Abstract
An effective intranasal (i.n.) vaccine against pneumonic plague was developed. The formulation employed two synthetic lipid A mimetics as adjuvant combined with Yersinia pestis-derived V- and F1- protective antigens. The two nontoxic lipid A mimetics, classed as amino-alkyl glucosaminide 4-phosphates (AGPs) are potent ligands for the Toll-like receptor (TLR) 4. Using a murine (BALB/c) pneumonic plague model, we showed a single i.n. application of the vaccine provided 63% protection within 21 days against a Y. pestis CO92 100LD50 challenge. Protection reached 100% by 150 days. Using a homologous i.n. 1°/2° dose regimen, with the boost administered at varying times, 63% protection was achieved within 7 days and 100% protection was achieved by 21 days after the first immunization. Little or no protection was observed in animals that received antigens alone, and no protection was observed when the vaccine was administered to BALB/c TLR4 mutant mice. Vaccine-induced serum IgG titers to F1 and V-antigen were reflected in high titers for IgG1 and IgG2a, the latter reflecting a bias for a cell-mediated (TH1) immune response. This intranasal vaccine showed 90% protection in Sprague-Dawley rats challenged with 1000LD50. We conclude that lipid A mimetics are highly effective adjuvants for an i.n. plague vaccine.
1. Introduction
The plague bacterium, Yersinia pestis, is classed as a bioterrorism Category A agent. This Center for Disease Control classification has been assigned because the organism can be spread by aerosol, has a high mortality rate [1, 2] and its illicit release could have a major impact on public health (http://www.bt.cdc.gov/agent/agentlist-category.asp). Previously U.S.-licensed Y. pestis whole cell vaccines only provided protection against the bubonic form of the disease that is acquired from the bite of the rodent flea vector and were not protective against pneumonic exposure [3, 4]. For this reason, the whole cell vaccine was removed from the market and efforts to develop an efficient vaccine for pneumonic plague have been an important focus in recent years.
It is now well established that immunity generated against two Y. pestis antigens, F1 (capsule protein) and V-antigen (type III secretin component), are protective against pneumonic plague [5–8]. Both of these antigens are virulence factors produced by Y. pestis at 37 °C; F1- capsules inhibit phagocytosis, and the V-antigen forms the distal tip of the type III secretin structure [9–14]. Subunit vaccines employing purified F1 antigen (F1) and V-antigen proteins, or a recombinant fusion protein combining epitopes of each antigen, administered with classical adjuvants such as alum (alhydrogel), can provide up to 100% protection in a murine or non-human primate pneumonic plague model [6, 15–17]. Other pneumonic plague vaccine candidates have utilized V-antigen alone which stimulates humoral protection in challenge studies with virulent Y. pestis and a Y. pestis F1 (caf1) mutant [9, 15, 18, 19]. Most of these successful vaccination studies employ a traditional alum-based adjuvant, require multiple subcutaneous injections, and do not provide protection from a Y. pestis aerosol challenge until 35 or more days post-immunization [6, 9, 20]. Presently alum is the only approved vaccine adjuvant in the U.S. and while effective, it has a humoral immune response bias (stimulating TH2 cells) versus promoting a cellular response (stimulating TH1 cells)[21]. As Smiley recently suggests [22], a more effective plague vaccine should be one that fosters a TH1 response because Y. pestis can survive and replicate within macrophages.
Several immunization studies have also been conducted using intranasal (i.n.) vaccine applications. Jones et al. [23] established that an intranasal vaccine comprised of F1 and V-antigen mixed with the mucosal adjuvant Protollin™ could provide high-level protection in a pneumonic plague model when mice were challenged with Y. pestis CO92 no sooner than 35 days post-immunization [23]. Other investigators have utilized a subunit vaccine against pneumonic plague in a 1°/2° dose regimen that evaluated intranasal application and injection of the vaccine preparations, but only two dose regimens that included an injected dose provided 100% protection [24, 25].
More recently, alternative adjuvants including Sigma adjuvant system (an oil-in-water emulsion containing generic monophosphoryl lipid A plus trehalose dicorynomycolate), CpG (synthetic unmethylated dinucleotides), or ADP-ribosylating enterotoxins, have been examined [7]. The former two rely on stimulation of Toll-like receptors (TLRs); TLR4 for lipid A and TLR9 for CpG DNA, both of which induce TH1 immune responses. The ADP-ribosylating enterotoxins appear to elicit a more balanced TH1 and TH2 response. These studies demonstrate that adjuvant selection is important in directing the type of immune response elicited.
The ability of Y. pestis to evade the innate immune response is well established [26]. Temperature induced modulation of Y. pestis lipid A from hexa-acylated (25°C) to tetra-acylated (37°C) is an important aspect of this process because tetra-acylated lipid A does not activate TLR4 and Y. pestis mutants engineered to express hexa-acylated lipid A at 37°C are attenuated [27, 28]. In this report, we examined the efficacy of using two amino-alkyl glucosaminide 4-phosphates (AGPs) as adjuvant for an intranasal pneumonic plague vaccine. These synthetic compounds, designated CRX-524 and CRX-527, are immunostimulatory ligands for TLR 4, but lack the highly toxic properties of bacterial-derived lipid A [29, 30]. A variety of AGP lipid A mimetics have been synthesized with varying lengths of secondary acyl side chains and the functional group on the aglycon component. The AGPs employed in this study (CRX-524 and CRX-527) have acyl side chains of 10 carbons and contain either H or a carboxyl group respectively at the aglycon unit [31]. We have shown these compounds, when administered intranasally, induce high levels of TNF-α, IL-12p70, and IFN-γ in murine lung tissue [32]. Using a murine model of pneumonic plague, we tested an intranasal vaccine using AGPs as adjuvant with F1 and/or V-antigen to determine i) the best concentrations of AGPs; ii) the effect of primary and secondary vaccine regimens; iii) the shortest duration to protection; iv) the requirement for TLR4 stimulation; and v) protection in rats.
2. Materials and Methods
2.1. Bacteria and growth conditions
Y. pestis CO92 (CDC, Ft. Collins, CO) was grown in Brain Heart Infusion (BHI) broth overnight at 30°C, diluted 1:200, and incubated for 36 h at 30°C with aeration. For use in experiments, aliquots of a single stock culture (~1 × 108 CFU/ml) were mixed with 20% glycerol (v,v), and stored at −80°C. Challenge doses for each experiment were quantified by plate counts. All experiments were performed under CDC-certified BSL-3 conditions at the University of Idaho.
2.2. Vaccine preparation
F1 was purified from Y. pestis as described by Andrews et al. [4] with minor modifications. Briefly, Y. pestis KIM 6 pgm+/pCD1− was grown in flasks on a platform shaker (200 rpm) at 37°C in BHI broth (pH 8) for 48 h. Cells were removed by centrifugation, the culture supernatant filter sterilized, and F1 protein precipitated with 30% ammonium sulfate at 4°C for 12 h. The precipitate was resuspended in 0.10X volume of phosphate buffered saline (PBS) and dialyzed against PBS. Protein purity and identity was assessed by SDS-PAGE and immuno-blotting using anti-F1 antibody. From Y. pestis KIM 5 the pCD1 plasmid (courtesy of R. Perry, University of Kentucky, Lexington, KY) lcrV coding sequence was PCR amplified with primers lcrVFor (with PstI) 5’CGCGCTGCAGATGATTAGAGCCTACGAA3’ and lcrVRev (with EcoRI) 5’CGCGAATTCTCATTTACCAGACGTGTC 3’ and cloned into pBAD HisC (N-terminal His-tagged, Invitrogen) digested with EcoRI and PstI. The recombinant plasmids were transformed into E. coli Top 10 (Invitrogen) and re-isolated to verify lcrV inserts by DNA sequencing and detection on Western blot. LcrV (V-antigen) expression was induced with 0.002% arabinose for 4 h, the cells were lysed, and V-antigen was purified using a Ni2+-affinity column under non-denaturing conditions per the manufacturer’s specifications (His Bind Purification Kit, Novagen). V-antigen purity and identity was verified by SDS-PAGE and immunonoblot using anti-V antibody.
