Abstract
Rationale: The etiology and pathogenesis of angiogenesis in idiopathic pulmonary fibrosis (IPF) is poorly understood. Inter-α-trypsin inhibitor (IaI) is a serum protein that can bind to hyaluronan (HA) and may contribute to the angiogenic response to tissue injury.
Objectives: To determine whether IaI promotes HA-mediated angiogenesis in tissue injury.
Methods: An examination was undertaken of angiogenesis in IaI-sufficient and -deficient mice in the bleomycin model of pulmonary fibrosis and in angiogenesis assays in vivo and in vitro. IaI and HA in patients with IPF were examined.
Measurements and Main Results: IaI significantly enhances the angiogenic response to short-fragment HA in vivo and in vitro. lal deficiency Ieads to decreased angiogenesis in the matrigel model, and decreases lung angiogenesis after bleomycin exposure in mice. IaI is found in fibroblastic foci in IPF, where it colocalizes with HA. The colocalization is particularly strong in vascular areas around fibroblastic foci. Serum levels of IaI and HA are significantly elevated in patients with IPF compared with control subjects. High serum IaI and HA levels are associated with decreased lung diffusing capacity, but not FVC.
Conclusions: Our findings indicate that serum IaI interacts with HA, and promotes angiogenesis in lung injury. IaI appears to contribute to the vascular response to lung injury and may lead to aberrant angiogenesis.
Clinical trial registered with www.clinicaltrials.gov (NCT00016627).
Keywords: inter–α-trypsin inhibitor, hyaluronan, angiogenesis, pulmonary fibrosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Pulmonary fibrosis leads to respiratory failure and death. The pathogenesis of pulmonary fibrosis is still unclear, but angiogenesis is believed to be a significant component. Extracellular matrix plays an important role in angiogenesis.
What This Study Adds to the Field
Serum inter–α-trypsin inhibitor (IaI) and matrix hyaluronan are necessary for the angiogenic response to lung injury. IaI serum levels are inversely associated with gas exchange in fibrosis patients, indicating that IaI may be linked to aberrant angiogenesis.
Idiopathic pulmonary fibrosis (IPF) is a progressive, lethal interstitial lung disease, characterized by unremitting scarring of alveolar tissue and respiratory failure. The histological correlate of IPF is usual interstitial pneumonitis (UIP). The biological mechanism of UIP pathogenesis is unknown, but may involve an aberrant healing response to repetitive tissue injury (1). Angiogenesis is an essential component of wound healing, and is likely to be involved in lung repair and the pathogenesis of UIP. Both human and animal research has investigated angiogenesis in the development of fibrosis. A seminal article by Turner-Warwick more than 40 years ago demonstrated extensive angiogenesis in human IPF lungs (2). However, the existing literature offers a mixed picture, with some reports suggesting that a net-angiogenic imbalance exists in UIP lungs (3), and others showing that angiostatic factors are increased in fibroblastic foci of UIP lungs (4). Ultimately, these contrasting results could be explained by an overall temporal and spatial heterogeneity in angiogenic activity in pulmonary fibrosis (5, 6).
Several factors are likely to affect angiogenesis in IPF. Extracellular matrix deposition is significantly increased in IPF (7). Matrix components, such as fibronectin, vitronectin, versican, and hyaluronan, have known angiogenic properties (8–11). Hyaluronan, an abundant extracellular matrix component, is produced as a high–molecular weight polymer, and does not undergo additional modification (12). The angiogenic effects of hyaluronan are related to its size: high-molecular-weight hyaluronan (HA) has angiostatic properties (13), whereas short-fragment HA (sHA), which is produced in inflammation and tissue injury, is highly angiogenic (14), and mediates its effects via cell receptors, such as CD44 and RHAMM (10). HA binds to cells most efficiently in complex with inter–α-trypsin inhibitor (IaI) (15).
IaI is a composite protein containing a light chain (called urinary trypsin inhibitor [UTI] or bikunin), and two heavy chains that can bind HA (16). IaI is synthesized in the liver and secreted into the circulation, where it can be found in high concentrations of up to 0.5 mg/ml. From the serum, IaI reaches sites of injury via extravasation. The exact role of IaI is unclear. However, it is known that IaI has antiinflammatory properties (17, 18), and that it can bind HA, and thus stabilize extracellular matrix (19). Furthermore, it is known that HA can promote inflammation after lung injury (20). We therefore hypothesized that IaI could reach injured areas in the lung, bind HA, and have antiinflammatory activity there. We also hypothesized that IaI may interact with HA and promote angiogenesis in response to fibrotic lung injury.
In this study, we demonstrate that IaI significantly enhances the angiogenic effect of sHA in vitro. IaI is necessary in vivo for angiogenesis in a matrigel model of angiogenesis and the absence of IaI leads to enhanced inflammation and decreased angiogenesis in bleomycin-induced lung injury. In patients with IPF, serum concentrations of IaI and HA are elevated compared with control subjects, and are inversely correlated with lung diffusion capacity for carbon monoxide (DlCO), but not FVC measurements. IaI is found in fibroblastic foci in IPF, where it colocalizes with HA. The colocalization is particularly strong in vascular areas around fibroblastic foci. In aggregate, our findings indicate that serum IaI interacts with HA, and promotes angiogenesis in tissue injury and pulmonary fibrosis. Some of the results of these studies have been submitted in the form of an abstract at the International American Thoracic Society Conference, Toronto, Canada, 2008 (21).
