Abstract
Rationale: Recent studies have demonstrated that combined substitutions of rifapentine for rifampin and moxifloxacin for isoniazid in the standard, daily, short-course regimen of rifampin, isoniazid, and pyrazinamide produces stable cure in 12 weeks or less. This study was designed to more precisely evaluate the contribution of moxifloxacin and isoniazid to rifapentine-based regimens.
Objectives: We compared bactericidal activity and treatment-shortening potential between regimens consisting of isoniazid or moxifloxacin plus rifapentine and pyrazinamide administered either thrice-weekly or daily.
Methods: Using a mouse model of tuberculosis, we assessed bactericidal activity by performing quantitative cultures of lung homogenates over the first 12 weeks of treatment. Relapse rates were assessed after completing 8, 10, and 12 weeks of treatment to determine the duration of treatment necessary for stable cure.
Measurements and Main Results: After 4 weeks of treatment, daily and thrice-weekly therapy with rifapentine, moxifloxacin, and pyrazinamide was significantly more active than treatment with rifapentine, isoniazid, and pyrazinamide. By 8 weeks of treatment, all mice receiving the moxifloxacin-containing regimens were lung culture negative, whereas those mice receiving the isoniazid-containing regimens continued to be lung culture positive. However, the duration of treatment necessary to achieve stable cure was 10 weeks for daily regimens and 12 weeks for thrice-weekly regimens, regardless of whether isoniazid or moxifloxacin was used. All mice receiving standard daily therapy with rifampin, isoniazid, and pyrazinamide relapsed after 12 weeks of treatment.
Conclusions: These results suggest that regimens consisting of isoniazid or moxifloxacin plus rifapentine and pyrazinamide may dramatically shorten the duration of treatment needed to cure human tuberculosis.
Keywords: tuberculosis, rifapentine, treatment
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Recent evidence from murine models of tuberculosis suggests that treatment regimens consisting of moxifloxacin, rifapentine and pyrazinamide may halve the length of therapy needed to cure tuberculosis.
What This Study Adds to the Field
This study demonstrates that regimens including rifapentine and pyrazinamide may dramatically shorten tuberculosis therapy whether they are combined with isoniazid or moxifloxacin.
Treatment of tuberculosis (TB) with the standard, 6-month, short-course regimen of rifampin (R), isoniazid (H), and pyrazinamide (Z) is highly effective in curing drug-susceptible pulmonary TB (1). However, because the regimen is lengthy and complicated, improper provision of treatment and poor adherence are serious obstacles to effective control of TB (2, 3). New treatment regimens that radically shorten the duration of effective therapy are expected to improve treatment success rates and thereby play an important role in controlling the TB epidemic (4).
A recent experiment in the murine model demonstrated that increasing rifamycin drug exposure by replacing R with rifapentine (P) as well as replacing H with moxifloxacin (M) in the standard daily (5 out of 7 days [5/7]) regimen shortened the duration of treatment necessary to prevent relapse from 6 months to 3 months or less (5). Furthermore, decreasing the rhythm of drug administration from 5 days per week to 3 days per week did not significantly alter the efficacy of the PMZ regimen, provided that the dose of P was increased accordingly (5).
A second experiment examined the individual contributions of P and M to the overall activity of the daily PMZ regimen. Over the first 4 weeks of treatment, PMZ was more active than PHZ and RMZ, which were, in turn, more active than RHZ. By the 6-week time point, however, PHZ was more active than RMZ. Finally, the proportions of mice relapsing were not significantly different after treatment for 10 weeks with PMZ (0 of 15 [0%]) and PHZ (2 of 15 [13%]), but higher for mice treated with RMZ (8 of 15 [53%]). Hence, despite the clear benefit of replacing H with M in combinations containing a rifamycin and Z in the murine model (5–9), it was the individual replacement of R with P that contributed the greatest treatment-shortening potential to the PMZ regimen (5). This finding suggests that novel regimens based solely on replacing R with P have significant treatment-shortening potential, and could serve as highly effective alternatives to regimens based on replacing both R with P and H with M.