Both purified antigens were mixed with adjuvant AGP compounds, CRX-524 and CRX-527 (synthesized and provided by GlaxoSmithKline), on the day of immunization and instilled into mice or rats intranasally within 5 h of preparation. Unless specified otherwise, murine experiments used a vaccine formulation consisting of 2 µg F1 + 6 µg V-antigen + 20 µg AGPs (10 µg CRX-524 + 10 µg CRX-527) in a total volume of 28 µL. Control groups in vaccine trials included mice that received antigens only, adjuvant only, or PBS only. In rat trials, animals received an i.n. vaccine containing 4 µg F1 +12 µg V-antigen + 40 µg AGPs (20 µg CRX-524 + 20 µg CRX-527) in a total volume of 56 µL; control groups were mock vaccinated with PBS.
2.3. Animals
Eight to ten week old female BALB/c mice and Sprague-Dawley rats were obtained from Simonsen Labs. F1-F6 generations of BALB/c TLR4 mutant mice were obtained by harem mating BALB/c TLR4 mutant [33] males and females (Jackson Laboratories). Animals were provided food and water ad libitum and handled in accordance with the University of Idaho’s Animal Care and Use Committee guidelines. All animal experiments involving Y. pestis CO92 were performed in the BSL-3 laboratory under strict adherence to the requirements and procedures in the use of select agents and toxins and in accordance with the University of Idaho Biosafety Committee. Challenged mice were housed in a mouse containment apparatus (BioZone MiniRack, BioZone Ltd.) with cage mates receiving the same vaccination regimen. Challenged rats were housed in standard rat cages with micro-isolator lids inside the BSL-3 Biological Safety Cabinet Class II, Type IV. All animals were observed daily for 14 days for signs of illness (ruffled fur, weight loss, decreased mobility) and bodies removed at death. Cages, bedding, food, and expired animals were autoclaved at 121°C for 1 h prior to removal from BSL-3 containment for cleaning and/or disposal.
2.4. Immunization regimens
Female BALB/c mice were tested in groups of 8. Each group was inoculated with the i.n. vaccine, or control materials, while under anesthesia (3% isoflurane) using a rodent anesthesia system (EZ-Anesthesia 2000, Euthanex Corp.). For single vaccine dose experiments, a 28 µL total volume of vaccine was instilled into the nares of prone-positioned animals. For experiments involving a 1°/2° application, the second dose was administered 24 h, 3 days, or 10 days after the initial i.n. vaccination. In each experiment, control groups received antigens alone, AGPs alone, or PBS in the same volume, route, and vaccination schedule.
For rat vaccine trials, groups of 10 female, ten to twelve week-old pathogen-free Sprague-Dawley rats (Simonsen Labs) were immunized as above with 56 µL total volume of an i.n. vaccine. These animals received a second i.n. dose 10 days after the initial vaccination. Control animals received PBS in the same volume, route and vaccination schedule.
2.5 Bacterial challenge
Relative to vaccination on day 0, a 1 ml aliquot of virulent Y. pestis CO92 stock culture was thawed the day of challenge. The aliquot was diluted for the challenge dose and for verifying the cell numbers by LB agar plate counts done in triplicate. After being anesthetized as described above, mice were challenged intranasally with a Y. pestis CO92 dose of 100 LD50s (2 × 106 organisms) in 10µL (5µL/nare). Immunized mice were challenged 1, 3, 7, 14, 21, 28, 45, 60, 90, 120, 150, or 180 days after vaccination. Protection was measured over a 14 day interval with death as the endpoint.
To our knowledge, the LD50 for pneumonic plague in rats has not been reported. The LD50 was determined by the method of Reed and Muench [34]. Five groups of rats (n = 4) were given decimal dilutions over a range of 1.5 × 101 to 1.5 × 105 CFUs of Y. pestis CO92 by i.n. inoculation and the mortality was determined for each group over a period of ten days. For rat vaccine studies, vaccinated and control groups were challenged intranasally with a 50µL Y. pestis CO92 dose of 1 × 105 organisms (25µL/nare) 45 days after the 1° vaccine dose. Rats were observed over 14 days with death as the endpoint for determining percent protection.
2.6. Measurement of serum antibody
To measure mouse serum antibody titers, blood was obtained via facial cheek bleeds from animals in each experimental group before immunization, and 1 or 7 days prior to Y. pestis challenge. Whole blood was also taken from all survivors 14 days post-challenge. Rat blood was obtained via a saphaneous vein bleed from representative animals from each experimental group before immunization and 7 days prior to challenge. Sera were first separated by centrifugation and then filter sterilized. Anti-V and anti-F1 titers for total IgG were first measured by exposing prepared sets of antigen-bearing immunoblot strips to serial ten-fold dilutions (1:100 -1:1,000,000) of serum. These blots were prepared by electrophoretic separation of purified V and F1 antigens (10µg each) on a mini-protean (Biorad) SDS-PAGE gel cast with a modified comb containing a small well for molecular weight markers and a single continuous well to provide uniform distribution of antigens across the remaining surface of the gel. After electrophoresis, proteins were transferred to membranes and then cut into 8 strips per membrane (~1.25 µg of each antigen/strip). These blots were exposed to sera dilutions and developed with appropriate alkaline phosphatase-labeled secondary anti-mouse or anti-rat antibody. Titers were determined by a visual endpoint dilution. These titers were repeated 2–4 times per group of sera. Once the decimal titer range was determined, the titer was further defined by serial dilutions of sera within the determined decimal dilution range. Negative controls included pre-immune mouse or rat serum and positive controls included high titer anti-V and anti-F1 rat antibodies.
To differentiate IgG1 and IgG2a titers, blots, prepared as above, were exposed to sera dilutions and then were probed with anti-mouse IgG isotype-specific monoclonal antibodies (SouthernBiotech). Titers were determined by visual endpoints compared to negative and positive controls.
2.7. Statistics
Survival data were calculated in combined experiments with the same treatment regimen. Chi-square analysis of treatment group survival data were conducted using the web-based R Projects for Statistical Computing (www.r-project.org/index.html) with P values of ≤ 0.05 considered to be significant.
3. Results
AGPs were evaluated as adjuvants in vaccine preparations containing F1 capsule protein and/or V-antigen from Y. pestis to protect against pneumonic plague. Vaccination regimens that varied the amount, the route, the timing, and the number of administrations were tested in a mouse model of pneumonic plague. The most effective vaccine regimen was tested in rats and the mechanism of protection was analyzed using TLR4 mutant mice. When determining vaccine efficacy, the Food and Drug Administration’s Center for Biologics and Evaluation recommends the bacterial challenge doses be 100LD50 [35]. The LD50 for Y. pestis CO92 administered intranasally to BALB/c mice was determined to be 2 × 104 CFU (data not shown). This value was consistent with previous determinations by others [18, 36–38] and was used in all mouse experiments.
