Abstract
Onconase (Onc), a ribonuclease from oocytes or early embryos of Northern Leopard frog (Rana pipiens), is cytostatic and cytotoxic to a variety of tumor lines in vitro, inhibits growth of tumors in animal in vivo models and is currently in Phase IIIb clinical trials for malignant mesothelioma where it displays antitumor activity with minor overall toxicity to the patient. One of the characteristic features of Onc is a synergism with a variety of other antitumor modalities. Cepharanthine (Cep), a biscoclaurine alkaloid from Stephania cepharantha Hayata, is widely used in Japan to treat variety of ailments. It also shows low toxicity to patients. The aim of the present study was to assess the interaction of these two drugs on different tumor cell lines. When human promyelocytic leukemia HL-60, histiomonocytic lymphoma U937, multiple myeloma RPMI-8228, prostate carcinoma DU 145 and prostate adenocarcinoma LNCaP cells were exposed to relatively low concentrations of Onc or Cep their growth rates were somewhat suppressed but the cells were still able to proliferate. Cell growth, however, was totally abolished in each of these cell lines when treated with Onc and Cep combined. The frequency of apoptosis was also many-fold higher in cultures treated with a combination of Onc and Cep than in respective cultures treated with Onc or Cep alone. The mechanism of the observed synergism is unclear but it may be associated with the Onc activity in targeting microRNAs and/or NFkappaB and Cep activity also targeting NFkappaB. The data suggest that the combination of these two drugs, that individually express a low toxic profile, may have strong antitumor potential.
Keywords: RNA interference, microRNAs, mesothelioma, apoptosis, bisbenzlisoquinoline, alkaloid, nuclear factor kappaB, reactive oxygen species
Introduction
Onconase (Onc), also known under names ranpirnase and Pannon®,1 is a ribonuclease isolated from oocytes or early embryos of the Northern Leopard frog (Rana pipiens).2-4 This basic protein of ∼12,000 MW shows weak ribonucleolytic activity and is a member of the pancreatic RNase A superfamily.5-8 Unlike RNase A, Onc is cytostatic and cytotoxic to a variety of tumor cell lines1,9-12 and inhibits growth of tumors in animal models.13,14 Onc is currently in Phase IIIb clinical trials for unresectable malignant mesothelioma where it displays antitumor activity with minor overall toxicity to the patient.15-18
The mechanism of the anticancer activity expressed by Onc is not entirely clear. Onc is internalized by an endocytic pathway19 and its ribonucleolytic activity is essential for cytostatic and cytotoxic effects.12 One reason for its anticancer selectivity could be explained as due to a greater electronegativity of the plasma membrane surface of cancer cells thus facilitating electrostatic interactions with this very basic protein.8 It has been postulated that tRNA,20 rRNA12 and/or microRNAs21 are the intracellular targets of Onc. The cytostatic effect of Onc, observed after 24–48 h of the treatment, presents as an arrest in the G1 phase of the cell cycle.1,22 The Onc-induced arrest of lymphoma U-937 cells in G1 was shown to be mediated by downregulation of cyclin D3, upregulation of p27KIP1, p16INK4A and p21WAF1/CIP1 and hypophosphorylation of pRb.22 Prolonged exposure of tumor cells to Onc leads to apoptosis, associated with classical changes in morphology,26 extensive DNA fragmentation, and activation of caspases, serine proteases and transglutaminase.1,22-25
Intriguingly, a strong enhancement in cytotoxicity has been observed when Onc was combined with other antitumor modalities, such as tamoxifen,10 lovastatin,10,27 cisplatin,10 vincristine,11 tumor necrosis factor α (TNFα),28 interferons,29 ionizing radiation,30 differentiation-inducing agents,31 or mild hyperthermia.32 We postulated that Onc enhances cytotoxicity of many antitumor drugs by suppressing translation of the “survival genes” activated by NFκB during drug treatment.28 Specifically, since Onc targets intracellular RNA the likely target in this case could be the NFkappaB-induced mRNAs coding for proteins that protect cells from apoptosis.33-35 It should be stressed that individual drugs at the doses used in the studies had relatively low toxicity by themselves but in combination with Onc were strongly cytotoxic.10,11,27-31 This observation suggested that anticancer effectiveness of such drug combinations can be enhanced with an acceptable increase in toxicity to the patient.