METHODS
In Vitro Vascular Tube Formation Assay
Human microvascular endothelial cells type 1 (22) were seeded on 96-well plates at 1.5 × 104 cells/well over 50 ml of gelled, reduced growth factor Basal Membrane Extract (Trevigen, Inc., Gaithersburg, MD), and grown to 50–70% confluence. Endotoxin-free long-fragment HA (Healon; Advanced Medical Optics, Santa Ana, CA) was sonicated to produce sHA (see Figure E1 in the online supplement), and added to some wells at 0.5 mg/ml, with or without IaI (30 μg/ml). Tubular structures were photographed after 10 hours' incubation, and branching points per node were measured for quantification of angiogenesis.
Experimental Animals
IaI-deficient mice have been backcrossed onto C57BL/6 for more than 10 generations (19). Littermates were used as control animals. C57BL/6 mice from Jackson Laboratories (Bar Harbor, ME) were used for some experiments examining expression of IaI heavy chains (ITIH). Mice were kept in a specific pathogen-free facility. Experiments were approved by the Duke and National Institute of Environmental Health Sciences Animal Care and Use Committee.
In Vivo Matrigel Angiogenesis Assay
Matrigel (BD BioSciences, San Jose, CA) mixed with 20 U/ml heparin and 100 ng bFGF (R&D Systems, Minneapolis, MN) was instilled subcutaneously (0.5 ml) into isoflurane-anesthetized mice. One plug was injected per mouse. After 7 days, mice were killed via CO2 asphyxiation, the matrigel plugs were removed and fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained. Some plugs were weighed, homogenized in Dulbecco's phosphate-buffered saline (DPBS), the hemoglobin content was measured with Drabkin's solution (Sigma, St. Louis, MO) according to the manufacturer's instructions, and the results were normalized to 100 mg of matrigel plug (23).
Fibrotic Lung Injury
Mice received 2 U/kg intratracheal bleomycin (Sigma), and were killed by CO2 asphyxiation at different time points after instillation. Lung lavage was performed with 3 ml cold saline. Cell counts were performed using the Shandon Cytospin 3 setup (Thermo-Fisher, Waltham, MA) after cell staining with hematoxylin. The right lungs and liver snips were snap-frozen and stored at −80°C until used, and the left lungs were inflated with 10% buffered formalin and paraffin embedded.
Histology and Immunohistochemistry
Formalin-fixed human lungs (from surgical biopsies or autopsies) and mouse lungs were sectioned at 5 μm and stained as indicated with Alcian blue, hematoxylin and eosin, or with rabbit anti-IaI (Dako, Carpinteria, CA), biotinylated HA binding protein (Seikagaku America, East Falmouth, MA), CD31 (Dako), or Factor 8 (Abcam, Cambridge, MA). Matrigel vessels were stained with CD34 (eBioscience, San Diego, CA), as described elsewhere (24).
mRNA Expression Analysis
mRNA was isolated from frozen lungs and livers by the Trizol method. Real-time reverse transcriptase–polymerase chain reaction was performed for the IaI heavy chain 1 (forward GGTCTTTGGCTCTAAAGTGCAATC and reverse GGTGGCTTCCTTGAGCTTTGT) and heavy chain 2 (forward GCCATCCACATCTTCAATGAGAG and reverse CGCTTGAGAAAGCTGTAGAGCTG) using SYBR-Green assay and the 7900HT sequence detection system (Applied Biosystems, Foster City, CA). The housekeeping protein 18s RNA was used as the reference mRNA. Fluorescence values for each gene were normalized to those of 18s, and expressed as fold change over control groups using the comparative Ct method (25).
Patient Selection
Patient recruitment and selection was described in detail elsewhere (26). Briefly, patients and unaffected control subjects were recruited at Vanderbilt University (Nashville, TN) and National Jewish Medical and Research Center (Denver, CO), after approval by the institutional review boards and issue of a certificate of confidentiality from the National Institutes of Health. Standard criteria were used to establish the diagnosis of IPF (27, 28). After informed consent, all subjects underwent blood draw and FVC and DlCO measurements.
Serum IaI Measurement
Serum IaI levels were determined by using an inhibitory ELISA for the IaI light-chain UTI/bikunin. ELISA plates were coated with purified UTI (Mochida Pharmaceutical, Tokyo, Japan) at 4°C overnight, and blocked with 3% bovine serum albumin. Totals of 50 μl each of sample or standard (0–25 μg/ml) and 50 μl each of diluted rabbit anti-human UTI antiserum were added to the well, and the plates were incubated at 37°C for 1 hour. Detection was performed with horseradish peroxidase–conjugated goat anti-rabbit IgG. Color development was performed with TMB solution (Kirkegaard and Perry, Gaithersburg, MA), and the absorbance at 450 nm was measured on a Molecular Devices VersaMAX spectrophotometer (Sunnyvale, CA). Assays were performed in triplicate.
Statistical Analysis
Analysis of variance, Student's t test, and the chi-square test were performed using SPSS (SPSS, Inc., Chicago, IL) and GraphPad (GraphPad, Inc., San Diego, CA) software. A P value less than 0.05 was considered statistically significant. Results are represented as mean (±SEM).