To directly address the respective contributions of H and M to P-based regimens, the current study was designed to fully characterize the differences in bactericidal activity (as determined by serial lung colony-forming unit [cfu] counts) and treatment-shortening potential (as determined by relapse rates after decreasing durations of treatment) between regimens consisting of PHZ and PMZ administered either daily (5/7) or thrice-weekly (3/7) in the murine model. Due to the lack of clinical data on the safety and tolerability of daily and thrice-weekly administration of P, we evaluated a range of doses that are under consideration for human trials.
METHODS
Antimicrobials
Drugs were obtained and stock solutions prepared as previously described (5).
Aerosol Infection
Six-week-old female BALB/c mice (Charles River, Wilmington, MA) were aerosol infected with Mycobacterium tuberculosis H37Rv using the Middlebrook Inhalation Exposure System (Glas-Col, Terre Haute, IN) with a log-phase broth culture. Mice were infected in four consecutive runs. All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Chemotherapy
Treatment began 14 days after infection. Drugs were administered by gavage either 5/7 or 3/7 at the following doses : H, 25 and 50 mg/kg when administered 5/7 and 3/7, respectively; M, 100 mg/kg; Z, 150 and 225 mg/kg when administered 5/7 and 3/7, respectively; R, 10 mg/kg; and P, 7.5, 10, 15, and 20 mg/kg (P7.5, P10, P15, and P20, respectively), as indicated.
At the start of treatment (Day 0 [D0]), mice were block randomized by infection run into 10 groups (Table 1). Negative-control mice went untreated (group 1) and positive-control mice received standard therapy with 2 months of RHZ followed by 1 month of RH 5/7 (group 2) or 3/7 (group 3). To assess the impact of substituting P for R in the standard 5/7 regimen, mice in groups 4 and 5 received 2 months of PHZ followed by 1 month of PH, with P7.5 and P10, respectively. To assess the impact of substituting P for R and M for H in the standard 5/7 regimen, mice in group 6 received 2 months of P10MZ followed by 2 weeks of P10M. In order to assess the impact of substituting P for R in the standard 3/7 regimen, mice in groups 7, 8, and 9 received 2 months of PHZ followed by 1 month of PH with P10, P15, and P20, respectively. Finally, to assess the impact of substituting P for R and M for H in the standard 3/7 regimen, mice in group 10 received 2 months of P15MZ followed by 1 month of P15M.
TABLE 1.
SCHEME OF EXPERIMENT
| No. of Mice Killed
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regimens* | D −13 | D 0 | Wk 4 | Wk 8 | Wk 10 | Wk 12 | Wk 8 (+3) | Wk 10 (+3)† | Wk 12 (+3) |
| Control animals | |||||||||
| (1) Infected, untreated | 12 | 12 | |||||||
| (2) 8 wk R10HZ + R10H (5/7) | 5 | 5 | 5 | 5 | 15 | ||||
| (3) 8 wk R10HZ + R10H (3/7) | 5 | 5 | 5 | 5 | |||||
| Treatment groups | |||||||||
| Daily | |||||||||
| (4) 8 wk P7.5HZ + P7.5H (5/7) | 5 | 5 | 5 | 5 | 15 | 15 | |||
| (5) 8 wk P10HZ + P10H (5/7) | 5 | 5 | 5 | 15 | 15 | 15 | |||
| (6) 8 wk P10MZ + P10M (5/7) | 5 | 5 | 15 | 15 | |||||
| Thrice-weekly | |||||||||
| (7) 8 wk P10HZ + P10H (3/7) | 5 | 5 | 5 | 5 | 15 | 15 | |||
| (8) 8 wk P15HZ + P15H (3/7) | 5 | 5 | 5 | 15 | 15 | ||||
| (9) 8 wk P20HZ + P20H (3/7) | 5 | 5 | 5 | 15 | 15 | ||||
| (10) 8 wk P15MZ + P15M (3/7) | 5 | 5 | 5 | 15 | 15 | ||||
Definition of abbreviations: H = isoniazid; M = moxifloxacin; P = rifapentine; R = rifampin; Z = pyrazinamide.
Drugs were given orally at the following doses: H, 25 and 50 mg/kg when administered daily (5 out of 7 days [5/7]) and thrice-weekly (3/7), respectively; R, 10 mg/kg; Z, 150 and 225 mg/kg when administered 5/7 and 3/7, respectively; M, 100 mg/kg; P, 10, 15, and 20 mg/kg, as indicated.
