Abstract
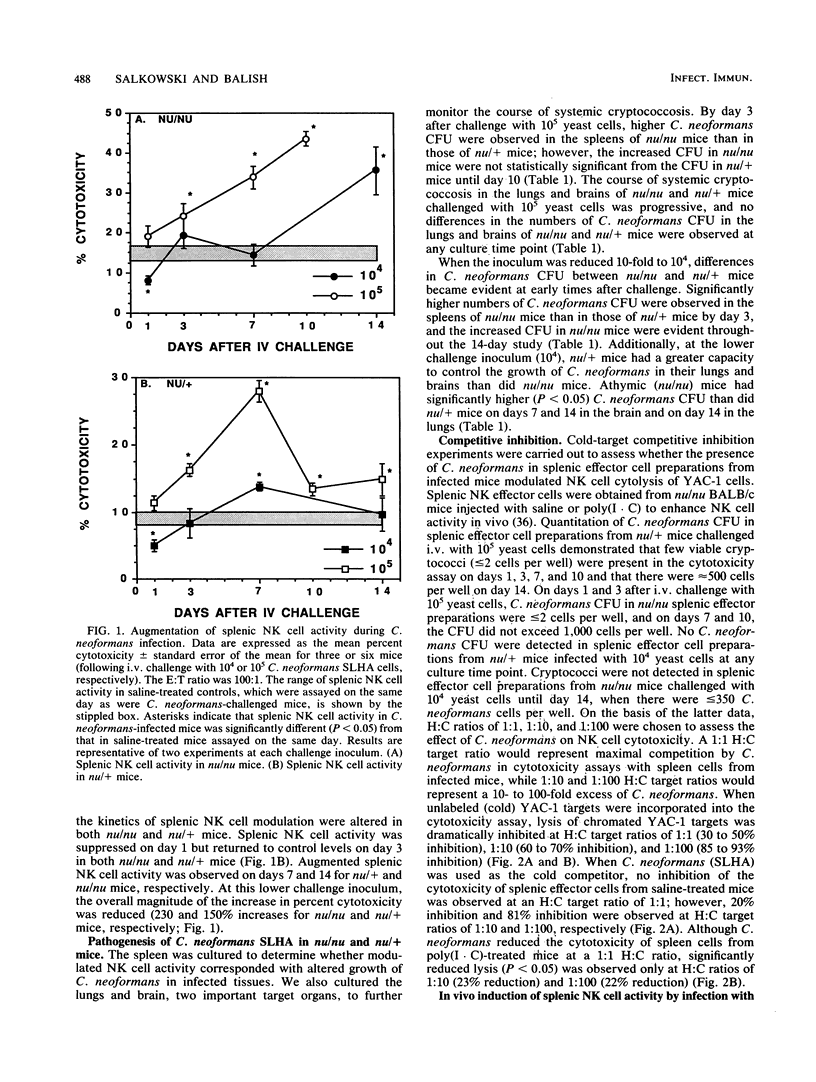
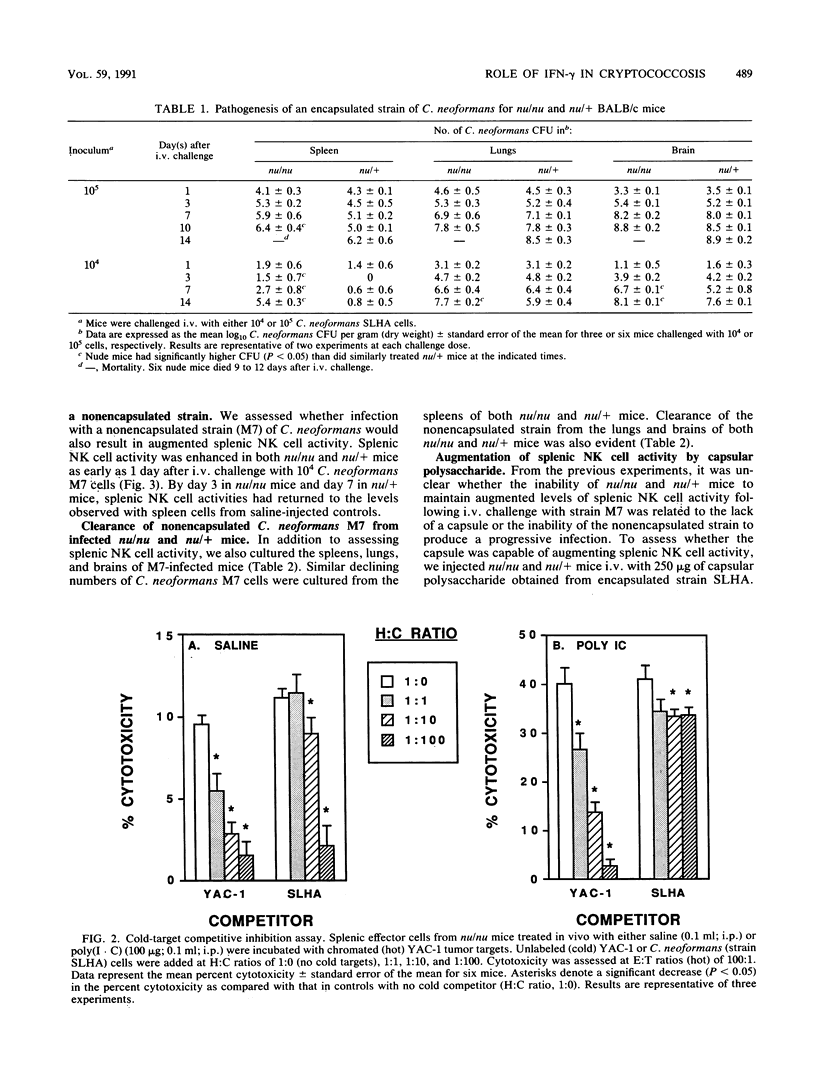
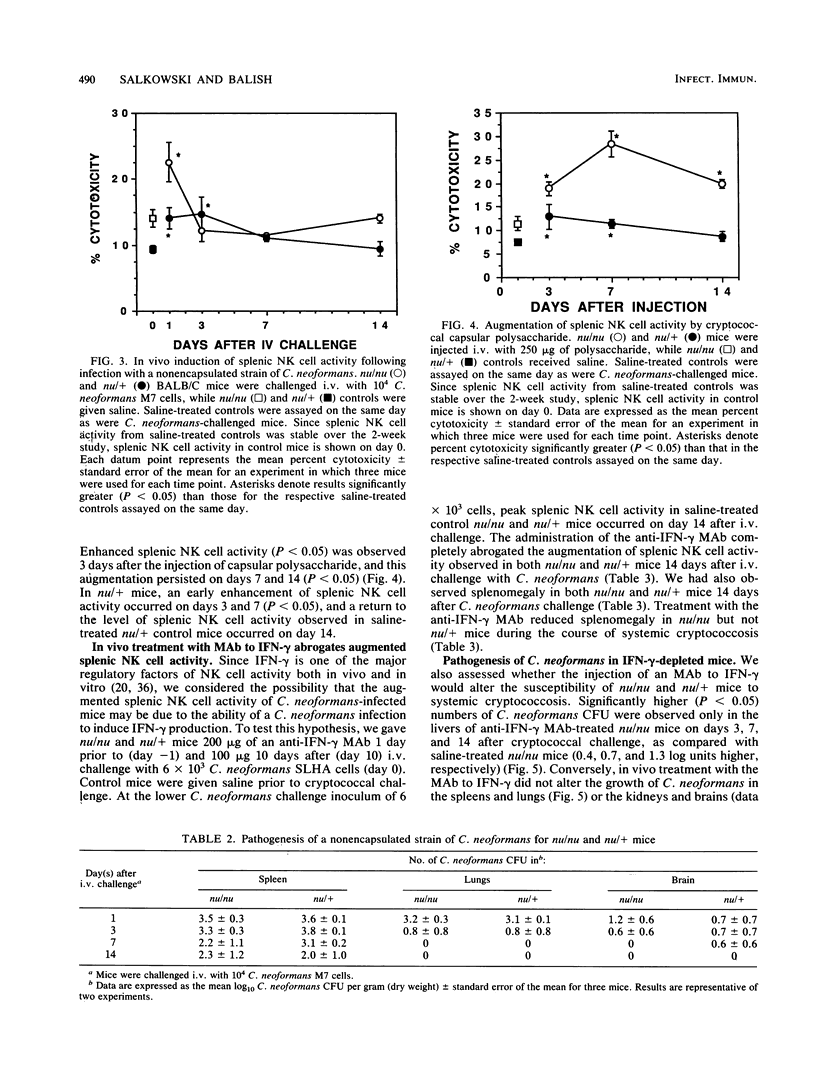
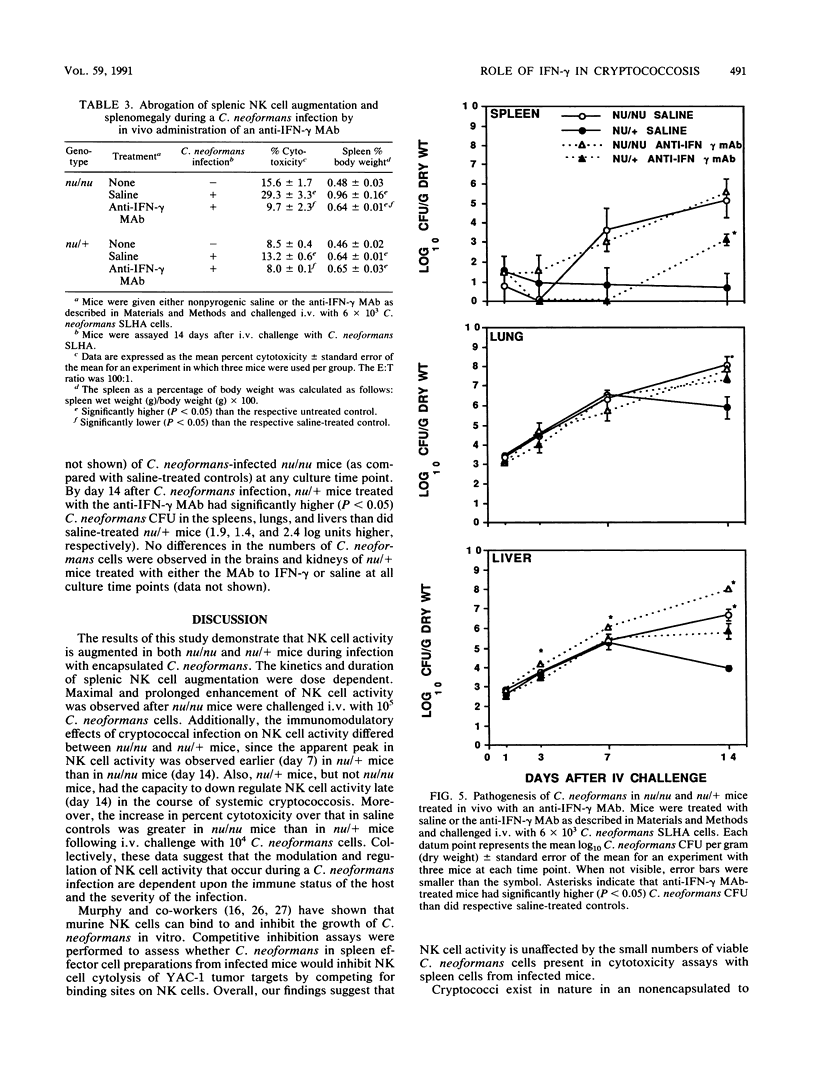
These studies demonstrate that the cytotoxic activity of splenic natural killer (NK) cells is augmented in both nu/nu and nu/+ mice during systemic cryptococcosis. Both the kinetics and the regulation of NK cell activity differed in Cryptococcus neoformans-infected nu/nu and nu/+ mice. Greater augmentation was observed following challenge with 10(5) cells than with smaller inocula, and augmented NK cell activity was not always associated with enhanced control of systemic cryptococcosis. Infection with a nonencapsulated strain of C. neoformans induced an early but transient increase in splenic NK cell activity in nu/nu and nu/+ mice. Injection of capsular polysaccharide induced a transient augmentation of splenic NK cell activity in nu/+ mice but caused a persistent increase in splenic NK cell activity in nu/nu mice. In vivo treatment with monoclonal antibody to gamma