A successful vaccine formulation requires efficacy be established in more than one animal model. For this purpose, we tested the most protective murine vaccine regimen in rats. Although rats have been used extensively as a model for bubonic plague an intranasal LD50 for Y. pestis CO92 has not been established. Groups of immunologically naïve rats (n = 4 per group) were intranasally challenged with Y. pestis CO92 at concentrations ranging from 1.5 × 101 to 5 × 107 CFU. All rats challenged with doses of ≥ 5 × 104 CFU succumbed within 4 days, similar to the time-to-death of mice receiving a lethal dose. At a dose of 1.5 × 102 CFU, 1 of 4 rats died, while 3 of 4 rats succumbed to the next higher dilution of 1.5 × 103 CFU. Using the formula of Reed and Muench [34] we determined the rat LD50 of intranasally administered Y. pestis CO92 to be 4.7 × 102 CFU.
3.1. A single i.n. vaccination protects against pneumonic plague
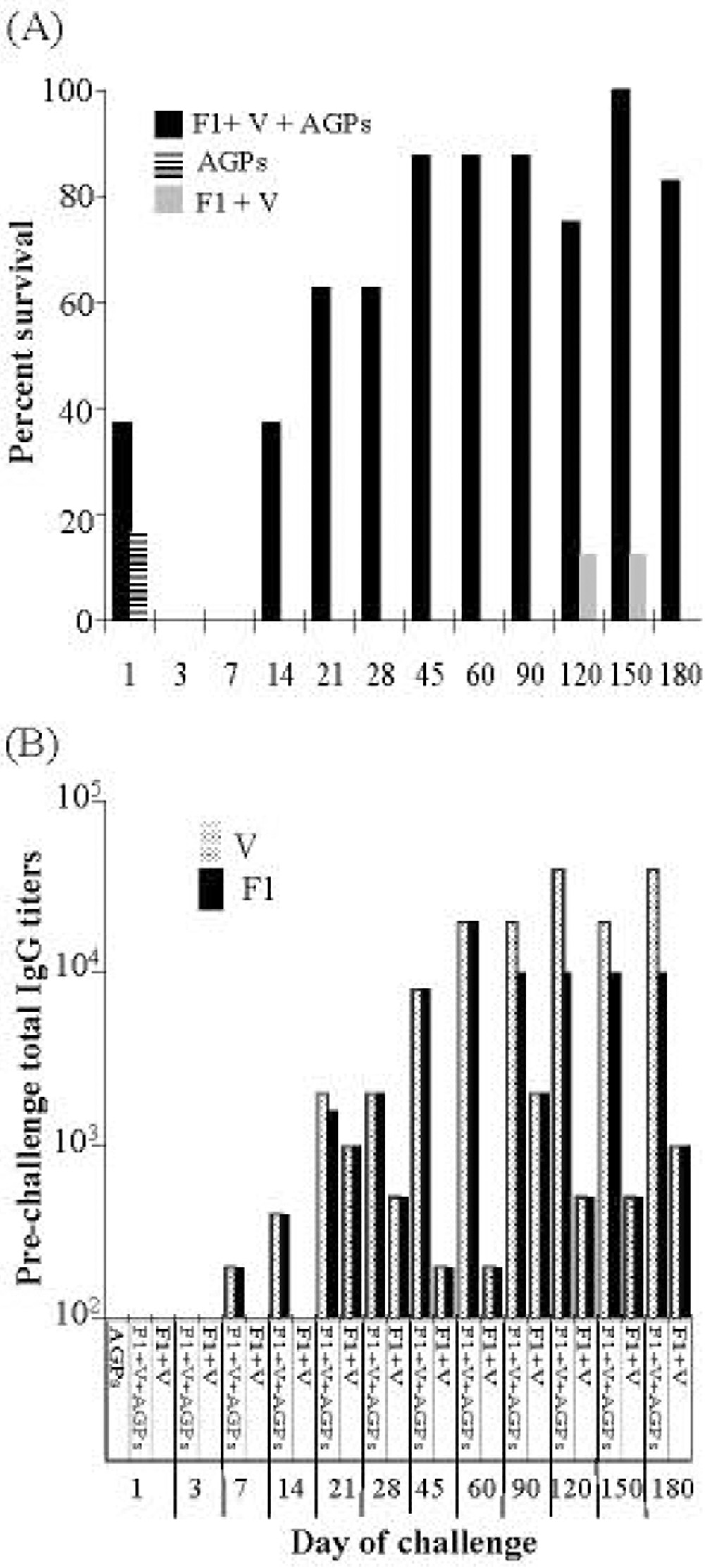
A series of vaccinations were conducted using various concentrations of purified Y. pestis F1 and V-antigen combined with varying concentrations of AGP adjuvants CRX-524 and CRX-527 (data not shown). A combination of these two lipid A mimetics was used based on our previous results which showed high level induction in murine lung tissue of the pro-inflammatory cytokines TNF-α, IL-12p70 and IFN-γ [32]. These initial experiments showed that an i.n. vaccine consisting of 2 µg F1 and 6 µg of V-antigen combined with 10 µg of each AGP provided protection. This vaccine formulation was further evaluated. Groups of mice (n = 8) were given a single i.n. vaccine dose, challenged with 100 LD50 of Y. pestis CO92 at timed intervals extending over 6 months and monitored for survival (Fig. 1A). Mice that received either the AGPs alone, or AGPs with antigens, showed 12.5% and 38% survival, respectively, 24 hrs after vaccination. This early protection was likely due to AGP stimulation of innate immunity [32]. By 2 weeks protection had returned; 38% of the animals receiving a single i.n. vaccine survived challenge. This level of protection increased to 90% 45 days post-immunization and continued to provide ≥ 75% protection through 180 days post vaccine (Fig. 1A). The effect of the AGPs as adjuvant was evident because most mice receiving only F1 and V-antigen succumbed to infection; only 2 of 96 mice survived pathogen challenge during the course of this experiment. Mice receiving PBS did not survive bacterial challenge at any time. Statistical analysis comparing all treatment control groups to vaccinated animals showed that vaccine protection was significant (P < 0.0001).
Fig. 1. A single i.n. vaccination protects against pneumonic plague.
Groups of BALB/c mice (n = 8) received an i.n. vaccine of 2 µg F1, 6 µg V, and 10ug each CRX-524 and CRX-527 on day 0. At various times (day 1 to day 180) animals were challenged intranasally with 100LD50s of Y. pestis CO92, and survival measured at 14 days post challenge. Actual i.n. challenge doses ranged from 2 to 3 × 106 CFU: days 1 and 3, 2 × 106 CFU; days 7 and 14, 2.8 × 106 CFU; days 21 and 28, 2.1 × 106 CFU; days 45, 60, 90, and 120, 3 ×106 CFU; days 150 and 180, 2.4 × 106 CFU.
A: On day 0, mice received an i.n vaccine of (F1 + V + AGPs, ■) and control groups received i.n. (AGPs, ), (F1 + V,
), (F1 + V, ), or PBS. Only animals receiving the i.n. vaccine were significantly protected from pneumonic plague (P < 0.0001).
), or PBS. Only animals receiving the i.n. vaccine were significantly protected from pneumonic plague (P < 0.0001).
