-R respiratory, at Day 28, P = 0.021 for AZLI-BID versus placebo and P = 0.092 for AZLI-TID versus placebo. For FEV1 at Day 28, P = 0.006 for AZLI-BID versus placebo and P = 0.005 for AZLI-TID versus placebo. For PA density (log10 PA cfu/g sputum) at Day 28, P = 0.011 for AZLI-BID versus placebo and P = 0.031 for AZLI-TID versus placebo. BID = twice daily; TID = three times daily.
Adjusted mean FEV1 improved 6.3% in the AZLI-pooled group compared with placebo (Day 28; 95% CI, 2.5–10.1; P = 0.001). Significant improvements were observed for both AZLI-BID and AZLI-TID groups compared with placebo (Figure 3). Responses of the AZLI-BID and AZLI-TID groups were comparable. FEV1 decreased during the follow-up period for all groups.
All patients received open-label TIS during the run-in period (Days −28 to 0). By chance, only the group that later received AZLI-TID had a notable improvement in observed FEV1 (Figure E1 of the online supplement).
Adjusted mean relative FEV1% predicted also improved in the AZLI-pooled group compared with placebo (Day 28; adjusted means; AZLI-pooled, 4.1%; placebo, −2.5%; treatment effect = 6.6%; 95% CI, 2.8–10.4; P < 0.001).
Changes in CFQ-R respiratory scores at end of treatment (Day 28) were modestly correlated with changes in FEV1 (Pearson correlation coefficients = 0.33, 0.24, 0.33, for AZLI-BID, AZLI-TID, placebo groups, respectively; Figure E2) and with Global Rating of Change Questionnaire respiratory domain responses (Pearson correlation coefficient = 0.46; all groups combined, P < 0.001).
Adjusted mean PA sputum density decreased 0.66 log10 PA cfu/g sputum in the AZLI-pooled group compared with the placebo group (Day 28: 95% CI. −1.13 to −0.19; P = 0.006). Significant decreases were observed for both AZLI-BID and AZLI-TID compared with placebo groups (Figure 3). At Day 14, responses of the AZLI-TID group were larger than those of the AZLI-BID group and, by Day 28, the responses of the AZLI-BID and AZLI-TID groups were comparable. PA density increased for all groups during the follow-up period.
During the 28-day TIS run-in, mean (SE) CFQ-R respiratory scores decreased −1.47 (0.98), mean FEV1 increased 0.9% (0.8%), and mean PA density decreased 0.28 (0.14) log10 PA cfu/g sputum.
Time to first hospitalization and median days per number of patients hospitalized did not differ significantly between treatment groups (Days 0–84). Weight increased 0.77% for the AZLI-pooled group compared with placebo (Day 28: 95% CI, 0.00–1.55; P = 0.051).
Safety
The incidence of treatment-emergent adverse events was generally comparable for the three groups (Table 2); any differences were not statistically significant. Nine patients were hospitalized because of serious adverse events occurring during the AZLI/placebo treatment period: seven for pulmonary exacerbation (AZLI-BID: 2; AZLI-TID: 4; placebo: 1) and one each for small bowel obstruction (AZLI-BID) and hyponatremia (AZLI-TID). There were no deaths during this study and no reports of anaphylaxis. Airway reactivity after treatment (acute FEV1 decrease ⩾15% within 30 min post-treatment; Days 0, 14) occurred in six patients (AZLI-pooled, 4 [3.0%]; placebo, 2 [2.6%]); none of these patients withdrew for this reason.
TABLE 2.