Extending this area of research we presently investigated cytostatic and cytotoxic effects of Onc in combination with the the biscoclaurine alkaloid cepharanthine (Cep). Cep is the drug isolated from the plant Stephania cepharantha Hayata used primarily in Japan to treat a variety of acute and chronic diseases.36 Specifically, Cep is used to treat: alopecia areata,37 venomous snakebites,38 radiotherapy-caused leukopenia,39 malaria40 and septic shock.41,42 Other pharmacological activities reported to be mediated by CEP are: inhibition of plasma membrane lipid peroxidation,43 inhibition of histamine release,44 anti-inflammatory effect,41 anti-allergic effect,45 multidrug resistance-reversing effect,46 inhibition of platelet aggregation47 and antitumor activity.48,49
The diversity of biological activities of Cep suggests that some may be mediated by a common mechanism. One mechanism that appears to have many consequences involves the oxidant-radicals scavenging property of this alkaloid. There is strong evidence that Cep effectively scavenges radicals such as superoxide anion, hydroxyl radical and nitric oxide.50-52 We have recently reported that Cep effectively scavenges endogenous oxidants generated during aerobic metabolism, protecting DNA from oxidative damage.53,54 The radicals scavenging properties of Cep may mediate several of the effects listed above, such as inhibition of the lipid peroxidation,43 protection from carcinogens,49 anti-inflammatory properties41 or protection from radiotherapy-induced leucopenia.39
It was recently reported that Cep sensitizes human oral carcinoma cells to radiation by inhibiting activation of NFκB.55 As mentioned, the NFκB is the most likely target of Onc as well.28 In analogy to Cep Onc also shows antioxidant properties.56 Since oxidative stress activates NFκB57-60 and thereby increases cells resistance to apoptosis33-35 its reduction may have pro-apoptotic consequences. Intriguingly, thus, both Cep and Onc, although so different in their structure and in mechanism of molecular interaction with intracellular targets, induce similar effects i.e., the reduction of oxidative stress and inhibition of NFκB activation. Relatively low toxicity also characterizes both these drugs. Given diversity of possible targets and mechanisms of action as well as the similarities in the present study we explored interaction of Onc and Cep on several tumor cell lines. The remarkable enhancement of cytotoxicity observed when Onc and Cep were used in combination suggests an attractive new approach for treating cancer.
Results
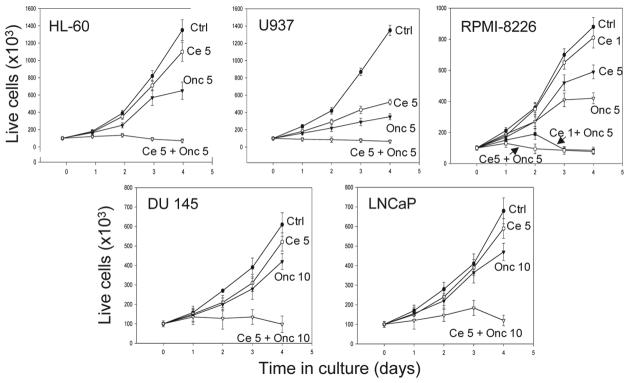
Analysis of growth curves of each of the five cell lines demonstrates that a combination of Onc and Cep had a potent effect on cell growth, much stronger than expected if the effects of Onc and Cep have been additive (Fig. 1). Growth of each cell line was distinctly suppressed in the presence of either 5 μg/ml (HL-60, U937 and RPMI-8228) or 10 μg/ml (DU 145, LNCaP) Onc, respectively. Cell growth was also suppressed in the presence of 5 μg/ml of Cep. The most sensitive to Onc or Cep alone were histiomonocytic lymphoma U937 cells, whereas the least sensitive were prostate cancer cell lines DU 145 and LNCaP. The data thus indicate that in the presence of Onc or Cep cells of each of the lines proliferated albeit at a reduced rate. In contrast, cell growth was abolished in cultures treated simultaneously with Onc and Cep as the number of live cells after four days of treatment was actually lower than at the time zero. This was the case for each of the five cell lines.