B: Pooled sera IgG titers against V  and F1
and F1  were measured in animals one day prior to challenge (days 1 to 28 challenges) or one week prior to challenge (days 45 to 180 challenges). Day 1 titers for animals receiving AGPs alone were below 10.
were measured in animals one day prior to challenge (days 1 to 28 challenges) or one week prior to challenge (days 45 to 180 challenges). Day 1 titers for animals receiving AGPs alone were below 10.
Pooled sera were obtained from each group of animals prior to Y. pestis challenge and IgG titers against F1 or V-antigen were determined (Fig. 1B). The data suggested that IgG titers ≥ 10,000 were required for protection against the pneumonic plague challenge. This specific antibody level was achieved 45 days post-vaccination and was maintained for at least six months, the duration of the trial. Animals that received F1 and V-antigen without AGPs had fluctuating IgG titers that never exceeded a titer of 3,000 (Fig. 1B).
3.2. Vaccine protection against pneumonic plague is enhanced by a homologous 1°/2° application
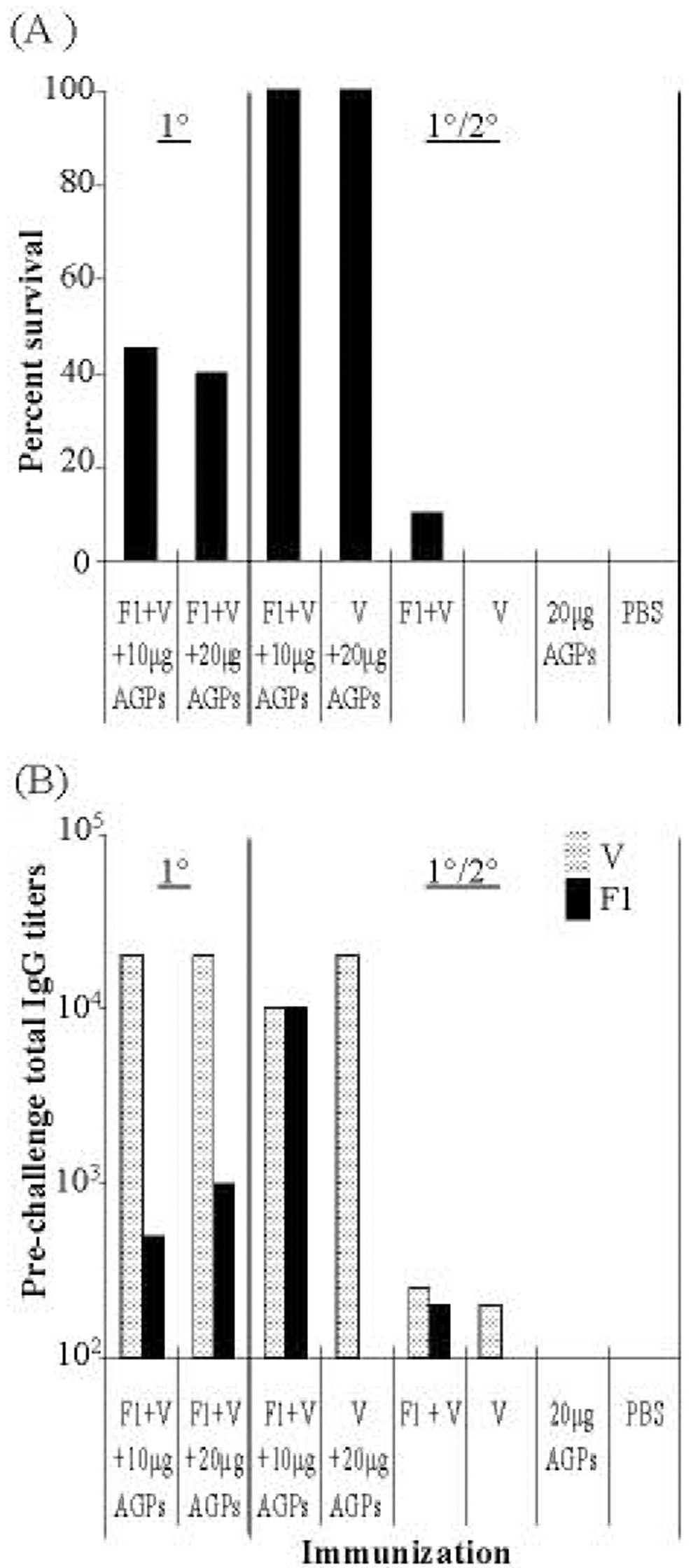
The efficacy of a vaccine regimen with 1°/2° doses was tested. A variety of F1 and V-antigen concentrations were tested (data not shown) to determine the effective dose of F1 and V-antigen. Based on this initial data, vaccine formulations containing 1 µg F1 + 3 µg V-antigen mixed with either 10 µg or 20 µg of AGPs, and i.n. doses administered twice (ten days apart) were selected for further study. Protection provided by vaccination with AGPs and V-antigen alone (without F1) was also examined. All animals were challenged 45 days after the 1° dose with 100 LD50 of Y. pestis CO92. Survival of mice receiving 1°/2° doses was compared with mice receiving a 1° vaccine alone, AGPs alone, F1 + V antigens alone, or PBS (Fig. 2). A 1°/2° i.n. vaccine containing 3 µg V-antigen + 10–20 µg AGPs, with or without F1, protected all mice (Fig. 2A, P < 0.0001). Also, the two groups of mice which received only a 1° vaccination of 1 µg F1 + 3 µg V-antigen + 10 or 20 µg AGPs had fewer survivors (≤ 42%) compared to mice receiving a 1° vaccine with higher amounts of F1 + V-antigen (2 µg F1+ 6µg V-antigen; 90% survival at day 45; Fig. 1A).
Fig. 2. Vaccine protection against pneumonic plague is enhanced by a 2° application.
Groups of BALB/c mice (n = 8) that received a 1° i.n. vaccination alone or a 1° and a 2° i.n. vaccination 10 days later were compared. The vaccine consisted of combinations of 1 µg F1, 3 µg V, and 5 -10 µg each CRX-524 and CRX-527. Forty-five days after 1° vaccination, all animals were challenged with 100LD50s Y. pestis CO92 and survival measured at 14 days post challenge. Actual i.n. challenge doses were 1.7 × 106 CFU for 1° vaccine group; 2.4 × 106 CFU for the 1° and 2° vaccine group; and 3.0 × 106 CFU for the 1° and 2° V-antigen alone group.
A: Mice received a 1° i.n. vaccine on day 0 or a 1° and 2° vaccine on day 0 and day 10; control groups received 1° and 2° i.n. (F1 + V), (V alone), (AGPs alone), or (PBS). Only those animals receiving the 1°/2° vaccine were completely protected (P < 0.0001)
B: Pooled sera total IgG titers against V  and F1
and F1  were measured in immunized animals 35 days after 1° vaccination.
were measured in immunized animals 35 days after 1° vaccination.
Pooled sera were obtained from each group of animals prior to Y. pestis challenge and total IgG titers against F1 or V-antigen were determined (Fig. 2B). The 1° vaccination with F1 + V-antigen + AGPs generated IgG titers to V-antigen >10,000 and to F1 ≤ 1,000. In comparison, animals given a 1°/2° i.n. vaccine (with or without F1) had equivalent or lower IgG titers to V-antigen but had higher survival rates (100% vs. = 42%). A 1°/2° vaccine regimen significantly increased IgG titers to F1 compared to a single i.n exposure. These results indicated that a lower dose i.n. vaccine administered in a 1°/2° schedule provided better protection than a single 1° dose even with equivalent total antigen exposure. Furthermore, IgG titers did not absolutely correlate with the level of protection. In part, this may be artifactual since we measured pooled sera titers from each group vs. titers from individual animals.