Months 8 (+3), 10 (+3), and 12 (+3) indicate that mice were killed 3 months after completing 8, 10, and 12 months of treatment.
Assessment of Treatment Efficacy
Treatment efficacy was assessed on the bases of lung cfu counts and the proportion of mice with culture-positive relapse after treatment completion. Untreated mice were killed the day after infection and 14 days later (D0), at the initiation of treatment. Treated mice were killed for quantitative culture of lung homogenates after completing 4, 8, 10, and 12 weeks of treatment, as previously described (5). The proportion of mice with relapse was assessed by killing a group of mice (n = 15 per group) 3 months after completion of treatment. Culture-positive relapse was defined by isolation of 1 cfu or more after plating the entire lung homogenate.
Statistical Analysis
Cfu counts were log10 transformed before statistical analysis. Mean cfu counts were compared using one-way analysis of variance followed by Dunnett's multiple comparison test (GraphPad Prism; GraphPad Software, Inc., La Jolla, CA). The proportions of mice relapsing after completing treatment were compared using Fisher's exact test (STATA 8.2; Stata Corporation, College Station, TX). The type I error rate for multiple comparisons was adjusted by Bonferroni's procedure.
RESULTS
Lung Colony-Forming Unit Counts before Treatment
The day after infection (D −13) the mean lung log10 cfu count (±SEM) was 3.66 ± 0.04. By the initiation of treatment on D0, the mean cfu counts had increased to 7.29 ± 0.09, 7.13 ± 0.08, 7.27 ± 0.05 and 7.13 ± 0.04 (P = 0.26) in mice infected during infection runs 1, 2, 3, and 4, respectively. All infected, untreated mice (n = 5) were killed when they became moribund at 3 weeks after infection.
Lung Colony-Forming Unit Counts during Treatment
After 4 weeks of treatment, all 5/7 P-based regimens had significantly greater bactericidal activity than R10HZ 5/7 (P < 0.001) (Figure 1a). Mice treated with P7.5HZ, P10HZ, and P10MZ 5/7 had mean lung cfu counts that were 0.62, 1.00, and 2.22 log10 cfu lower than those of mice treated with R10HZ 5/7, respectively (P < 0.01). Likewise, all 3/7 P-based regimens were significantly more active than R10HZ 3/7 (P < 0.0001). Mice treated with P10HZ, P15HZ, P20HZ, and P15MZ 3/7 had mean lung cfu counts that were 1.26, 1.51, 1.75, and 2.99 log10 lower than those of mice treated with R10HZ 3/7, respectively (P < 0.01).
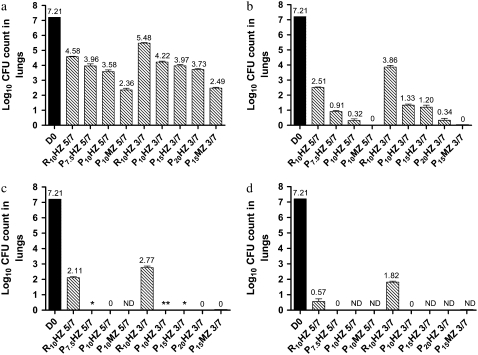
Figure 1.
Lung log10 colony-forming unit (CFU) counts at treatment initiation (Day 0 [D0; black bars]) and after (a) 4 weeks, (b) 8 weeks, (c) 10 weeks, and (d) 12 weeks in Mycobacterium tuberculosis–infected mice. Data are presented as means and SD (n = 5 mice per time point). H = isoniazid; M = moxifloxacin; ND = not determined; P = rifapentine; R = rifampin; Z = pyrazinamide. *One of five mice harbored 1 cfu/lung; **three of five mice harbored 1 cfu/lung.