TREATMENT-EMERGENT ADVERSE EVENTS OF 5.0% OR MORE IN ANY TREATMENT GROUP DURING THE AZLI/PLACEBO TREATMENT PERIOD
| TEAEs ⩾5% in any Treatment Group* | Placebo (n = 76) | AZLI-BID (n = 69) | AZLI-TID (n = 66) | AZLI-Pooled (n = 135) |
|---|---|---|---|---|
| Cough, n (%) | 26 (34.2) | 19 (27.5) | 24 (36.4) | 43 (31.9) |
| Productive cough, n (%) | 13 (17.1) | 9 (13.0) | 9 (13.6) | 18 (13.3) |
| Wheezing, n (%) | 6 (7.9) | 5 (7.2) | 9 (13.6) | 14 (10.4) |
| Hemoptysis, n (%) | 7 (9.2) | 7 (10.1) | 6 (9.1) | 13 (9.6) |
| Nasal congestion, n (%) | 6 (7.9) | 5 (7.2) | 5 (7.6) | 10 (7.4) |
| Rhinorrhea, n (%) | 2 (2.6) | 5 (7.2) | 5 (7.6) | 10 (7.4) |
| Headache, n (%) | 5 (6.6) | 2 (2.9) | 6 (9.1) | 8 (5.9) |
| Pharyngolaryngeal pain, n (%) | 7 (9.2) | 3 (4.3) | 5 (7.6) | 8 (5.9) |
| Dyspnea, n (%) | 3 (3.9) | 2 (2.9) | 5 (7.6) | 7 (5.2) |
| Pyrexia, n (%) | 2 (2.6) | 4 (5.8) | 3 (4.5) | 7 (5.2) |
| Respiratory tract congestion, n (%) | 5 (6.6) | 5 (7.2) | 2 (3.0) | 7 (5.2) |
| Abdominal pain, upper, n (%) | 3 (3.9) | 4 (5.8) | 1 (1.5) | 5 (3.7) |
| Decreased appetite, n (%) | 5 (6.6) | 4 (5.8) | 1 (1.5) | 5 (3.7) |
| Fatigue, n (%) | 7 (9.2) | 3 (4.3) | 2 (3.0) | 5 (3.7) |
| Dysphonia, n (%) | 4 (5.3) | 1 (1.4) | 1 (1.5) | 2 (1.5) |
| Exercise tolerance decreased, n (%) | 4 (5.3) | 1 (1.4) | 1 (1.5) | 2 (1.5) |
| Sinus congestion, n (%) | 5 (5.6) | 0 (0.0) | 2 (3.0) | 2 (1.5) |
Definition of abbreviations: AZLI-BID = twice-daily dosage of aztreonam lysine for inhalation; AZLI-TID = three-times-daily dosage of aztreonam lysine for inhalation; TEAE = treatment-emergent adverse events.
TEAEs coded using the Medical Dictionary for Regulatory Activities (MedDRA) preferred term; for TEAEs with incidence ⩾10% in any group, % patients for each TEAE did not differ significantly between treatment groups (Fisher's exact test).
Mean changes in vital signs and in hematology and serum chemistry variables from Day −28 or Day 0 were comparable for all treatment groups during the study. Mean total white blood cell counts, neutrophil counts, % neutrophils, and serum glucose concentrations were near or above the upper limit of normal for all treatment groups throughout the study.
Clinical Pharmacology and Microbiology
Aztreonam concentrations in plasma 1 hour after dosing were as follows (median [range]): AZLI-BID, 533 (0–1,390) ng/ml, and AZLI-TID, 585 (0–2,920) ng/ml on Day 0 (n = 66, 63); and AZLI-BID, 581 (45–1,540) ng/ml, and AZLI-TID, 622 (31–1,710) ng/ml on Day 14 (n = 65, 59). Aztreonam concentrations in sputum 10 minutes after dosing were as follows: AZLI-BID, 422 (0–2,160) μg/g, and AZLI-TID, 537 (0.2–3,010) μg/g on Day 0 (n = 63, 58); and AZLI-BID, 429 (0.3–3,430) μg/g, and AZLI-TID, 406 (68–3,240) μg/g on Day 14 (n = 61, 51).
MIC50 and MIC90 values of aztreonam for PA remained unchanged between Days 0 and 56 except for a transient fourfold increase on Day 14 in the AZLI-TID group (Table 3). The proportion of patients who had PA isolates with aztreonam MIC values greater than 8 μg/ml (parenteral breakpoint) increased during AZLI treatment; the increase was transient in the AZLI-TID group (Day 0, 28, 42: AZLI-BID: 27%, 44%, 39%; AZLI-TID: 33%, 43%, 28%; placebo: 38%, 37%, 30%, respectively) (1). MIC50 and MIC90 values of tobramycin for PA isolates changed less than fourfold (Days −28 to 56). The proportion of patients who had PA with tobramycin MIC values of 8 μg/ml or more (parenteral breakpoint) did not increase (Days −28 to 42). No persistent increases were observed for the prevalence of Staphylococcus aureus, Stenotrophomonas maltophilia, or Achromobacter xylosoxidans (Days 0–28); B. cepacia complex was not isolated.