RESULTS
IaI Enhances the Angiogenic Effect of sHA In Vitro
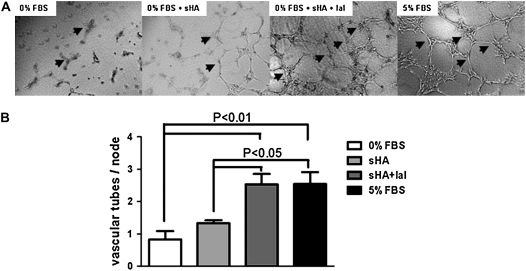
It is known that IaI binds to HA in the extracellular matrix and potentiates CD44–HA binding (29). We therefore hypothesized that IaI promotes angiogenesis through enhanced sHA binding. We first examined the effect of IaI on vascular tube formation in vitro. We found that human microvascular endothelial cells type 1 have more robust angiogenic response to sHA when IaI is added to the medium (Figure 1A). We quantified the effect by counting the number of vascular tubes per “node” (i.e., branching point), a measure of network complexity. At a concentration of 0.5/ml, sHA had no significant effect on vascular network formation compared with control. Addition of IaI to sHA significantly enhanced vascular tube formation and complexity (Figure 1B). Long-fragment HA and IaI alone had no effect on angiogenesis in these experiments. Addition of IaI to the long-fragment HA medium did not change the long-fragment HA response (data not shown), indicating that IaI is important for the angiogenic effect of sHA binding. Interestingly, although the IaI-sHA addition produced similar branch points per node as the positive control (fetal bovine serum), the vascular tubes had a less mature appearance, suggesting that other factors besides HA are important for the development of mature angiogenesis.
Figure 1.
In vitro vascular tube formation by human microvascular endothelial cells type 1. (A) Short-fragment hyaluronan (sHA) induces vascular tube formation, an effect that is significantly enhanced by inter–α-trypsin inhibitor (IaI). Negative control was 0% fetal bovine serum (FBS). Positive control was 5% FBS. (B) Quantification of vascular tube formation (Dunnett's multiple comparison testing; n = 6 wells/group). Arrowheads indicate vascular nodes. Error bars represent SEM.
IaI Is Necessary for In Vivo Angiogenesis in the Murine Matrigel Model
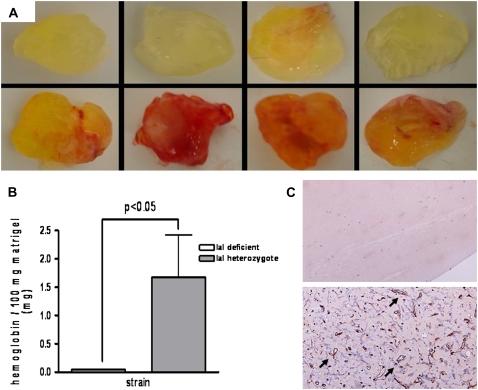
To further examine whether IaI was necessary for angiogenesis in the whole animal, we used an in vivo matrigel angiogenesis model. We found that IaI-deficient mice (Figure 2A, top row) had minimal angiogenesis into the matrigel plug as compared with IaI-sufficient mice (Figure 2A, bottom row). When we measured the hemoglobin content of the plugs as a means of quantification of angiogenesis, we saw a statistically significant difference between IaI-sufficient and -deficient mice. This was wholly attributable to the absence of hemoglobin and angiogenesis in plugs from IaI-deficient mice (Figures 2B–2C).
Figure 2.
In vivo matrigel angiogenesis assay. (A) Photographs of matrigel plugs explanted from inter–α-trypsin inhibitor (IaI)–deficient mice (top panels) or IaI-sufficient mice (bottom panels) 7 days after injection. There is a virtual absence of vascularization in plugs from IaI-deficient mice. (B) Quantification of plug hemoglobin content. No hemoglobin was detected in plugs from IaI-deficient mice. The difference from IaI-sufficient littermates is statistically significant. Error bars represent SEM. (C) Histologic examination of matrigel plugs. There were no visible cells or angiogenesis in plugs removed from IaI-deficient mice (top panel) compared with IaI-sufficient mice (bottom panel, arrows) (CD34 staining; original magnification = ×200; n = 7 mice/group).
IaI Is Expressed in the Liver in Response to Lung Injury, but Reaches the Lung during Inflammation and Fibrosis
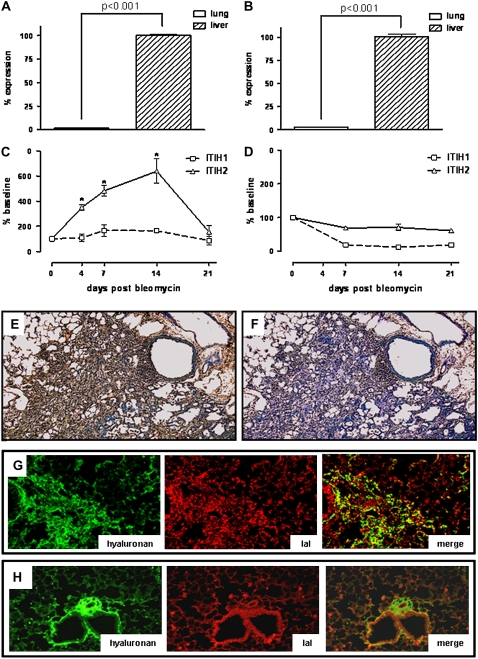
Angiogenesis is considered important in the pathogenesis of pulmonary fibrosis (4–6). We therefore evaluated the effect of IaI in a murine model of fibrotic lung injury. First, we determined the origin of IaI in injured lung by examining IaI heavy-chain expression after intratracheal bleomycin exposure. We examined lung and liver mRNA expression of IaI heavy chains ITIH1 and ITIH2, because they are components of circulating IaI in the mouse. At baseline, there was a roughly 50-fold higher mRNA expression of IaI heavy chains ITIH1 and ITIH2 in the liver compared with the lung (Figures 3A and 3B). We noted a significant up-regulation of heavy-chain mRNA expression in the liver in response to bleomycin, peaking between 7 and 14 days after bleomycin lung injury (Figure 3C). In contrast, the initially low mRNA expression of heavy chains in the lung declined even further, possibly due to influx of inflammatory cells and fibroblasts that do not express IaI mRNA (Figure 3D). In nonfibrotic lungs, IaI and HA staining is evident only in the subbronchial and subendothelial space (30). However, fibrotic lungs stained strongly for IaI after bleomycin exposure (Figures 3E–3F), and IaI colocalized with HA in fibrotic areas of the lung (Figure 3G). Similar results were obtained at 7 and 14 days after bleomycin exposure (data not shown). This was in contrast to naive lungs, where there is little HA–IaI colocalization (Figure 3H). These results suggest that, after bleomycin lung injury, IaI message is primarily expressed in the liver, and IaI protein is reaching the lung via the circulation rather than through local lung production.