3.3. A 1°/2° i.n. vaccination provides early complete protection against pneumonic plague
Given the success observed in the previous set of experiments, we repeated the 1°/2° dose regimen with bacterial challenges at earlier time points to determine how soon protection is achieved. The time interval between 1° and 2° vaccination was also shortened in a series of trials to determine the level of protection obtainable within one week post-1° dose.
Mice were given 20 µg AGPs + 2 µg F1 + 6 µg V-antigen on days 0 and 10. Groups of mice (n = 8) were challenged with 100 LD50 Y. pestis CO92 on days 21, 28, 60, or 90 following the 1° vaccine dose. As seen in Fig. 3A, this vaccine regimen provided 100% protection as early as 21 days post-1° vaccination and complete protection was maintained for the 90 day duration of the trial (P <0.0001). Animals receiving AGPs alone or PBS did not survive bacterial challenge (data not shown), and those receiving F1 + V-antigen alone had survival rates that never exceeded 25% (Fig. 3A).
Fig. 3. A 1° and 2° i.n. vaccination provides early complete plague protection.
Groups of BALB/c mice (n = 8) received a 1° and 2° i.n. vaccination of 2 µg F1, 6 µg V, and 10 µg each CRX-524 and CRX-527 on day 0 and day 10. At various times after the 1° vaccine (day 21 to day 90), groups were challenged intranasally with 100LD50s Y. pestis CO92 (2.3 × 106 CFU) and survival measured at 14 days post challenge.
A: Mice received a 1° and 2° i.n. vaccine on day 0 and day 10 (■); control groups received i.n. F1 + V ( ), AGPs, or PBS. Only animals receiving the i.n. vaccine were significantly protected (P < 0.0001)
), AGPs, or PBS. Only animals receiving the i.n. vaccine were significantly protected (P < 0.0001)
B: Pooled sera total IgG titers against V  and F1
and F1  were measured in animals one week prior to challenge.
were measured in animals one week prior to challenge.
Pooled sera were obtained from each group of animals prior to Y. pestis challenge and IgG titers were determined (Fig. 3B). Titers to F1 and V-antigen were higher among mice receiving the vaccine containing AGP (50,000) compared to mice receiving F1 and V-antigen without AGPs (6,000). We concluded that this i.n. 1°/2° AGP-based plague vaccine provided complete protection against a 100 LD50 pneumonic plague challenge within three weeks.
The timing between 1°/2° vaccinations were further compressed to 24 h or 72 h and protection from pneumonic plague challenge tested at 7 days. Table 1 shows the results of representative regimens tested. Sixty-three % of the mice survived the challenge administered just one week after the 1° vaccine dose. This high rate of survival was dependent on AGPs in the vaccine preparation as only 12.5% of the animals that did not receive AGPs survived bacterial challenge. In addition, the time-to-death among these mice was 6–10 days. There were no survivors or extended time to death among the control animals receiving PBS or AGPs alone.
Table 1.
Survival of vaccinated mice challenged at seven days with Y. pestis CO92a
| 1°/2° Vaccineb | Dose (µg) | ||||
|---|---|---|---|---|---|
| Interval (h) | F1c | Vd | AGPse | % Survivalf | TTDg |
| 24 | 1 | 3 | 10 | 63 | 10 |
| 72 | 2 | 6 | 20 | 63 | 6 |
| 72 | 2 | 6 | - | 12.5 | 6 |
| 72 | - | - | 20 | 0 | 4 |
| 72h | - | - | - | 0 | 3 |
Intranasal challenge seven days after 1° vaccine, 100LD50s Y. pestis CO92 (2 × 106 CFU)
Two doses of intranasal vaccine given 24 or 72 h apart
F1, Capsule F1 protein
V, V-antigen
AGPs, amino-alkyl glucosaminide phosphates; 20 µg contains 10 µg each CRX-524 and CRX-527, 10 µg contains 5 µg each CRX-524 and CRX-527
% Survival at 14 days post challenge of asymptomatic BALB/c mice, n = 8
TTD, Time to death
Control groups received i.n. PBS in equal volumes
The efficacy of administering AGP-based vaccines subcutaneously was also tested. Mice received 2 µg F1 + 6 µg V-antigen + 20 µg AGPs, subcutaneously as a single injection followed by a 100 LD50 Y. pestis i.n. challenge one week later. Among eight mice, 1 expired (87% survival) and the animal that succumbed died 12 days after challenge (data not shown). Control groups included animals that received antigens alone (12% survival), AGPs alone (0 survivors), and a mock PBS vaccine (0 survivors). We concluded that an AGP-based vaccine administered intranasally or subcutaneously provided significant protection (P < 0.0001) within 1 week post-vaccination.
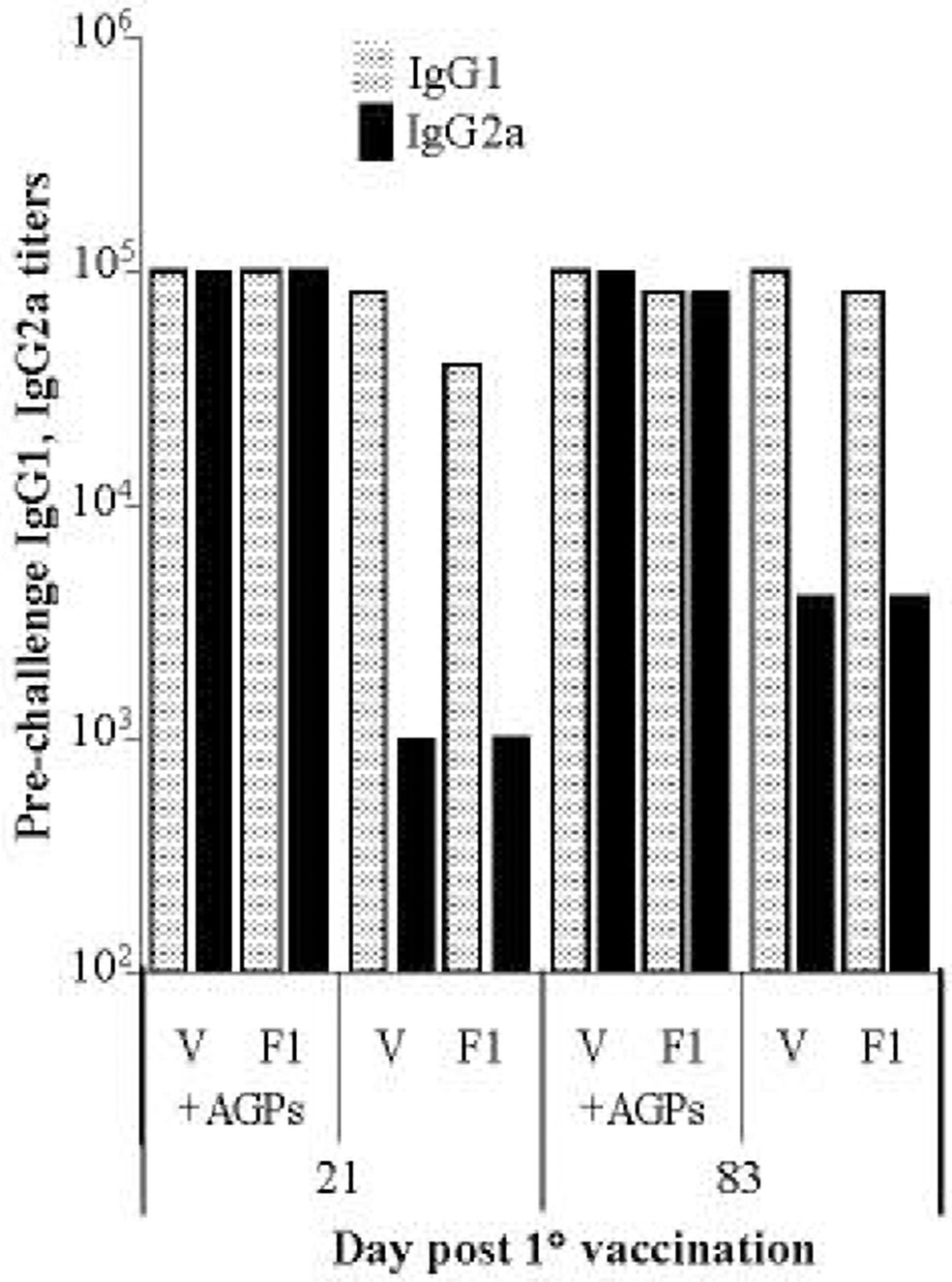
3.4. Vaccination with AGP as adjuvant promotes a TH1 response
To determine if the high IgG titers in immunized animals were indicative of a TH1 or TH2 response, we compared sera IgG1 and IgG2a titers. Animals were given an i.n. vaccination (2 µg F1 + 6 µg V-antigen + 20 µg AGPs) on days 0 and 10 and sera titers analyzed on days 21 or 83 post 1° vaccination. As seen in Fig. 4, animals that received the AGP-based vaccine had high IgG1 and IgG2a titers to both V and F1 (≥80,000). Mice immunized with F1 and V without the AGP adjuvant had at least a 20-fold lower IgG2a response to both V and F1 (≤ 4000). PBS or AGP alone vaccinated control animals had no measurable IgG1 or IgG2a response to V or F1 (data not shown). This data showed that AGPs promoted a cellular immune response, reflected by increased IgG2a titers.