After 8 weeks of treatment, the greater bactericidal activity of P-based regimens was even more apparent (Figure 1b). Mice that received R10HZ 5/7 harbored 2.51 log10 cfu per lung, whereas those that received P7.5HZ and P10HZ 5/7 harbored 0.91 and 0.32 log10 cfu, respectively (P < 0.001), and all five mice treated with P10MZ 5/7 had negative cultures. Mice that received R10HZ 3/7 had more than 3.5 log10 cfu per lung, whereas those that received P10HZ, P15HZ, and P20HZ 3/7 had 1.33, 1.20, and 0.34 log10 cfu per lung, respectively (P < 0.001), and all mice treated with P15MZ 3/7 had negative cultures. Therefore, in the standard daily or thrice-weekly regimen of R10HZ, the replacement of R with P resulted in a significant increase in bactericidal activity. Further increases in activity were observed with the additional replacement of H with M.
After 10 weeks of treatment, all mice that received P-containing regimens had negative lung cultures, with the exception of one mouse each in the P7.5HZ 5/7 and P15HZ 3/7 groups, and three mice in the P10HZ 3/7 group (Figure 1c). Each of the five culture-positive mice harbored only 1 cfu in the entire lung. In contrast, mice that received R10HZ 5/7 or 3/7 had more than 2 log10 cfu per lung.
Finally, after 12 weeks of treatment, all mice that received P7.5HZ 5/7 and P10HZ 3/7 were culture negative, whereas mice that received R10HZ 5/7 and 3/7 still harbored 0.57 and 1.82 log10 cfu per lung, respectively (Figure 1d).
Culture Status 3 Months after Treatment Completion
Among mice that received 8 weeks of treatment with P10HZ and P10MZ 5/7, relapse occurred in 10 of 15 (67%) and 4 of 15 (27%), respectively (Table 2) (P = 0.07). After 2 additional weeks of treatment, no mouse treated with P10HZ or P10MZ 5/7 relapsed. However, 9 of 15 (60%) mice that received the lower P7.5 relapsed (P = 0.0007, compared with P10HZ and P10MZ). The 12-week treatment with P7.5HZ 5/7 prevented relapse in all mice. As expected, all mice that received R10HZ 5/7 for 12 weeks were culture positive after 3 months of follow-up (P < 0.0001, compared with 12 wk of P7.5HZ and P10HZ [5/7]).
TABLE 2.
RELAPSE AFTER COMPLETING TREATMENT
| No. of Mice Relapsing after Treatment % (Proportion)
|
|||
|---|---|---|---|
| Treatment Group* | 8 wk | 10 wk | 12 wk |
| Daily | |||
| R10HZ 5/7 | — | — | 100 (15/15) |
| P7.5HZ 5/7 | — | 60 (9/15) | 0 (0/15) |
| P10HZ 5/7 | 67 (10/15) | 0 (0/15) | 0 (0/15) |
| P10MZ 5/7 | 27 (4/15) | 0 (0/15) | — |
| Thrice-weekly | |||
| R10HZ 3/7 | — | — | — |
| P10HZ 3/7 | — | 87 (13/15) | 33 (5/15) |
| P15HZ 3/7 | — | 53 (8/15) | 0 (0/15) |
| P20HZ 3/7 | — | 7 (1/15) | 0 (0/15) |
| P15MZ 3/7 | — | 7 (1/15) | 0 (0/15) |
See Table 1 for definition of abbreviations.
Z administered for the first 2 months of therapy only.
Among mice treated with 3/7 P-containing regimens, a similar dose-dependent effect of P on relapse was observed (Table 2). After 10 weeks of treatment with P10HZ, P15HZ, and P20HZ 3/7, relapse was observed in 13 of 15 (87%), 8 of 15 (53%), and 1 of 15 (7%) mice, respectively. When M was substituted for H in the P15HZ regimen, the proportion of mice with relapse fell to 1 of 15 (7%) (P = 0.014), indicating the additional benefit of replacing H with M. The 12-week treatment with thrice-weekly P-containing regimens prevented relapse in all mice, except in 5 of 15 (33%) mice treated with P10HZ (P = 0.04).
DISCUSSION
The present experiment was designed to determine the differences in bactericidal activity and treatment-shortening potential between PHZ- and PMZ-based regimens in the murine model of TB. Our results demonstrate that daily administration of P10HZ or P10MZ resulted in stable cure of all mice after only 10 weeks of treatment, and that thrice-weekly administration of P15HZ or P15MZ was able to achieve stable cure of all mice after only 12 weeks of treatment. These results reaffirm those of previous studies demonstrating the dramatic treatment-shortening potential of daily and thrice-weekly PMZ regimens (5), and extend those results by demonstrating that similar PHZ regimens may also be capable of shortening the duration of treatment to 12 weeks or less.