Figure 3.
Expression of inter–α-trypsin inhibitor (IaI) heavy chains in liver and lung at baseline and after intratracheal bleomycin exposure in C57BL/6 mice. (A) inter–α-trypsin inhibitor heavy chain (ITIH1) and (B) ITIH2 are expressed in liver (arbitrarily set at 100% reference) more than 50-fold than in lung at baseline. (C) After bleomycin exposure, ITIH1 expression in the liver (open squares) is up-regulated twofold and ITIH2 expression (open triangles) is up-regulated more than sixfold. The highest up-regulation is seen between Days 7 and 14 after bleomycin (*P < 0.001). (D) Lung ITIH1 and ITIH2 expression decreases after bleomycin exposure. Error bars represent SEM. (E) IaI is detectable through immunohistochemistry (brown staining) in fibrotic lungs 3 weeks after bleomycin exposure (original magnification = ×40). (F) Isotype control for (E). (G) Staining of bleomycin-exposed lung for hyaluronan (left panel), and IaI (middle panel). There is hyaluronan–IaI colocalization evident in yellow (right panel) (original magnification = ×200). (H) Staining of unexposed lung for hyaluronan (left panel) and IaI (middle panel). Hyaluronan is mainly in the subepithelial space, and IaI mainly in bronchial epithelia, as well as in vessels (a result of serum IaI) with little colocalization (original magnifcation, ×200).
IaI Ameliorates Inflammation after Bleomycin Exposure and Promotes Angiogenesis in Response to Lung Injury
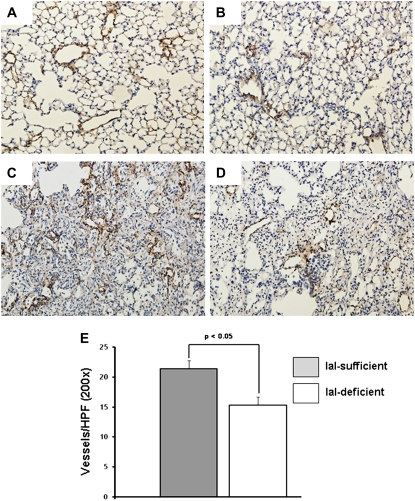
We then examined the effect of IaI on lung inflammation after bleomycin exposure. IaI-deficient mice demonstrated significantly increased cellular inflammation after bleomycin exposure compared with IaI-sufficient mice (Figures E2A and E2B). This effect persisted throughout 21 days of observation (Figure E2C). Whole-lung lavage HA levels were significantly higher in IaI-deficient mice compared with IaI-sufficient mice (Figure E2D). There was no difference in the degree of fibrosis by histology and lung collagen quantification at 21, 28, and 42 days after bleomycin exposure (data not shown). We examined angiogenesis in IaI-deficient and -sufficient mice at baseline, 7, 14, and 21 days after bleomycin lung injury through factor VIII staining. No difference was observed at baseline (Figures 4A and 4B). IaI-sufficient mice (Figure 4C) had higher vascular density in fibrotic areas than IaI-deficient mice (Figure 4D), a difference that was statistically significant after quantification (Figure 4E) at Days 14 and 21 after bleomycin exposure.
Figure 4.
Angiogenesis in bleomycin-exposed mice. No difference was observed in baseline vessels between (A) inter–α-trypsin inhibitor (IaI)-sufficient and (B) IaI-deficient mice. At 3 weeks after intratracheal bleomycin exposure, (C) IaI-sufficient mice show more vascularity in fibrotic areas than (D) IaI-deficient mice (factor VIII immunohistochemistry; original magnification = ×100). (E) The difference in vascularity is statistically significant when quantified. Two independent observers were involved in this measurement. One observer took photographs of all fibrotic areas in a longitudinal section of lung, and a second, blinded observer measured vessels in 10 high-power (×200 original magnification) fields (HPF) per mouse. The average per HPF for every mouse was used for statistical calculations (n = 10–14 per group). Error bars represent SEM.