Figure 1.
Effect of Cep (Ce), or Onc either alone or in combination (Ce + Onc) on growth of HL-60, U937, RPMI-8228, DU 145 and LNCaP cells. The drugs were administered into cultures at time 0, Cep at concentration either 1 or 5 μg/ml (Ce; Ce 5) and Onc at concentration either 5 or 10 μg/ml (Onc 5, Onc 10), and frequency of live (trypan blue excluding) cells was estimated after 1, 2, 3 and 4 days of culturing. Note no cell growth in cultures treated concurrently with Cep and Onc.
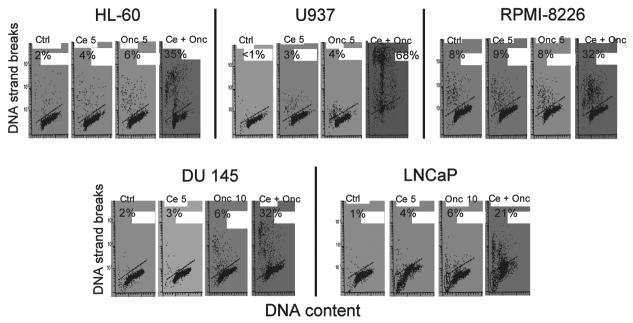
Figure 2 presents the data summarizing the cytotoxic effects of Onc and Cep either alone or in combination on all five cell lines. Cell death was estimated as frequency of apoptosis; apoptotic cells were identified by the presence of extensive DNA breakage which was detected by the TUNEL assay.61,62 Because in the drug treated cultures of HL-60, U937 and RPMI-8228 cells apoptosis occurred more rapidly than in DU 145 and LNCaP cultures the first three lines were examined 24 h after drug administration whereas the latter after 72 h. Concentrations of Onc and Cep as well as the duration of exposure to these drugs was adjusted so that the effect of the combined treatment would be more evident. The results reveal a striking enhancement of cytotoxicity for each of these cell lines in the presence of the combined drugs. In fact, the data show a synergistic effect of Onc and Cep given simultaneously when expressed as the apoptotic index. This was even more apparent than the results reflected by the growth curves (Fig. 1). The most dramatic enhancement was observed in the case of U937 cells. These cells had a relatively low apoptotic index when treated with 5 μg/ml of Onc (4%) or Cep (3%) alone, but the combined treatment with Onc and Cep for 24 h resulted in apoptotic index as high as 68%. HL-60 cells were also very sensitive when treated simultaneously with Onc and Cep; the frequency of apoptotic cells in the culture containing both drugs was nearly six- and nine-fold higher than in the cultures containing either Onc or Cep alone, respectively. A similar enhancement of cytotoxicity, well above the additive effects of the individual drugs, was seen in cultures of RPMI-8228, DU 147 and LNCaP cells.
Figure 2.
Frequency of apoptosis in cultures of HL-60, U937, RPMI-8228, DU 145 and LNCaP cells treated with Cep (Ce) or Onc either alone or in combination (Ce + Onc). The drugs were added into cultures at time zero, Cep at concentration 5 μg/ml (Ce; Ce 5) and Onc at concentration either 5 or 10 μg/ml (Onc 5, Onc 10), as shown, and the frequency of apoptotic (TUNEL positive; percent shown in each panel) cells in cultures of HL-60, U937 and RPMI-8228 was estimated 24 h, and in cultures of DU 145 and LNCaP 72 h-after drug administration.