TABLE 3.
MIC50 AND MIC90 OF AZTREONAM FOR ALL Pseudomonas aeruginosa ISOLATES (μg/ml) FOR THE TOBRAMYCIN INHALATION SOLUTION AND AZLI/PLACEBO TREATMENT PERIODS
| Result (μg/ml)
|
Change* in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Day | MIC50 | MIC90 | No. of PA Isolates | Minimum MIC | Maximum MIC | MIC50 | MIC90 |
| Placebo | Day −28 | 2 | 64 | 137 | ⩽1 | >2,048 | ||
| (n = 76) | Day 0 | ⩽1 | 64 | 125 | ⩽1 | 1,024 | No | No |
| Day 14 | ⩽1 | 32 | 140 | ⩽1 | 512 | No | No | |
| Day 28 | ⩽1 | 64 | 113 | ⩽1 | 512 | No | No | |
| Day 42 | ⩽1 | 32 | 97 | ⩽1 | 512 | No | No | |
| Day 56 | 2 | 64 | 84 | ⩽1 | 1,024 | No | No | |
| AZLI-BID | Day −28 | 2 | 32 | 104 | ⩽1 | 1,024 | ||
| (n = 69) | Day 0 | 2 | 64 | 105 | ⩽1 | >2,048 | No | No |
| Day 14 | 4 | 128 | 101 | ⩽1 | >2,048 | No | No | |
| Day 28 | 4 | 128 | 93 | ⩽1 | >2,048 | No | No | |
| Day 42 | 2 | 128 | 94 | ⩽1 | 512 | No | No | |
| Day 56 | 2 | 64 | 90 | ⩽1 | 1,024 | No | No | |
| AZLI-TID | Day −28 | 4 | 64 | 107 | ⩽1 | 1,024 | ||
| (n = 66) | Day 0 | 2 | 32 | 111 | ⩽1 | 1,024 | No | No |
| Day 14 | 8 | 128 | 92 | ⩽1 | 1,024 | Increased | Increased | |
| Day 28 | 4 | 64 | 95 | ⩽1 | 1,024 | No | No | |
| Day 42 | 2 | 64 | 78 | ⩽1 | 1,024 | No | No | |
| Day 56 | ⩽1 | 64 | 70 | ⩽1 | 2,048 | No | No | |
Definition of abbreviations: AZLI-BID = twice-daily dosage of aztreonam lysine for inhalation; AZLI-TID = three-times-daily dosage of aztreonam lysine for inhalation; MIC = minimum inhibitory concentration; PA = Pseudomonas aeruginosa.
Changes on Day 0 were from Day −28 and changes on Days 14 to 56 were from Day 0. A change of fourfold or more was considered an increase in MIC.
DISCUSSION
AZLI at a dose of 75 mg, twice or three times daily for 28 days significantly delayed time to need for additional inhaled or intravenous antipseudomonal antibiotics to treat respiratory symptoms indicative of pulmonary exacerbation in patients with CF. Compared with placebo, AZLI treatment also significantly improved respiratory symptoms and pulmonary function and significantly decreased log10 PA colony-forming units. AZLI was well tolerated; adverse events were generally consistent with the symptoms of CF lung disease.
In general, responses to twice- and three-times-daily AZLI were comparable. The reduction in PA density appeared sooner for the AZLI-TID than for the AZLI-BID group and time to antibiotic need was somewhat longer for the AZLI-BID than for the AZLI-TID group. The larger increase observed for the AZLI-TID group than for the other groups during the TIS pretreatment period likely occurred by chance, because all three groups were receiving open-label TIS. For the AZLI-TID group, the additive improvement in lung function responses resulting from back to back treatment with TIS and three-times-daily AZLI approached 10%. Upon withdrawal of therapy, the subsequent rapid decrease in lung function may have been apparent to both patients and investigators, and may have contributed to the shorter time to antibiotic need observed for the AZLI-TID group than for the AZLI-BID group. These results suggest that a time-to-need study design may be more applicable for a continuous rather than an episodic therapeutic intervention if the intervention leads to short-term improvements that decrease rapidly after therapy is withdrawn. Overall, the two AZLI dosing regimens in this study appeared to be generally comparable; no AZLI dose–response was observed. It will be interesting to see if similar results are obtained from the ongoing open-label, multicycle AZLI study of twice- and three-times-daily dosing.