IaI and HA Colocalize in UIP Lungs
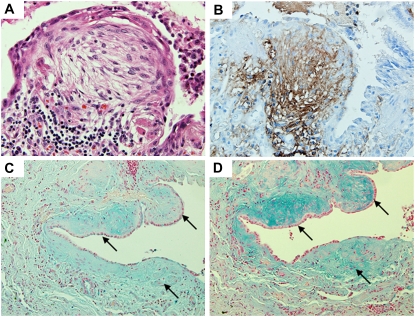
We examined whether IaI is found in the lung tissue of patients with IPF. We stained lungs for IaI and HA in patients with histologically verified UIP. We found that fibroblastic foci in UIP lungs (Figure 5A) stained positive for IaI (Figure 5B). HA staining was also strongly positive in fibroblastic foci, evidenced by positive Alcian blue staining, which was abolished by hyaluronidase pretreatment (Figures 5C and 5D). Confocal immunohistochemistry showed that IaI and HA colocalized in fibroblastic foci (Figure 6). Interestingly, colocalization appeared particularly strong in the periphery of fibroblastic foci, where blood vessels are found (Figures 7A–7D). Furthermore, colocalization of IaI and HA was most prominent in the subendothelial space of blood vessels adjacent to fibroblastic foci (Figures 7E–7G). We then stained lungs with different types of interstitial fibrotic lung diseases for IaI (Figure E3). Healthy lungs and lungs with hypersensitivity pneumonitis, acute interstitial pneumonia, and nonspecific interstitial pneumonia stained faintly for IaI, and the staining was found mostly in vessels and in the subendothelial basal membrane, as has been previously described (Figures E3A–E3C). Sarcoidosis lungs stained positive for IaI in the margins of sarcoidosis nodules (Figure E3D). UIP lungs stained positive in fibroblastic foci (Figure E3E). These results suggest that fibroblastic foci in UIP constitute a unique type of tissue injury among other interstitial lung diseases, where IaI can be found.
Figure 5.
Inter–α-trypsin inhibitor (IaI) and hyaluronan localize in fibroblastic foci in usual interstitial pneumonitis (UIP). (A) Representative photomicrograph of a fibroblastic focus in a patient with UIP (hematoxylin and eosin; original magnification = ×400). (B) Same fibroblastic focus stained for IaI (original magnification = ×400). IaI is present in the fibroblastic focus, staining most strongly in the margins. (C and D). Alcian blue staining of a lung with UIP fibroblastic foci (arrows) with (C) or without (D) hyaluronidase pretreatment (original magnification = ×200). Hyaluronidase treatment significantly diminishes the blue–green staining within the fibroblastic focus.
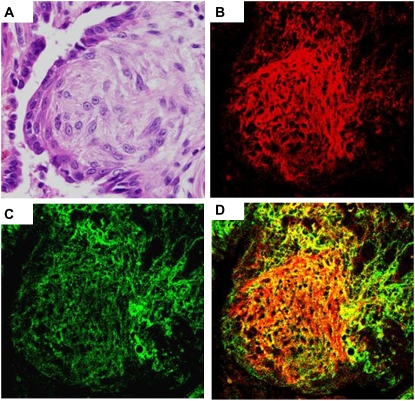
Figure 6.
Inter–α-trypsin inhibitor (IaI) and hyaluronan colocalize in fibroblastic foci in usual interstitial pneumonitis (UIP). (A) Hematoxylin–eosin stain of a fibroblastic focus (original magnification = ×400). (B) Immunohistochemistry for IaI (red; original magnification = ×400). (C) Immunohistochemistry for hyaluronan (green; original magnification = ×400). (D) Merged image for IaI and hyaluronan colocalization (yellow; original magnification = ×400).
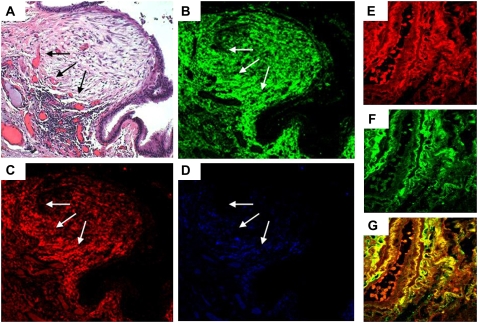
Figure 7.
Inter–α-trypsin inhibitor (IaI) and hyaluronan colocalize in the subendothelial space. (A) Fibroblastic focus stained for hematoxylin and eosin. The same fibroblastic focus stains positive for (B) hyaluronan and (C) IaI, particularly in the periphery (arrows), where vessels are marked with CD31 (D) (×200 original magnification). The subendothelial area and basal membrane of a small-sized blood vessel stain positive for (E) IaI and (F) hyaluronan, which colocalize in this location (G) (original magnification = ×630).
IaI Serum Concentrations Are Increased in Sera of Patients with IPF
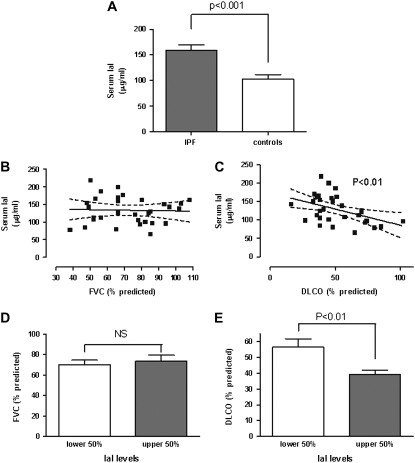
We measured the serum concentrations of IaI in 44 patients with IPF and 43 control subjects (Figure 8). We found that patients with IPF had significantly higher serum IaI concentrations compared with control subjects (Figure 8A). We then investigated the association of serum IaI concentration with parameters of lung function in 30 of the 44 patients, for whom complete lung function data were available. Serum IaI concentrations did not correlate with FVC (Figure 8B), but showed a significant inverse correlation with the DlCO measurements in patients with IPF (Figure 8C). To ensure that our correlations were not skewed by outliers, we compared patients with IPF in the lower 50% range of IaI levels to patients in the upper 50% range of IaI levels. There were no significant differences between groups with regard to male:female ratio, smoker:nonsmoker ratio, and age (Table 1). Patients with low or high IaI serum concentration did not differ in regard to FVC, indicating that serum IaI is not a specific marker of fibrosis severity (Figure 8D). However, patients with low serum IaI concentration had significantly higher DlCO levels than patients with high serum IaI (Figure 8E). In aggregate, these results indicate that serum IaI concentrations are associated with severity of deficits in gas exchange in patients with IPF.