Fig. 4. AGP-based vaccine promotes a TH1 response.
Sera from BALB/c groups of mice (n = 8) receiving a 1° and 2° i.n. AGP-based vaccine or F1 + V antigens alone was taken on days 21 or day 83, pooled, and titers measured for IgG1  and IgG2a
and IgG2a  against each antigen.
against each antigen.
3.5. AGP-based vaccine protection requires TLR4
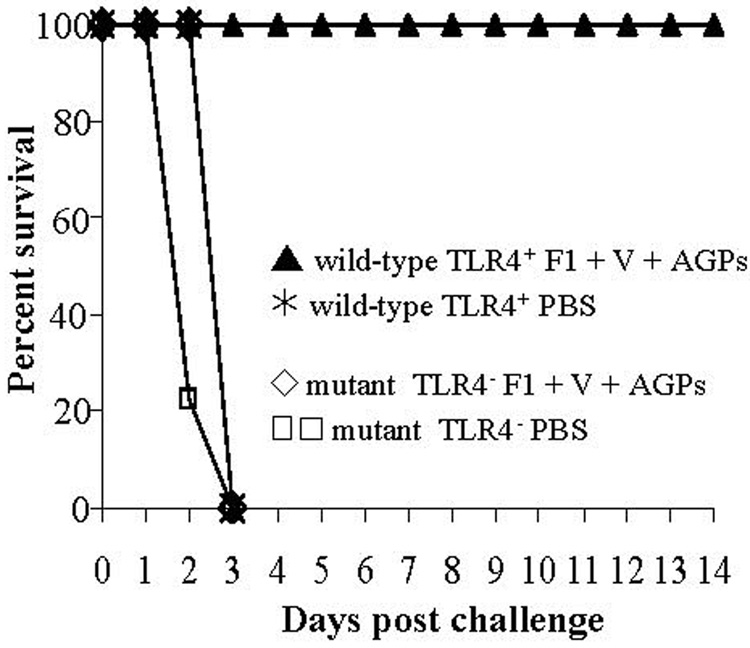
To determine if the AGP adjuvant activity was dependant on TLR4 activation, groups of BALB/c TLR4 wild-type and TLR4 mutant mice (n = 8) were vaccinated with 2 µg F1 + 6 µg V-antigen + 20 µg AGPs or mock-vaccinated with PBS in a 1°/2° regimen, administered on days 0 and 10. The mice were challenged with 15 LD50 Y. pestis CO92 on day 21. As seen in Fig. 5, the vaccinated TLR4 mutant animals did not survive challenge and did not exhibit delayed time-to-death. In contrast, 100% of the vaccinated TLR4 competent animals survived challenge. Control animals for both BALB/c TLR4 wild-type and mutant groups receiving mock vaccinations and then challenged with 15 LD50 Y. pestis CO92 all succumbed to infection by day 4. These experiments showed that AGPs employed as adjuvant were dependent upon TLR4.
Fig. 5. AGP-based vaccine protection requires Toll-like receptor 4.
Groups of BALB/c wild-type TLR4 (▲) and mutant TLR4 (◇) mice (n = 8) received a 1° and 2° i.n. vaccine of 2 µg F1, 6 µg V, and 10 µg each CRX-524 and CRX-527, or PBS (wild-type- , mutant-
, mutant-  ) on day 0 and day 10. Twenty-one days after 1° immunization, animals were challenged intranasally with 15LD50s Y. pestis CO92 (2.5 × 105 CFU). Mice were monitored for disease symptoms and survival through 14 days post challenge. Only wild-type TLR4 animals receiving the vaccine were protected (P < 0.0001).
) on day 0 and day 10. Twenty-one days after 1° immunization, animals were challenged intranasally with 15LD50s Y. pestis CO92 (2.5 × 105 CFU). Mice were monitored for disease symptoms and survival through 14 days post challenge. Only wild-type TLR4 animals receiving the vaccine were protected (P < 0.0001).
3.6. A 1°/2° i.n. vaccination protects rats against pneumonic plague
To determine if the vaccine formulation used in the mouse model was efficacious in another mammal, we immunized Sprague-Dawley rats (n = 10) with a 1°/2° i.n. vaccination on day 0 and day 10. To partially compensate for increased body weight, the antigen levels were increased to 4 µg F1 + 12 µg V-antigen + 40 µg AGPs (20 µg each of CRX-524 and CRX-527). Forty-five days after 1° immunization, all rats were intranasally challenged with 1 × 105 CFU Y. pestis CO92, equivalent to just over 1000 LD50. As shown in Fig. 6, this vaccine formulation provided 90% protection compared to 0% protection in a control group receiving PBS. Furthermore, the one vaccinated animal succumbed to infection 12 days post-pathogen challenge compared to control animals all of which died between days 3 and 4. In summary, an effective i.n. vaccine for pneumonic plague developed in a mouse model was effective in rats against a 1000 LD50 intranasal challenge.
Fig. 6. A 1° and 2° i.n. vaccination protects rats against pneumonic plague.
Groups of Sprague-Dawley rats (n = 10) received a 1° and 2° i.n. vaccine on day 0 and day 10 of 4 µg F1, 12 µg V, and 20 µg each CRX-524 and CRX-527(■), or PBS alone (○). Forty-five days after 1° immunization, all rats were challenged with ~1000LD50s Y. pestis CO92 (5 × 105 CFU). Rats were monitored for disease symptoms and survival through 14 days post challenge. Only those rats receiving the vaccine were protected from pneumonic plague (P < 0.0001).