Although the present data were not amenable for analysis by the classical Chou and Talalay approach63 because of the lack of IC50 for each treatment, the synergistic (greater than additive) effects of Onc and Cep combination are very apparent. Thus, by analysis of the percentage of apoptotic cells (after compensation for frequency of spontaneous apoptosis in untreated cultures) the additive effects of Onc and Cep when used alone were 6% for HL-60, 5% for U937, 1% for RPMI-8228, 5% for DU 145 and 8% for LNCaP cells (Fig. 2). However, in the respective cultures treated with Onc and Cep together the frequency of apoptotic cells was 33%, 67%, 24%, 30% and 20%, several-fold above that of the additive effects.
Discussion
The present data clearly demonstrate a synergistic effect when Onc and Cep were combined to treat all five tumor cell lines. Cep was used at relatively low concentrations of either 1 or 5 μg/ml, at which its effect on cell proliferation or on induction of apoptosis was rather minimal. The exception was the response of U937 cells whose proliferation was significantly reduced without evidence of extensive apoptosis. A relatively low, cytostatic rather than cytotoxic, concentration of Onc also was used. Specifically, based on our earlier observations1,22,23,28 and on the data of pilot experiments in the present study 5 μg/ml Onc concentration was chosen to treat the leukemic HL-60, U937 and RPMI-8228 cell lines, which were somewhat more sensitive to Onc, and 10 μg/ml to treat the more resistant prostate cancer lines. At those Onc concentrations the rate of cell growth was distinctly reduced while the frequency of apoptosis was relatively low. The effects of Onc and Cep, thus, when each was used alone, were primarily cytostatic with minimal cytotoxicity manifested by apoptosis. However, in combination the cytotoxic effect of Onc and Cep, become very apparent, as the percentage of apoptotic cells was many-fold higher compared with the added effects of Onc and Cep used alone. Also, no net cell growth (proliferation) was seen in cultures containing both Onc and Cep.
What may be the mechanism responsible for the observed enhancement of cytotoxicity when Onc was combined with Cep, and possibly for the synergism of Onc seen in combination with variety of antitumor modalities with diverse molecular structure and mode of action?10,11,28-32 Several mechanisms may contribute towards this remarkable synergism. We postulated before that microRNAs are likely the targets of Onc and their destruction may explain variety of facets of Onc activity.21 One possibility is targeting by this RNase members of the family of microRNAs that provide tumor resistance to cytotoxic drugs through mobilizing the cell defense mechanisms.64 Another target of Onc whose degradation is expected to enhance sensitivity to antitumor modalities is the family of microRNAs associated with the p53 tumor suppressor network that modulate proclivity of cells to undergo apoptosis.65 Still another target of Onc that can play a role in inducing cytotoxicity may be telomerase RNA.66,67 Telomeres are replenished by RNA-templated synthesis of telomeric DNA and there is strong evidence implicating length of telomerase RNA in cancer progression and “immortality” of tumor cells.66 Telomerase RNA confines the telomere length and even minute deletion of its length profoundly reduces cell ability to proliferate.67
Several lines of evidence also implicate NFκB as the target of both, Onc and Cep. NFκB is a highly inducible transcriptional factor which plays an essential role in triggering transcription of genes involved in stress and inflammatory responses.68 As mentioned in Introduction NFκB is often activated during treatment with antitumor drugs and the expression of “survival genes” induced by the factor protects cells from apoptosis. The classic example of such activity is protection from apoptosis induced along the extrinsic pathway by “death ligands” such as TNFα or CD95 (Fas).35 When transcription or translation of these genes is prevented by actinomycin D or cycloheximide the toxicity of the ligands is dramatically increased.69 In analogy, the enhancement of cytotoxicity of TNFα by Onc was explained by the targeting mRNA of the induced “survival genes” by this ribonuclease.28 In the case of Cep, activation of NFκB, which is a redox-sensitive transcription factor,70 seems to be prevented by the antioxidant action of this alkaloid.50-54 Antioxidant properties of Onc56 could additionally lower the redox potential within the cell providing further hindrance in activation of NFκB. Simultaneous inactivation of NFκB by diverse mechanisms as offered by Onc and Cep, respectively, may be so effective that it completely eliminates expression of the “survival genes.” The cytostatic effects of these drugs, otherwise seen when the drugs were used individually, become cytotoxic under these conditions.