Although treatment with either PHZ or PMZ yielded stable cure by 10–12 weeks, treatment with PMZ clearly displayed superior bactericidal activity during the first month of treatment, in which the rates of decline in bacterial colony-forming unit counts were 0.91 and 1.21 log10 cfu/week for P10HZ 5/7 and P10MZ 5/7, and 0.81 and 1.18 for P15HZ 3/7 and P15MZ 3/7, respectively. The benefit of substituting M for H remained evident after 8 weeks of treatment. All mice that received P10MZ 5/7 or P15MZ 3/7 had negative lung cultures, whereas those mice that received P10HZ 5/7 and P15HZ 3/7 had positive lung cultures. The beneficial impact of substituting M for H has previously been demonstrated in the murine model with both RHZ- and PHZ-based regimens (5, 7, 8). In these studies, the enhanced initial bactericidal activity of M over H after 8 weeks of treatment has ranged from 0.72 log10 cfu/lung for twice-weekly RMZ to 2.46 log10 cfu/lung for daily RMZ; similar results have been observed in studies replacing H with sparfloxacin (10). These findings, along with those presented here, clearly demonstrate that replacing H with M, in either the standard RHZ regimen or the experimental PHZ regimen, significantly increases initial bactericidal activity. Previous studies in the murine model demonstrate that H antagonizes the activity of regimens composed of RZ with and without a fluoroquinolone, and that removal of this antagonistic effect explains much of the beneficial impact of replacing H with M (7, 10, 11). Although the precise mechanism of this effect has not been described, it is unlikely to result from an adverse pharmacokinetic interaction (6). Whether this same antagonistic effect is observed in PZ-based regimens remains unknown, and is currently under investigation. Finally, it is reasonable to question the relevance of this apparent antagonism to the treatment of humans. Thus far, no human trial has been designed to address this issue, although an ongoing clinical trial comparing RHZE, RHZM, and RMZE may help to indirectly address whether this phenomenon is observed in humans.
The finding that both PHZ and PMZ regimens achieved stable cure by 10–12 weeks underscores that the overall treatment-shortening effect is largely driven by the greater sterilizing activity of P over R at the doses studied. This was clearly evident in comparing the results of 12 weeks of treatment with RHZ and P10HZ 5/7. All mice receiving RHZ remained culture positive at the completion of treatment, while all mice receiving PHZ were cured. This dramatic increase in sterilizing activity that occurs upon replacing R with P is likely due to the greater rifamycin drug exposure obtained with P (5, 8, 12). When comparing P to R at the same dose and dosing frequency in the murine model, P produces a superior pharmacodynamic profile, in which the free drug area under the concentration–time curve divided by the minimum inhibitory concentration is 2.5-fold higher, and P concentrations exceed the minimum inhibitory concentration for the entire dosing interval, as compared with 60% of the time for R (5). Overall, these finding suggest that regimens based solely on substituting P for R in the standard RHZ 5/7 or 3/7 regimens may prove, clinically, to be as effective in shortening the duration of therapy as regimens based on substituting both P for R and M for H.
Given the exposure-dependent activity of the rifamycins (13), we hypothesized that increasing the dose of P would result in enhanced sterilizing activity, and thereby decrease the proportion of mice relapsing after treatment. In mice treated with PHZ 3/7 for 10 weeks, increasing the dose from P10 to P20 decreased the occurrence of relapse in a dose-dependent fashion, from 87% with P10HZ to 7% with P20HZ. Likewise, increasing the dose from P7.5 to P10 in the PHZ 5/7 regimen reduced the proportion of mice relapsing after 10 weeks of treatment from 60 to 0%. Given that the P doses tested in the current experiment correspond to the same doses in humans, as measured by serum concentration-versus-time profiles, it is reasonable to speculate that increasing P doses in humans would also result in enhanced sterilizing activity (5, 8). Before addressing this hypothesis, however, it will be critical to establish the safety and tolerability profile of thrice-weekly and daily P.