Figure 8.
Inter–α-trypsin inhibitor (IaI) serum concentrations. (A) Significantly higher serum IaI concentrations in patients with idiopathic pulmonary fibrosis (IPF; gray bars) compared with control subjects (white bars). (B) No correlation exists between serum IaI concentrations and FVC. (C) Correlation between serum IaI concentrations and diffusion capacity for carbon monoxide (DlCO). (D) No difference in FVC between patients with high (white bar) and low (gray bar) serum IaI concentrations. (E) DlCO is significantly higher in patients with low serum IaI concentrations (white bar) than in patients with high serum IaI concentrations (gray bar). Error bars represent SEM.
TABLE 1.
PATIENT DEMOGRAPHICS BY SERUM INTER–α-TRYPSIN INHIBITOR LEVELS: LOWER 50% TO UPPER 50%
| Characteristics | Lower 50% IaI (n = 16) | Upper 50% IaI (n = 15) | Chi-Square | t Test |
|---|---|---|---|---|
| Male/female, % | 63/37 | 55/45 | 0.6 | |
| Smoker/nonsmoker, % | 63/37 | 60/40 | 0.7 | |
| Systemic steroid use, yes/no, % | 56/44 | 62/38 | 0.8 | |
| Other immunosuppressant use*, yes/no, % | 69/31 | 73/27 | 0.8 | |
| Other medication (bosentan), yes/no, % | 13/87 | 13/87 | 1.0 | |
| Mean age ± SEM, yr | 64 ± 4 | 67 ± 4 | 0.58 |
Definition of abbreviation: IaI = inter–α-trypsin inhibitor.
Cyclophosphamide, azathioprine, methotrexate, IFN-γ, etanercept, colchicine.
DISCUSSION
The development of lung fibrosis is believed to be associated with aberrant injury and repair. Angiogenesis is required for the normal healing response to tissue injury. Newly formed vessels deliver nutrients and inflammatory cells to the injury site, thus promoting wound healing. In chronic diseases like IPF, this balance may be disrupted, leading to aberrant angiogenesis with detrimental rather than salutary effects. Our findings indicate that serum IaI interacts with HA, and promotes angiogenesis in lung injury. This conclusion is supported by several complementary approaches. First, we show that, in vitro, IaI promotes the angiogenic effects of sHA. Second, we show that, in lung tissue injury, IaI interacts with HA to establish the angiogenic response. This underscores the synergistic roles of systemic and local factors in the course of chronic lung injury, and suggests that IaI may play a role in IPF. Third, we demonstrate that, among patients with IPF, serum IaI concentrations correlate with a disproportional decrease in DlCO, independent of restriction (FVC), and colocalize with HA in the fibroblastic foci. Our findings suggest that IaI contributes to the vascular response to lung injury, and may lead to aberrant angiogenesis.
This article is the first to document that IaI is important for HA-mediated angiogenesis in relevant models of tissue injury and repair. Our results suggest that circulating IaI is the source of the IaI found in injured tissue, and that extravasation of serum IaI in regions of tissue injury can bind local HA and facilitate neovascularization as a component of tissue repair. In this way, IaI may actually provide a positive angiogenic feedback loop in tissue injury–vessels deliver IaI in the area of injury, and IaI enhances local HA binding to promote further angiogenesis. It is interesting that IaI deficiency completely impairs angiogenesis in the matrigel model, whereas, in the bleomycin model, IaI-deficient mice still display some angiogenesis. We speculate that the explanation lies with HA levels. The enhancing effect of IaI in HA binding is particularly prominent at low and intermediate HA levels (29). Therefore, the high tissue HA levels after bleomycin injury (20) may counterbalance the effect of IaI deficiency. Indeed, we found that high concentrations of sHA had significant angiogenic effect in vitro, even in the absence of IaI (data not shown). In that sense, IaI may be particularly useful as a homeostatic agent, stimulating HA effects during low-level or chronic injury, such as in IPF. HA levels are elevated in IPF lung lavage fluid (31). HA can be degraded to lower molecular weight forms through reactive oxygen species or hyaluronidase activity, and de novo sHA is synthesized by fibroblasts and smooth muscle cells after cytokine or growth factor stimulation (32). Thus, although we did not have the opportunity to measure HA levels or sizes in lungs of patients with IPF, there is some evidence that sHA is the active HA component in IPF lungs (32). We speculate, therefore, that IaI likely binds to sHA and potentiates its angiogenic actions in IPF.