The increased CFQ-R respiratory scores indicated that patients perceived their respiratory symptoms as improving after AZLI treatment. CFQ-R respiratory scores appeared to detect change in this CF population with sensitivity equal to FEV1, the established efficacy endpoint. However, these endpoints are measuring different aspects of clinical efficacy, as indicated by the modest correlation between patient-reported changes in respiratory symptoms (CFQ-R respiratory) and measured changes in lung function (FEV1) (28).
The decrease in PA sputum density after AZLI treatment was small but statistically significant and was observed in clinically stable patients immediately after a course of TIS. The decrease was comparable to those observed in previous TIS studies enrolling intensively treated patients, but smaller than those observed in a previous AZLI study enrolling less intensively treated patients (29–31). Thus, for PA density, the magnitude of change appears to be dependent on recent antibiotic therapies. There was no evidence for development of microbial resistance to aztreonam during this study, but this will need to be examined over longer time periods and multiple treatment courses.
Patients in this study were predominantly adults (78% ⩾18 yr) and were largely compliant with the current guidelines of care for patients with CF (12). In addition to the TIS run-in, TIS use in the previous year averaged 5.3 courses, approaching the maximum of 6.5 courses approved per year (6). Thus, the magnitude of improvement in FEV1 (6.3%) and FEV1% predicted (6.6%) after AZLI treatment was unexpected, and suggests that lung disease in adults with CF may be more responsive to additional treatment than previously believed. The large proportion of adults enrolled also likely reflects the changing demographics of patients with CF; as the standard of care improves, there are fewer patients younger than 18 years with moderate to severe lung disease and chronic PA airway infection (1, 2, 5–8).
Although the study entry criteria were comparable, patients in this study were older (26 vs. 21 yr) with higher mean FEV1% predicted (55 vs. 50–51%) than patients in TIS studies a decade ago (5). This patient population, despite being older, had less lung disease progression; this also likely reflects the improved clinical management of CF (1, 2, 5–8).
Patient responses during the TIS run-in period appeared markedly attenuated compared with responses observed in previous TIS studies (5, 13, 14, 29, 32, 33). Further studies will be required to elucidate the mechanism(s) underlying this apparent attenuation in clinical efficacy resulting from chronic TIS use. An increase in FEV1 was observed after 28 days of AZLI treatment; therefore, AZLI appears to circumvent the mechanism(s) affecting patient responses to TIS. However, this study included only one treatment period and the effectiveness of AZLI needs to be examined over longer time periods and multiple treatment courses.
The results of this study indicate that AZLI may be an effective add-on therapy for patients with CF and chronic PA airway infection who are intensively treated with TIS; in 2005, this group included 58% of patients in the United States (>5 yr of age) with CF and PA airway infection (2). The improvements in FEV1 and PA sputum density decreased during the 2 weeks after therapy stopped. Thus, to maintain lung function, future strategies for managing patients may include rotating use of different inhaled antibiotics or use of combination therapies.
Supplementary Material
Acknowledgments
The authors thank the patients and their families as well as the site investigators (SI) and research coordinators (RC) for each study site, as listed below. Statistical analyses were performed by Kendle International and Gilead Sciences, Inc.