Regardless of mechanism of the observed synergism between Onc and Cep the present data demonstrate that a combination of these two relatively nontoxic agents exerts dramatic cytotoxic effect on tumor cells. These observations warrant further studies on in vivo animal models exploring anticancer activity and the overall toxicity of the drug combination.
Materials and Methods
Cell cultures
Human cell lines: promyelocytic leukemia HL-60, histiomonocytic lymphoma U937, multiple myeloma RPMI-8228, prostate carcinoma DU 145 and prostate adenocarcinoma LNCaP all were obtained from the American Type Culture Collection (ATCC; Manassas, VA). HL-60, U937, RPMI-8228 and LNCaP cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine (all from GIBCO/BRL Life Technologies, Inc., Grand Island, N.Y.). DU 145 cells were grown in Dulbecco's modified Eagle's medium. The cultures were kept in 25 ml FALCON flasks (Becton Dickinson Co., Franklin Lakes, NJ) at 37°C in an atmosphere of 5% CO2 in air. The cultures of cells growing in suspension (HL-60, U937, and RPMI-8228) were maintained to have fewer than 5 × 105 cells per ml in culture such that the cells were at an exponential and asynchronous phase of growth. The cultures of cells growing attached to flasks were also maintained in exponential and asynchronous phase of growth by repeated trypsinization and reseeding prior to reaching subconfluency.
Cell treatment
At the onset of the experiments the cells were seeded at 1 × 105 cells per 1 ml and were treated either with Cep, Onc or combination of these drugs at respective concentrations as presented in Figures legends. Preparations of Cep were kindly provided by Kakenshoyaku Company, Ltd, Osaka, Japan (Cepharanthin inj., Lot No KO22196). These preparations, prepared in sterile and buffered saline, are being clinically applied for different ailments in Japan. Onc was obtained from Alfacell Co., (Somerset, NJ), and its solutions were prepared in buffered saline, as described before.25,32 Control cultures were treated with equivalent volumes of buffered saline. Control and drug treated cultures were run in triplicate.
Assessment of cell viability and apoptosis
The cells growing in suspension were sampled every 24 h after drug administration and their viability was assessed by staining with trypan blue; the number of live cells excluding trypan blue were plotted to construct the growth curves (Fig. 1). The attached cells were trypsinized and their viability was also estimated by the trypan blue assay. Attention was paid to collecting and pooling both trypsinized and floating cells, as the latter population has a higher proportion of mitotic and apoptotic cells that detach in culture. To assess the frequency of apoptosis, the cells were fixed in 1% formaldehyde for 15 min on ice and then transferred into 70% ethanol. The presence of DNA strand breaks in apoptotic cells was then detected using the terminal transferase-mediated break labeling with Br-dUTP, as described.61,62 Cellular DNA was counterstained with propidium iodide (PI; Molecular Probes/Invitrogen, Eugene, OR) in the presence of RNase A (Sigma Chemical Co., St Louis, MO).62 Green (FITC) and red (PI) fluorescence of cells in was measured using a FASCcan flow cytometer (Becton-Dickinson, San Jose, CA). The red (PI) and green (FITC) fluorescence from each cell were separated and quantified using the standard optics and CELLQuest software (Becton-Dickinson).
Acknowledgements
Supported in part by NCI RO1 28 704.