That thrice-weekly treatment with P15HZ displayed a similar degree of bactericidal and sterilizing activity as treatment with daily P7.5HZ underscores the notion that intermittent regimens can be as active as daily regimens, as long as the P dose is increased accordingly. It is important to stress that this dosing strategy may only be applicable to regimens based on P, as previous clinical studies have reported a strong association between a potentially fatal hypersensitivity syndrome and increasing R doses in intermittent regimens (14). Therefore, P may have a dually beneficial role in the treatment of TB by simultaneously decreasing the duration of treatment and decreasing the frequency of administration without compromising efficacy. These results are especially encouraging, given that a recent systematic review of published clinical trials on RHZ-based regimens reported that decreasing the frequency of administration from daily to thrice weekly or twice weekly increases the risk of relapse (15).
In conclusion, we have used a well-established murine model to determine the treatment-shortening potential of regimens based on PHZ and PMZ. Our study demonstrates that both H- and M-containing regimens produce stable cure in a similar time frame, despite the greater initial bactericidal activity of the PMZ regimen. Therefore, replacing R with P may prove to be a simple alternative to replacing both R with P and H with M in shortening the duration of effective therapy. If daily and thrice-weekly P is shown to be safe and tolerable in humans, P-based regimens may have enormous potential to significantly shorten the treatment of TB.
Supported by National Institutes of Health contract AI40007 and grant AI58993.
Originally Published in Press as DOI: 10.1164/rccm.200807-1029OC on August 21, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.British Thoracic Society. A controlled trial of 6-months chemotherapy in pulmonary tuberculosis. Final report: results during the 36 months and after the end of chemotherapy and beyond. Br J Dis Chest 1984;78:330–336. [PubMed] [Google Scholar]
- 2.Maartens G, Wilkinson RJ. Tuberculosis. Lancet 2007;370:2030–2043. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson RE, Martinson NA. Tuberculosis in Africa: combating an HIV-driven crisis. N Engl J Med 2008;358:1089–1092. [DOI] [PubMed] [Google Scholar]
- 4.Stop TB Partnership and World Health Organization. Global Plan to Stop TB 2006–2015. Geneva: World Health Organization; 2006. Publication No. WHO/HTM/STB/2006.35.
- 5.Rosenthal IM, Zhang M, Williams KN, Peloquin CA, Tyagi S, Vernon AA, Bishai WR, Chaisson RE, Grosset JH, Nuermberger EL. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model [Internet] [accessed July 6, 2008]. PLoS Med 2007;4(12):e344. Available from: http://medicine.plosjournals.org/archive/1549-1676/4/12/pdf/10.1371_journal.pmed.0040344-L.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuermberger EL, Yoshimatsu T, Tyagi S., O'Brien RJ, Vernon AN, Chaisson RE, Bishai WR, Grosset JH. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med 2004;169:421–426. [DOI] [PubMed] [Google Scholar]
- 7.Nuermberger EL, Yoshimatsu T, Tyagi S, Williams K, Rosenthal I, O'Brien R, Vernon A, Chaisson R, Bishai W, Grosset J. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med 2004;170:1131–1134. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal IM, Williams K, Tyagi S, Peloquin CA, Vernon AA, Bishai W, Grosset JH, Nuermberger EL. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am J Respir Crit Care Med 2006;174:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal I, Williams K, Tyagi S, Vernon A, Peloquin C, Bishai W, Grosset J, Nuermberger E. Weekly moxifloxacin and rifapentine is more active than the Denver regimen in murine tuberculosis. Am J Respir Crit Care Med 2005;172:1457–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalande V, Truffot-Pernot C, Paccaly-Moulin A, Grosset J, Ji B. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother 1993;37:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosset J, Truffor-Pernot C, Lacroix C, Ji B. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 1992;36:548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burman W, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 2001;40:327–341. [DOI] [PubMed] [Google Scholar]
- 13.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 2003;47:2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosset J, Leventis S. Adverse effects of rifampin. Rev Infect Dis 1983;5:S440–S446. [DOI] [PubMed] [Google Scholar]
- 15.Chang K, Leung C, Yew W, Ho S, Tam C. A nested case–control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med 2004;170:1124–1130. [DOI] [PubMed] [Google Scholar]