Atlantic Health System Hospital Corporation and Morristown Memorial Hospital, Summit/Morristown, NJ—SI: Stanley Fiel; RC: Paula Lomas; Alamo Clinical Research Associates, San Antonio, TX—SI: Carlos Orozco; RC: Terri Phillips, Albany Medical College, Albany, NY—SI: Jonathan Rosen; RC: Paula Malone and Katharine Mokhiber; Baptist Medical Center, Oklahoma City, OK—SI: Santiago Reyes; RC: Teresa Orf; Baylor College of Medicine, Houston, TX—SI: Christopher Oermann; RC: Charles Sellers; Chicago Children's Asthma Respiratory and Exercise Specialists, Glenview, IL—SI: Steven Boas; RC: Melinda Bicchinella; Children's Hospital and Regional Medical Center, Seattle, WA—–SI: Ron Gibson; RC: Sharon McNamara; Children's Hospital Boston, Boston MA—–SI: David Waltz and Thomas Martin; RC: Summer Adams; Children's Hospital Los Angeles, Los Angeles, CA—–SI: Marlyn Woo; RC: Lynn Fukushima; Children's Hospital Medical Center of Akron, Akron, OH—SI: Gregory Omlor; RC: Debbie Ouellette; Children's Hospital of Michigan and Wayne State University, Detroit, MI—SI: Debbie Toder; RC: Catherine Van Wagnen; Children's Hospital of Orange County, Orange, CA—SI: Bruce Nickerson; RC: Candice Ramos and Melissa Mendoza; Children's Hospital of Pittsburgh, Pittsburgh, PA—–SI: Joseph Pilewski; RC: Elizabeth Hartigan; Children's Lung Specialists, Las Vegas, NV—–SI: Craig Nakamura; RC: Tara Brascia; Children's Medical Center of Dayton, Dayton, OH—–SI: Robert Finkt; RC: Sandy Bartosik; Children's Memorial Hospital/Northwestern University, Chicago, IL—–SI: Susanna McColley; RC: Catherine Powers; Columbia University Medical Center, New York, NY—SI: Emily DiMango; RC: Jennifer Sormillon; Columbus Children's Hospital and Ohio State University, Columbus, OH—SI: Karen McCoy; RC: M. Terri Johnson; Connecticut Children's Medical Center, Hartford, CT—SI: Craig Lapin; RC: Ginny Drapeau; Drexel University College of Medicine, Philadelphia, PA—SI: Michael Sherman; RC: Judy Hillman; Emory Healthcare, Atlanta, GA—SI: Daniel Caplan; RC: Tedra Flynn; Indiana University Medical Center, Indianapolis, IN—SI: Aruna Sannuti; RC: Annette Hempfling; Kaiser Oakland Medical Center, Oakland, CA—SI: Gregory Shay; RC: Julie Lee; Long Island College Hospital, Brooklyn, NY—SI: Robert Giusti; RC: Christine Mavaro; Long Island Jewish Medical Center, New Hyde Park, NY—SI: Rubin Cohen; RC: Maryanne Gannon; Loyola University Medical Center, Maywood, IL—SI: Sean M. Forsythe; RC: Cathy Kalnicky and Theresa Krause; Maine Medical Center, Portland, ME—SI: Jonathan Zuckerman; RC: Sue Mortenson; Massachusetts General Hospital, Boston, MA—SI: Henry Dorkin; RC: Jane Solomon and Monica Ulles; Medical College of Georgia, Augusta, GA—SI: Margaret Guill; RC: Kathy Dyer and Juan Reyes; Medical University of South Carolina, Charleston, SC—SI: C. Michael Bowman; RC: Terry Byars; New England Medical Center, Boston, MA—SI: Thomas Martin and William Yee; RC: Karen Murray and Corri Nelson; Nemours Children's Clinic, Jacksonville, FL—SI: Kathryn Blake; RC: Betty DeLuca; Nemours Children's Clinic, Orlando, FL—SI: Mark Weatherly; RC: Sondra Sadler; New York Medical College, Valhalla, NY—SI: Nikhil Amin; RC: Ingrid Gherson; North Suburban Pulmonary and Critical Care Consultants, Niles, IL—SI: Arvey Stone; RC: Suellen Moen; Oregon Health and Science University, Portland, OR—SI: Michael Wall; RC: Tamee Blankenship; Pediatric Infectious Diseases, Morgantown, WV—SI: Kathryn Moffett; RC: Susan Collins; Pediatric Pulmonary Associates, Columbia, SC—SI: Roxanne Marcille and Daniel Brown; RC: Carolyn Turner; Pediatric Pulmonary Associates, St. Petersburg, FL—SI: Magdalen Gondor; RC: Melanie Newkirk; Penn State Milton S. Hershey Medical Center, Hershey, PA—SI: Gavin Graff; RC: Diane Kitch; Phoenix Children's Hospital, Phoenix, AZ—SI: Peggy Radford; RC: Natalia Argel; Rhode