The results presented here suggest that IaI plays a role in the pathogenesis of IPF. Based on our presented animal data, we believe that, in the setting of IPF, IaI reaches the fibroblastic focus via the surrounding vessels, which explains the strong IaI staining in the perivascular area of fibroblastic foci. Our findings suggest that IaI in and around fibroblastic foci is not just a bystander but has angiogenic properties in IPF for several reasons. First, in the mouse matrigel model, IaI is essential for angiogenesis. Second, in bleomycin lung fibrosis, IaI deficiency decreases angiogenesis in the fibrotic area. Third, IaI and HA colocalize specifically in the subendothelial basal membrane. Finally, IaI serum levels correlate inversely with DlCO in patients with IPF.
Our findings indicate that the interaction between IaI and HA may play a role in aberrant angiogenesis in the pathogenesis of IPF. Angiogenesis is a part of the healing response after tissue injury; however, aberrant angiogenesis may instead support fibroproliferation and inhibit tissue repair (33). The presence of neovascularization in fibrotic lungs was pointed out decades ago (2). Newly formed vessels in IPF are fully integrated into the fibrotic tissue lattice (5, 34) and form a vascular network, which extends from the pleura to the parenchyma (35). The factors triggering IPF-related angiogenesis are incompletely understood. Studies have demonstrated the presence of both angiogenic factors, such as IL-8, macrophage inflammatory protein-2, epithelial neutrophil–activating protein (ENA)-78 and vascular endothelial growth factor (3, 36–38), and angiostatic factors, such as pigment epithelium–derived factor (PEDF) and CXCL11 (4, 39), in fibrotic lungs. Thus, a debate exists whether there is too much, too little, or simply heterogeneous angiogenesis in IPF (6). We observed that the presence of IaI led to significantly more angiogenesis in a murine model of fibrotic lung injury, and IaI colocalized with HA in vascular areas in UIP lungs. However, in patients with IPF, high IaI concentrations were associated not with higher, but with significantly lower gas exchange capacity (DlCO), independently of lung restriction (FVC). This suggests the presence of ongoing lung injury and repair in IPF lungs. We speculate that, in fibrotic areas, angiogenic and angiostatic factors are competing. Epithelia in fibrotic lungs may play an important role in this aspect. Epithelia overlying the fibroblastic foci are a rich source of chemokines and cytokines, which, in aggregate, promote a fibrotic microenvironment (40). However, it is possible that distressed epithelia send “conflicting signals” to the underlying stroma. On one hand, epithelial cells express proangiogenic signals, such as vascular endothelial growth factor and ENA-78 (36, 38), which stimulate angiogenesis. On the other hand, epithelial cells also release antiangiogenic factors, such as PEDF (4), thereby inhibiting angiogenesis. The leading edge of the fibroblastic focus may therefore be the edge of the angiostatic environment, with compensatory angiogenic activity promoted by IaI and HA. The net effect is the formation of vessels in the leading edge of fibroblastic foci. These vessels are not connected to ventilated parts of the lung, thus increasing shunt and ventilation–perfusion mismatch in patients with IPF (41, 42). The physiologic correlate is decreased DlCO, disproportionate to the degree of restriction (FVC).
DlCO and gas exchange are independent predictors of mortality in IPF (43–45). The degree of gas exchange impairment in IPF is likely a result of several coexisting processes (i.e., alveolar obliteration, pulmonary arterial hypertension, and vascular shunting). These processes are related, but not always interdependent: for example, pulmonary arterial pressures do not always correlate with FVC, and they are only loosely related to DlCO (44, 46). Furthermore, DlCO correlates poorly with histological abnormalities in IPF (47, 48). Our findings suggest that, in chronic lung injury, the ongoing angiogenic response to persistent lung injury may not be directly associated with the degree of restriction. In our study, serum concentrations of IaI were associated with decreased DlCO, but not FVC (Figure 8). Shunt flow is a major contributor to hypoxemia in IPF (41, 42). Taken together, our results imply that aberrant angiogenesis may independently contribute to impaired gas exchange in IPF.
In summary, we have shown that IaI is a novel mediator of angiogenesis, and that this action is predicated on binding to HA in the extracellular matrix. IaI is a systemic factor, and reaches areas of injury via the circulation. Overall, serum IaI concentrations are elevated in patients with IPF, indicating that IaI may contribute to tissue repair and angiogenesis in this disease. Serum IaI concentrations are associated with the severity of impaired gas exchange (DlCO), independent of the degree of lung restriction (FVC). Because gas exchange deficits predict mortality in IPF, our findings suggest that aberrant angiogenesis, mediated at least partly by matrix HA and serum IaI, may be an independent contributor of morbidity and mortality in IPF.
Supplementary Material
Supported in part by grants from National Institute of Environmental Health Services (ES11961) and National Heart, Lung, and Blood Institute (HL91335), and by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences, the National Cancer Institute, and the National Heart, Lung, and Blood lnstitute.