References
- 1.Darzynkiewicz Z, Carter SP, Mikulski SM, Ardelt WJ, Shogen K. Cytostatic and cytotoxic effects of Pannon (P-30 Protein), a novel anticancer agent. Cell Tissue Kinetics. 1988;21:169–82. doi: 10.1111/j.1365-2184.1988.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 2.Ardelt W, Mikulski SM, Shogen K. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. Homology to pancreatic ribonuclease. J Biol Chem. 1991;266:245–51. [PubMed] [Google Scholar]
- 3.Ardelt W, Lee HS, Randolph G, Viera A, Mikulski SM, Shogen K. Enzymatic characterization of onconase, a novel ribonuclease with anti-tumor activity. Protein Sci. 1994;3:137–47. [Google Scholar]
- 4.Mosimann SC, Ardelt W, James MNG. Refined 1.7Å X-ray crystallographic structure of P-30 protein, an amphibian ribonuclease with antitumor activity. J Mol Biol. 1994;236:1141–53. doi: 10.1016/0022-2836(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 5.Ardelt W, Shogen K, Darzynkiewicz Z. Onconase and amphinase, the anti-tumor ribonucleases from Rana pipiens oocytes. Curr Pharm Biotechnol. 2008 doi: 10.2174/138920108784567245. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold U, Ulbrich-Hofmann R. Natural and engineered ribonucleases as potential cancer therapeutics. Biotechnol Lett. 2006;28:1615–22. doi: 10.1007/s10529-006-9145-0. [DOI] [PubMed] [Google Scholar]
- 7.Benito A, Ribo M, Vilanova M. On the track of antitumor ribonucleases. Mol BioSyst. 2005;1:294–302. doi: 10.1039/b502847g. [DOI] [PubMed] [Google Scholar]
- 8.Lee JE, Raines RT. Ribonucleases as novel chemotheraupeutics: the ranpirnase example. BioDrugs. 2008;22:53–8. doi: 10.2165/00063030-200822010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikulski S, Viera A, Darzynkiewicz Z, Shogen K. Synergism between a novel amphibian oocyte ribonuclease and lovastatin in inducing cytostatic and cytotoxic effects in human lung and pancreatic carcinoma cell lines. Br J Cancer. 1992;66:304–10. doi: 10.1038/bjc.1992.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikulski S, Viera A, Shogen K. In vitro synergism between a novel amphibian oocytic ribonuclease (ONCONASE) and tamoxifen, lovastatin and cisplatin in human OVCAR-3 ovarian carcinoma cell line. Int J Oncol. 1992;1:779–85. [PubMed] [Google Scholar]
- 11.Rybak SM, Pearson J, Fogler W, Volker K, Spence S, Newton DL, Mikulski SM, Ardelt W, Riggs C, Kung H, Longo D. Enhancement of vincristine cytotoxicity in drug-resistant cells by simultaneous treatment with ONCONASE, an antitumor ribonuclease. JNCI. 1996;88:747–53. doi: 10.1093/jnci/88.11.747. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Mikulski SM, Ardelt W, Rybak SM, Youle RJ. A cytotoxic ribonuclease. Study of the mechanism of onconase cytotoxicity. J Biol Chem. 1993;268:10686–93. [PubMed] [Google Scholar]
- 13.Mikulski SM, Bernstein E, Ardelt W, Shogen K, Menduke K. Striking increase in survival of mice bearing M109 Madison carcinoma treated with a novel protein from amphibian embryos. JNCI. 1990;82:151–3. doi: 10.1093/jnci/82.2.151-a. [DOI] [PubMed] [Google Scholar]
- 14.Lee I, Kalota A, Gewirtz AM, Shogen K. Antitumor efficacy of the cytotoxic RNase, ranpirnase, on A549 human lung cancer xenografts of nude mice. Anticancer Res. 2007;27:299–307. [PubMed] [Google Scholar]
- 15.Mikulski SM, Constanzi JJ, Vogelzang NJ, McCachren S, Taub RN, Chun H, Mittelman A, Panella T, Puccio C, Fine R, Shogen K. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J Clin Oncol. 2002;20:274–81. doi: 10.1200/JCO.2002.20.1.274. [DOI] [PubMed] [Google Scholar]