Island Hospital, Providence, RI—SI: Michael Schechter; RC: Pam Marciniak; St. Christopher's Hospital for Children, Philadelphia, PA—SI: Laurie Varlotta; RC: Ignacio Tapia; Stanford University Medical Center, Stanford, CA—SI: Richard Moss; RC: Colleen Dunn and Zoe Davies; Stony Brook University Medical Center, Stony Brook, NY—SI: Catherine Kier; RC: Teresa Carney; The Children's Hospital Association, Denver, CO—SI: Frank Accurso; RC: Meg Anthony and Churee Pardee; University of Arkansas for Medical Sciences, Little Rock, AR—SI: Paula Anderson; RC: Adam Taggart; University of California at Davis Medical Center, Sacramento, CA—SI: Brian Morrissey; RC: Doug Elliot and Ellen Vlastelin; University of California, San Diego, San Diego, CA—SI: Douglas Conrad; RC: Laura Koenig and Bobbie Munden; University of Florida Health Sciences Center, Gainesville, FL—SI: L. Terry Spencer; RC: Dawn Baker and Margaret Humphries; University of Kansas Medical Center, Kansas City, KS—SI: Timothy Williamson; RC: Karen Conyers; University of Miami School of Medicine and Batchelor Children's Research Institute, Miami, FL—SI: Michael Light; RC: Maribeth Velasco; University of Michigan, Ann Arbor, MI—SI: Samya Nasr; RC: Ermee Sakmar; University of Minnesota, Minneapolis, MN—SI: Carlos Milla; RC: Jacquelyn Zirbes and Brooke Noren; Virginia Commonwealth University, Richmond, VA—SI: Greg Elliott; RC: Juellisa Gadd; Women and Children's Hospital of Buffalo, Buffalo, NY—SI: Drucy Borowitz; RC: Nadine Caci.
Supported by Gilead Sciences, Inc.; U.S. Food and Drug Administration grant 1R01FD003016-01; and National Institutes of Health General Clinical Research Center grants M01 RR00188, M01 RR00037, M01 RR02172, M01 RR00043, M01 RR000489, M01 RR00034, M01 RR00039, M01 RR00750, M01 RR01066, M01 RR001070, M01 RR10733, M01 RR00070, M01 RR10710, M01 RR00069, M01 RR00827, M01 RR00082, M01 RR023940, M01 RR00042, M01 RR00400, and M01 RR00065.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200712-1804OC on July 24, 2008
Conflict of Interest Statement: K.S.M., from 2004 to present, has received grant support for multicenter trial participation from Inspire Pharmaceuticals ($67,472), Chiron ($52,801), Genentech ($40,367), Corus ($120,178), Corus/Gilead Sciences, Inc. ($130,496); and reimbursement for airfare, hotel, and meeting attendance to the 2007 CF Conference ($7,306) from Gilead Sciences. A.L.Q. received consulting fees from Gilead Sciences, Genentech, Inc., and Novartis; travel funds were provided by Gilead Sciences and Genentech, Inc. She serves on the NASAG Board, which oversees the Epidemiological Study of the CF database and is funded by Genentech. C.M.O. has grants for participation in multicenter studies from Gilead Sciences (formerly Corus), Inspire Pharmaceuticals, and Altus Biologics; he has consulted for the France Foundation, a Graduate Medical Education firm, on CF care–based learning activities for $2,000. R.L.G. has received grant funding from Corus Pharma, Inspire Pharmaceuticals, and Cystic Fibrosis Foundation Therapeutics, Inc., to conduct clinical investigations; he also served on an advisory board for Genentech, Inc., to discuss the use of Pulmozyme in the management of early CF lung disease. G.Z.R-.B. has received funding from Gilead Sciences, Inc. (Corus Pharma), Inspire Pharmaceuticals, and Cystic Fibrosis Foundation Therapeutics, Inc., to conduct clinical trials. A.B.M. is an employee of Gilead Sciences, is an author of a patent on Aztreonam Lysine, and holds significant equity interest of 300,000 options or shares in Gilead Sciences.
References
- 1.Gibson RL, Burns JL, Ramsey BW. State of the art: pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–951. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry. 2005 annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2006.