4. Discussion
At present, plague is a modest contributor to human morbidity and mortality. A total of 36,876 cases of plague were reported by the WHO between 1987 and 2001, the majority being the bubonic form of the disease [39]. Of this total, 2,847 cases were fatal. Nevertheless, because of the potential application of Y. pestis as an aerosolized bioterrorist agent, plague remains a significant concern. This concern is highlighted by the lack of a fast acting vaccine that provides protection against the pneumonic form of the disease. Previously employed whole-cell killed and the live attenuated vaccines were discontinued in the U.S. because they did not provide long-term protection, had significant side-effects, and did not protect against pneumonic plague. The focus of this work was to investigate the efficacy of formulating a vaccine to meet this need. The strategy adopted was to develop a vaccine based on the wealth of information showing that an immune response against Y. pestis V- and F1 antigens is protective against pneumonic disease [6, 25, 40, 41] coupled with new TLR4 stimulating synthetic lipid A mimetic adjuvants that promote a TH1 immune response [23,24]. Stimulation of a TH1 immune response provides the best protection against viral and intracellular bacterial infections because activation of cell-mediated immunity neutralizes cells harboring replicating pathogens. Furthermore, TH1 induced production of IgG2a isotypes yield more effective opsonizing antibodies [42–45]. This immune response is in contrast to that induced by alum, the only approved vaccine adjuvant in the U.S. Alum promotes primarily a TH2 immune response with a bias in production of the IgG1 subclass [21, 46]. Generally, alum-based vaccines require multiple injections with high antigen concentrations.
Lipid A mimetics can be strong agonists or antagonists of TLR4-mediated activity depending on the length and number of acyl chains on the carbohydrate backbone [25]. Recognition that lipid A toxicity could be alleviated while maintaining TLR4 stimulatory effects by modification of the structure, has stimulated further interest in these compounds for both direct therapeutic and vaccine adjuvant applications. Related to the synthetic lipid A mimetics is the modified, monophosphoryl lipid A (MPL) from Salmonella minnesota R595. MPL is currently employed as a vaccine adjuvant for several human infectious agents (hepatitis B and papilloma viruses) licensed in European and South American countries [47, 48]. Recent studies suggest that MPL, and by inference, AGPs, are less inflammatory due to TLR4 stimulation by the TRIF pathway rather than the MyD88 cascade that is induced by natural lipopolysaccharide (LPS) [49]. Synthetic AGP derivatives such as the CRX-524 and CRX-527, used in this study, have low toxicity but heightened TLR4 stimulatory effects in comparison to MPL and therefore may have greater efficacy for use as adjuvants. Their stimulation of IL-12, TNF-α and IFN-γ is consistent with promoting a cell-mediated TH1 immune response [50]. The fact that they are synthetic molecules reduces the variability in standardizing naturally-derived products.
We showed that providing a single i.n. vaccination with synthetic lipid A mimetics as adjuvant with low µg amounts of V- and F1 antigens, provided significant long-term protection in a murine pneumonic plague model. Significant early protection (38%) was also afforded to animals challenged within 24 hrs of immunization. This very early protection was primarily due to adjuvant alone which induces a strong rapid innate immune response that includes induction of TNF-α, IL-12 and IFN-γ known to bias the development of TH0 cells to TH1 maturation [37, 50]. In other work from our laboratory, we have shown that AGPs alone can be protective against pneumonic plague infection [32], similar to what has been reported for other infectious agents [24,25,51]. AGP stimulation of an innate immune response is time- and pathogen dose-dependent. These results may be significant if vaccines against select agents employing TLR-stimulatory adjuvants are administered after initial Y. pestis release documentation. AGPs thus have direct therapeutic function as well as potent adjuvant activity.
By employing a homologous 1°/2° i.n. vaccine regimen, with a 10 day interval between vaccinations, 100% protection was achieved within 21 days. Compressing the prime/boost schedule to administration on days 1 and 3 provided 63% protection in mice by day 7. We also showed that this AGP-based vaccine was effective when administered subcutaneously. This versatility provides an advantage over alum-based vaccines. Specifically, the AGP-based vaccine can be effective if applied directly to mucosal surfaces without needle use, but also functions well if administered by the traditional parenteral route. Mutant mice lacking TLR4 show no protection by this AGP-adjuvant vaccine, thus AGP stimulation of innate immunity is critical for the success of this vaccine formulation.
In summary, the use of a synthetic lipid A mimetic adjuvant combined with Y. pestis protective antigens demonstrated rapid and prolonged protection against a 100 LD50 challenge to lung tissue. The advantage of this method over traditional adjuvants is that innate immunity is stimulated through TLR4 signaling and promotes a cellular immune response, likely required for protection against bacterial pathogens like Y. pestis that can invade and survive within macrophages.
Acknowledgements
This work was supported, in part, by the Idaho Agriculture Experiment Station and Public Health Service grants U54-AI-57141, P20-RR16454, and P20-RR15587 from the National Institutes of Health. The authors thank Dr. Jay Evans (GlaxoSmithKline, Hamilton, MT) for helpful discussions and the for providing the AGPs used in this study. Sam Miller and Sean Skerritt provided helpful discussion during the course of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perry RD, Fetherston JD. Yersinia pestis-etiological agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welkos S, Pitt MLM, Martinez M, Friedlander AM, Vogel P, Tammariello R. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in non-human primate and mouse models of pneumonic plague. Vaccine. 2002;20:2206–2214. doi: 10.1016/s0264-410x(02)00119-6. [DOI] [PubMed] [Google Scholar]
- 3.Russell P, Eley SM, Hibbs SE, Manchee RJ, Stagg AJ, Titball RW. A comparison of plague vaccine USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine. 1995;13:1551–1556. doi: 10.1016/0264-410x(95)00090-n. [DOI] [PubMed] [Google Scholar]
- 4.Andrews GP, Heath DG, Anderson GW, Jr, Welkos SL, Friedlander AM. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect Immun. 1996;64(6):2180–2187. doi: 10.1128/iai.64.6.2180-2187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynn A, Freytag LC, Clements JD. Effect of homologous and heterologous prime-boost on the immune response to recombinant plague antigens. Vaccine. 2005;23:1957–1965. doi: 10.1016/j.vaccine.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Williamson ED, Stagg AJ, Eley SM, Taylor R, Green M, Jones SM, Titball RW. Kinetics of the immune response to the (F1 + V) vaccine in models of bubonic and pneumonic plague. Vaccine. 2007;25(6):1142–1148. doi: 10.1016/j.vaccine.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Uddowla S, Freytag LC, Clements JD. Effect of adjuvants and route of immunizations on the immune response to recombinant plague antigens. Vaccine. 2007;25:7984–7993. doi: 10.1016/j.vaccine.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodin JL, Nellis DF, Powell BS, Vyas VV, Enama JT, Wang LC, et al. Purification and protective efficacy of monomeric and modified Yersinia pestis capsular F1-V antigen fusion proteins for vaccination against plague. Protein Expr Purif. 2007;53:63–79. doi: 10.1016/j.pep.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GW, Heath DG, Bolt CR, Welkos SL, Friedlander AM. Short- and long-term efficacy of single dose subunit vaccines against Yersinia pestis in mice. Am J Trop Med Hyg. 1998;58(6):793–799. doi: 10.4269/ajtmh.1998.58.793. [DOI] [PubMed] [Google Scholar]