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200803-386OC on August 14, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Garantziotis S, Steele MP, Schwartz DA. Pulmonary fibrosis: thinking outside of the lung. J Clin Invest 2004;114:319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner-Warwick M. Precapillary systemic-pulmonary anastomoses. Thorax 1963;18:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keane MP, Arenberg DA, Lynch JP III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 1997;159:1437–1443. [PubMed] [Google Scholar]
- 4.Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, Schwarz MI, Cool CD, Worthen GS. Pigment epithelium–derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med 2004;170:242–251. [DOI] [PubMed] [Google Scholar]
- 5.Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, Sasano H, Kondo T, Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2004;169:1203–1208. [DOI] [PubMed] [Google Scholar]
- 6.Keane MP. Angiogenesis and pulmonary fibrosis: feast or famine? Am J Respir Crit Care Med 2004;170:207–209. [DOI] [PubMed] [Google Scholar]
- 7.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:1819–1828. [DOI] [PubMed] [Google Scholar]
- 8.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993;119:1079–1091. [DOI] [PubMed] [Google Scholar]
- 9.Jang YC, Tsou R, Gibran NS, Isik FF. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery 2000;127:696–704. [DOI] [PubMed] [Google Scholar]
- 10.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 2007;26:58–68. [DOI] [PubMed] [Google Scholar]
- 11.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J 2004;18:754–756. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997;242:27–33. [DOI] [PubMed] [Google Scholar]
- 13.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells: inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer 1997;71:251–256. [DOI] [PubMed] [Google Scholar]
- 14.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985;228:1324–1326. [DOI] [PubMed] [Google Scholar]
- 15.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 2003;278:47223–47231. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein–glycosaminoglycan–protein complex. J Biol Chem 2004;279:38079–38082. [DOI] [PubMed] [Google Scholar]
- 17.Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, Schwartz DA. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol 2007;179:4187–4192. [DOI] [PubMed] [Google Scholar]
- 18.Wu R, Cui X, Lim YP, Bendelja K, Zhou M, Simms HH, Wang P. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med 2004;32:1747–1752. [DOI] [PubMed] [Google Scholar]
- 19.Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K. Defect in SHAP-hyaluronan complex causes severe female infertility: a study by inactivation of the bikunin gene in mice. J Biol Chem 2001;276:7693–7696. [DOI] [PubMed] [Google Scholar]
- 20.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 2002;296:155–158. [DOI] [PubMed] [Google Scholar]
- 21.Garantziotis S, Zudaire E, Hollingsworth JW, Jiang D, Loyd JE, Richardson E, Zhuo L, Noble PW, Kimata K, Schwartz DA. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan mediate aberrant angiogenesis in idiopathic pulmonary fibrosis. AM J Respir Crit Care Med 2008;177:A726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992;99:683–690. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Cao H, Rao GN. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt signaling in 14,15-epoxyeicosatrienoic acid–induced angiogenesis. J Biol Chem 2006;281:905–914. [DOI] [PubMed] [Google Scholar]
- 24.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 2003;120:501–511. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 26.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA III, Sporn TA, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 2005;172:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 29.Zhuo L, Kanamori A, Kannagi R, Itano N, Wu J, Hamaguchi M, Ishiguro N, Kimata K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J Biol Chem 2006;281:20303–20314. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Sun GW, Terao T. Immunolocalization of hyaluronic acid and inter-alpha-trypsin inhibitor in mice. Cell Tissue Res 1999;296:587–597. [DOI] [PubMed] [Google Scholar]
- 31.Bjermer L, Lundgren R, Hallgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax 1989;44:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages: the role of HA size and CD44. J Clin Invest 1996;98:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzouvelekis A, Anevlavis S, Bouros D. Angiogenesis in interstitial lung diases: a pathogenetic hallmark or a bystander? Respir Res 2006;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renzoni EA, Walsh DA, Salmon M, Wells AU, Sestini P, Nicholson AG, Veeraraghavan S, Bishop AE, Romanska HM, Pantelidis P, et al. Interstitial vascularity in fibrosing alveolitis. Am J Respir Crit Care Med 2003;167:438–443. [DOI] [PubMed] [Google Scholar]
- 35.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med 2006;174:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keane MP, Belperio JA, Burdick MD, Lynch JP, Fishbein MC, Strieter RM. ENA-78 is an important angiogenic factor in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:2239–2242. [DOI] [PubMed] [Google Scholar]
- 37.Keane MP, Belperio JA, Moore TA, Moore BB, Arenberg DA, Smith RE, Burdick MD, Kunkel SL, Strieter RM. Neutralization of the CXC chemokine, macrophage inflammatory protein-2, attenuates bleomycin-induced pulmonary fibrosis. J Immunol 1999;162:5511–5518. [PubMed] [Google Scholar]
- 38.Simler NR, Brenchley PE, Horrocks AW, Greaves SM, Hasleton PS, Egan JJ. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax 2004;59:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strieter RM, Starko KM, Enelow RI, Noth I, Valentine VG. Effects of interferon-γ 1b on biomarker expression in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2004;170:133–140. [DOI] [PubMed] [Google Scholar]
- 40.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 2006;3:364–372. [DOI] [PubMed] [Google Scholar]
- 41.Agusti AG, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1991;143:219–225. [DOI] [PubMed] [Google Scholar]
- 42.Gunther A, Enke B, Markart P, Hammerl P, Morr H, Behr J, Stahler G, Seeger W, Grimminger F, Leconte I, et al. Safety and tolerability of bosentan in idiopathic pulmonary fibrosis: an open label study. Eur Respir J 2007;29:713–719. [DOI] [PubMed] [Google Scholar]
- 43.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med 2006;174:803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, Mishima M, Kitaichi M, Izumi T. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007;131:650–656. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994;149:450–454. [DOI] [PubMed] [Google Scholar]
- 46.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006;129:746–752. [DOI] [PubMed] [Google Scholar]
- 47.Cherniack RM, Colby TV, Flint A, Thurlbeck WM, Waldron JA Jr, Ackerson L, Schwarz MI, King TE Jr. Correlation of structure and function in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1995;151:1180–1188. [DOI] [PubMed] [Google Scholar]
- 48.Fulmer JD, Roberts WC, von Gal ER, Crystal RG. Morphologic–physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J Clin Invest 1979;63:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.