- 3.Pamukcu A, Bush A, Buchdahl R. Effects of Pseudomonas aeruginosa colonization on lung function and anthropometric variables in children with cystic fibrosis. Pediatr Pulmonol 1995;19:10–15. [DOI] [PubMed] [Google Scholar]
- 4.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol 1992;12:158–161. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-KM, Borowitz D, Bowman CM, Marshall BC, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 1999;341:23–30. [DOI] [PubMed] [Google Scholar]
- 6.TOBI [prescribing information] [Internet] [accessed April 24, 2007]. East Hanover, NJ: Novartis. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/tobi.pdf
- 7.Pulmozyme [prescribing information] [Internet] [accessed May 23, 2007]. San Francisco: Genentech. Available from: http://www.gene.com/gene/products/information/opportunistic/pulmozyme/insert.jsp
- 8.Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais J-P. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax 2006;61:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson RL, Retsch-Bogart GZ, Oermann C, et al. Microbiology, safety and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol 2006;41:656–665. [DOI] [PubMed] [Google Scholar]
- 10.Azactam [prescribing information] [Internet] [accessed April 23, 2007]. Princeton, NJ: Bristol-Meyers-Squibb Co. Available from: http://www.fda.gov/cder/foi/label/2002/50632slr011lbl.pdf
- 11.Dietzsch HJ, Gottschalk B, Heyne K, Leupold W, Wunderlich P. Cystic fibrosis: comparison of two mucolytic drugs for inhalation treatment (acetylcysteine and arginine hydrochloride). Pediatrics 1975;55:96–100. [PubMed] [Google Scholar]
- 12.Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr., Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007;176:957–969. [DOI] [PubMed] [Google Scholar]
- 13.Moss RB. Administration of aerosolized antibiotics in cystic fibrosis patients. Chest 2001;120:107S–113S. [DOI] [PubMed] [Google Scholar]
- 14.Moss RB. Long term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest 2002;121:55–63. [DOI] [PubMed] [Google Scholar]
- 15.Döring G, Elborn JS, Johannesson M, de Jonge H, Griese M, Smyth A, Heijerman H, Consensus Study Group. Clinical trials in cystic fibrosis. J Cyst Fibros 2007;6:85–99. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery AB, Ramsey B. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr 2001;139:359–365. [DOI] [PubMed] [Google Scholar]
- 17.Henry B, Aussage P, Grosskopf C, Goehrs JM. Development of the Cystic Fibrosis Questionnaire (CFQ) for assessing quality of life in pediatric and adult patients. Qual Life Res 2003;12:63–76. [DOI] [PubMed] [Google Scholar]
- 18.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol 2003;28:535–546. [DOI] [PubMed] [Google Scholar]
- 19.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest 2005;128:2347–2354. [DOI] [PubMed] [Google Scholar]
- 20.McCoy K, Retsch-Bogart G, Oermann C, Gibson, R, Montgomery, AB. Aztreonam lysine for inhalation (AZLI) for CF patients with P aeruginosa (PA) infection. J Cyst Fibros 2007;6:S10. [Google Scholar]
- 21.American Thoracic Society Committee on Diagnostic Standards for Non-Tuberculous Respiratory Diseases. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 22.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 1983;127:725–734. [DOI] [PubMed] [Google Scholar]
- 23.Bucholski A, Keller M, Balcke A, Lintz FC, Seeann S, Flitter WD, Hofmann T, Stapleton KW. In vitro performance of eFlow, an electronic inhaler for administration of a novel aztreonam formulation to CF patients. Pediatr Pulmonol 2003;35:321. [Google Scholar]
- 24.Guyatt GH. Making sense of quality-of-life data. Med Care 2000;II:175–179. [DOI] [PubMed] [Google Scholar]
- 25.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–415. [DOI] [PubMed] [Google Scholar]
- 26.Quittner A, McCoy K, Montgomery B. Inhaled antibiotics to treat stable patients with cystic fibrosis and Pseudomonas aeruginosa (PA): measuring patient perception of symptom improvement. Pediatr Pulmonol 2007;42:301. [Google Scholar]
- 27.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 1998;27:158–163. [DOI] [PubMed] [Google Scholar]
- 28.Goss CH, Quittner AL. Patient-reported outcomes in cystic fibrosis. Proc Am Thorac Soc 2007;4:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J 2002;20:658–664. [DOI] [PubMed] [Google Scholar]
- 30.Lamb HM, Goa KL. Drugs in disease management: management of patients with cystic fibrosis: defining the role of inhaled tobramycin. Dis Manage Health Outcomes 1999;6:93–108. [Google Scholar]
- 31.Retsch-Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, Gibson R. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol 2008;43:47–58. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey BS, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 1993;328:1740–1746. [DOI] [PubMed] [Google Scholar]
- 33.Smith AL, Ramsey B, Hedges DL, Hack B, Williams-Warren J, Weber A, Gore EJ, Redding GJ. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr Pulmonol 1989;7:265–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.