- 10.Weeks S, Hill J, Friedlander AM, Welkos S. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb Pathog. 2002;32:227–237. doi: 10.1006/mpat.2002.0498. [DOI] [PubMed] [Google Scholar]
- 11.Abramov VM, Khlebnikov VS, Vasiliev AM, Kosarev IV, Vasilenko RN, Kulikova NL, et al. Attachment of LcrV from Yersinia pestis at dual binding sites to human TLR-2 and human IFN-γ receptor. J Proteome Res. 2007;6:2222–2231. doi: 10.1021/pr070036r. [DOI] [PubMed] [Google Scholar]
- 12.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Hessemann J. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002;196(8):1017–1024. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Rosqvist R, Forsberg Å. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect Immun. 2002;70(3):1453–1460. doi: 10.1128/IAI.70.3.1453-1460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller CA, Broz P, Müller SA, Ringler P, Erne-Brand F, Sorg I, et al. The V-antigen of Yersinia forms a distinct structure at the tip of the injectisome needles. Science. 2005;310(5748):674–676. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- 15.Overheim KA, DePaolo RW, DeBord KL, Morrin EM, Anderson DM, Green NM, et al. LcrV plague vaccine with altered immunomodulatory properties. Infect Immun. 2005;73(8):5152–5159. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson GW, Jr, Heath DG, Bolt CR, Welkos SL, Friedlander AM. Short- and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice. Am J Trop Med Hyg. 1998;58(6):793–799. doi: 10.4269/ajtmh.1998.58.793. [DOI] [PubMed] [Google Scholar]
- 17.Williamson ED, Flick-Smith HC, Waters E, Miller J, Hodgson I, Le Butt CS, et al. Immunogenicity of the rF1 + RV vaccine for plague with identification of potential immune correlates. Microb Pathog. 2007;42:11–21. doi: 10.1016/j.micpath.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Heilman D, Liu F, Giehl T, Joshi W, Huang X, et al. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine. 2004;22:3348–3357. doi: 10.1016/j.vaccine.2004.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GW, Leary SE, Williamson ED, Titball RW, Welkos SL, Worsham PL, et al. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-positive and -negative strains of Yersinia pestis. Infect Immun. 1996;64:4580–4585. doi: 10.1128/iai.64.11.4580-4585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones SM, Day F, Stagg AJ, Williamson ED. Protection conferred by a fully recombinant sub-unit vaccine against Yersinia pestis in male and female mice of four inbred strains. Vaccine. 2001;19:358–366. doi: 10.1016/s0264-410x(00)00108-0. [DOI] [PubMed] [Google Scholar]
- 21.Khajuria A, Gupta A, Malik F, Singh S, Singh J, Gupta BD, et al. A new vaccine adjuvant (BOS 2000) a potent enhancer mixed Th1/Th2 immune responses in mice immunized with HBsAg. Vaccine. 2007;25:4586–4594. doi: 10.1016/j.vaccine.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008;7(2):209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones T, Adamovicz JJ, Cyr SL, Bolt CR, Bellerose N, Pitt LM, Lowell GH, Burt DS. Intranasal Protollin™/V1-V vaccine elicits respiratory and serum antibody responses and protects mice against lethal aerosolized plague infection. Vaccine. 2006;24(10):1625–1632. doi: 10.1016/j.vaccine.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 24.Reed DS, Martinez MJ. Respiratory immunity is an important component of protection elicited by subunit vaccination against pneumonic plague. Vaccine. 2006;24(13):2283–2289. doi: 10.1016/j.vaccine.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 25.Glynn A, Roy CJ, Powell BS, Adamovicz JJ, Freytag LC, Clements JD. Protection against aerosolized Yersinia pestis challenge following homologous and heterologous prime-boost with recombinant plague antigens. Infect Immun. 2005;73(8):5256–5261. doi: 10.1128/IAI.73.8.5256-5261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brubaker RR. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V-antigen) Infect Immun. 2003;71:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebeil R, Ernst RK, Gowen BB, Miller SI, Hinnebusch BJ. Variation in lipid A structure in the pathogenic yersiniae. Mol Microbiol. 2004;52(5):1363–1373. doi: 10.1111/j.1365-2958.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 28.Montminy SW, Khan N, McGrath S, Malkowicz MJ, Sharp F, Conlan JE, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;112:521–530. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 29.Cluff CW, Baldridge JR, Stover AG, Evans JT, Johnson DA, Lacy MJ, et al. Synthetic Toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect Immun. 2005;73(5):3044–3052. doi: 10.1128/IAI.73.5.3044-3052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson DJ, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4(7):1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 31.Stover AG, Da Silva Corriea J, Evans JT, Cluff CW, Elliott MW, Jeffery EW, et al. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J Biol Chem. 2004;279(6):4440–4449. doi: 10.1074/jbc.M310760200. [DOI] [PubMed] [Google Scholar]
- 32.Airhart CL, Rohde H, Bohach GA, Hovde CH, Deobald CF, Lee SS, et al. Induction of innate immunity by lipid A mimetics increases survival from pneumonic plague. Microbiol. 2008;154(7):2131–2150. doi: 10.1099/mic.0.2008/017566-0. [DOI] [PubMed] [Google Scholar]
- 33.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57Bl/10ScCr mice: mutation in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 34.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27(3):493–497. [Google Scholar]
- 35.Geary NL. FDA Heath and Human Services Public Workshop on Animal Models and Correlates of Protection for Plague Vaccines. Gaithersburg, Maryland: FDA-Center for Biologics Evaluation and Research; 2004. Animal models and correlates of protection for plague vaccines; pp. 1–300. [Google Scholar]
- 36.Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: A mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci USA. 2005;102(49):17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006;74(6):3381–3386. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bubeck SS, Cantwell AM, Dube PH. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect Immun. 2007;75(6):697–705. doi: 10.1128/IAI.00403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Human plague in 2002 and 2003. Wkly Epidem Record. 2004;79(33):301–308. [PubMed] [Google Scholar]
- 40.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006;72:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alpar HO, Eyles JE, Williamson ED, Somavarapu S. Intranasal vaccination against plague, tetanus, and diphtheria. Adv Drug Deliv Rev. 2001;51:173–201. doi: 10.1016/s0169-409x(01)00166-1. [DOI] [PubMed] [Google Scholar]
- 42.Gessener JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 43.Unkeless JC, Eisen HN. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975;142:1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 45.Heusser CH, Anderson CL, Grey HM. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977;145:1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N, et al. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine. 2005;23(46–47):5450–5456. doi: 10.1016/j.vaccine.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 47.Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6(2):133–140. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- 48.Crosbie EJ, Kitchener HC. Cervarix--a bivalent L1 virus-like particle vaccine for prevention of human papillomavirus type 16- and 18-associated cervical cancer. Expert Opin Biol Ther. 2007;7(3):391–396. doi: 10.1517/14712598.7.3.391. [DOI] [PubMed] [Google Scholar]
- 49.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami K. Promising immunotherapies with Th1-related cytokines against infectious diseases. J Infect Chemother. 2003;9(3):201–209. doi: 10.1007/s10156-003-0263-5. [DOI] [PubMed] [Google Scholar]
- 51.Lembo A, Pelletier M, Iyer R, Timko M, Dudda JC, West TE, et al. Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J Immunol. 2008;108:7574–7581. doi: 10.4049/jimmunol.180.11.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]