interferon abrogated the augmentation of splenic NK cell activity induced during cryptococcal infections in both nu/nu and nu/+ mice and enhanced the susceptibility of nu/+ mice to C. neoformans to a greater extent than it did that of nu/nu mice. These results suggest that gamma interferon is an important mediator of resistance to C. neoformans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bartizal K. F., Salkowski C., Balish E. The influence of a gastrointestinal microflora on natural killer cell activity. J Reticuloendothel Soc. 1983 May;33(5):381–390. [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D., Gunn C. M. Cryptococcus neoformans. I. Nonencapsulated mutants. J Bacteriol. 1967 Nov;94(5):1475–1479. doi: 10.1128/jb.94.5.1475-1479.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geus B., Van den Enden M., Coolen C., Nagelkerken L., Van der Heijden P., Rozing J. Phenotype of intraepithelial lymphocytes in euthymic and athymic mice: implications for differentiation of cells bearing a CD3-associated gamma delta T cell receptor. Eur J Immunol. 1990 Feb;20(2):291–298. doi: 10.1002/eji.1830200210. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Farhi F., Bulmer G. S., Tacker J. R. Cryptococcus neoformans IV. The Not-So-Encapsulated Yeast. Infect Immun. 1970 Jun;1(6):526–531. doi: 10.1128/iai.1.6.526-531.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985 Aug;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect Immun. 1989 Jul;57(7):1990–1997. doi: 10.1128/iai.57.7.1990-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kofler H., Fritsch P. Cryptococcosis in the immunocompromised host. Curr Probl Dermatol. 1989;18:193–202. doi: 10.1159/000416856. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Gulley W. F., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977 Dec;18(3):701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988 Oct;56(10):2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Asano M., Kohanawa M., Chen Y., Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990 Jul;58(7):2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R. Cryptococcosis. Infect Dis Clin North Am. 1989 Mar;3(1):77–102. [PubMed] [Google Scholar]
- Powell K. E., Dahl B. A., Weeks R. J., Tosh F. E. Airborne Cryptococcus neoformans: particles from pigeon excreta compatible with alveolar deposition. J Infect Dis. 1972 Apr;125(4):412–415. doi: 10.1093/infdis/125.4.412. [DOI] [PubMed] [Google Scholar]
- Small J. M., Mitchell T. G. Strain variation in antiphagocytic activity of capsular polysaccharides from Cryptococcus neoformans serotype A. Infect Immun. 1989 Dec;57(12):3751–3756. doi: 10.1128/iai.57.12.3751-3756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Herberman R. B., Chirigos M. A., Maluish A. E., Schneider M. A., Adams J. S., Philips H., Thurman G. B., Varesio L., Long C. Hyporesponsiveness to augmentation of murine natural killer cell activity in different anatomical compartments by multiple injections of various immunomodulators including recombinant interferons and interleukin 2. J Immunol. 1985 Oct;135(4):2483–2491. [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