- 16.Constanzi J, Sidransky D, Navon A, Goldsweig H. Ribonucleases as a novel pro-apoptotic anticancer strategy: review of the preclinical and clinical data for ranpirnmase. Cancer Invest. 2005;23:643–50. doi: 10.1080/07357900500283143. [DOI] [PubMed] [Google Scholar]
- 17.Vogelzang NJ, Porta C, Mutt L. New agents in the management of advanced mesothelioma. Semin Oncol. 2005;32:336–50. doi: 10.1053/j.seminoncol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Pavlakis N, Vogelzang NJ. Ranpirnase—an antitumor ribonuclease: its potential role in malignant mesothelioma. Expert Opin Biol Ther. 2006;6:1391–9. doi: 10.1517/14712598.6.4.391. [DOI] [PubMed] [Google Scholar]
- 19.Haigis MC, Raines RT. Secretory ribonucleases are internalized by a dynamin-independent endocytic pathway. J Cell Sci. 2003;116:313–24. doi: 10.1242/jcs.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena SK, Sirdeshmukh R, Ardelt W, Mikulski SM, Shogen K, Youle RJ. Entry into cells and selective degradation of tRNA by a cytotoxic member of the RNase A family. J Biol Chem. 2002;277:15142–6. doi: 10.1074/jbc.M108115200. [DOI] [PubMed] [Google Scholar]
- 21.Ardelt B, Ardelt W, Darzynkiewicz Z. Cytotoxic ribonucleases and RNA interference (RNAi) Cell Cycle. 2003;2:22–4. doi: 10.4161/cc.2.1.232. [DOI] [PubMed] [Google Scholar]
- 22.Juan G, Ardelt B, Li X, Mikulski SM, Shogen K, Ardelt W, Mittelman A, Darzynkiewicz Z. G1 arrest of U-937 cells by onconase is associated with suppression of cyclin D3 expression, induction of p16INK4A, p21WAF1/CIP1 and p27KIP and decreased pRb phosphorylation. Leukemia. 1998;12:1241–8. doi: 10.1038/sj.leu.2401100. [DOI] [PubMed] [Google Scholar]
- 23.Grabarek J, Ardelt B, Du L, Darzynkiewicz Z. Activation of caspases and serine proteases during apoptosis induced by onconase (Ranpirnase) Exp Cell Res. 2002;278:61–71. doi: 10.1006/excr.2002.5568. [DOI] [PubMed] [Google Scholar]
- 24.Grabarek J, Ardelt B, Kunicki J, Darzynkiewicz Z. Detection of in situ activation of transglutaminase during apoptosis: correlation with the cell cycle phase by multiparameter flow- and laser scanning-cytometry. Cytometry. 2002;49:83–9. doi: 10.1002/cyto.10150. [DOI] [PubMed] [Google Scholar]
- 25.Ardelt B, Ardelt W, Pozarowski P, Kunicki J, Shogen K, Darzynkiewicz Z. Cytostatic and cytotoxic properties of Amphinase, a novel cytotoxic ribonuclease from Rana pipiens oocytes. Cell Cycle. 2007;6:3097–102. doi: 10.4161/cc.6.24.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami Traganos F. Cytometry in cell necrobiology. Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 27.Mikulski SM, Viera A, Darzynkiewicz Z. Synergism between a novel amphibian oocyte ribonuclease and lovastatin in inducing cytostatic and cytotoxic effects in human lung and pancreatic carcinoma cell lines. Br J Cancer. 1992;66:304–10. doi: 10.1038/bjc.1992.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deptala A, Halicka HD, Ardelt W, Mikulski SM, Shogen K, Darzynkiewicz Z. Potentiation of tumor necrosis factor induced apoptosis by onconase. Int J Oncol. 1998;13:11–6. doi: 10.3892/ijo.13.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Tsai SY, Hsieh TC, Ardelt B, Darzynkiewicz Z, Wu JM. Combined effects of onconase and IFNbeta on proliferation, macromolecular syntheses and expression of STAT-1 in JCA-1 cancer cells. Int J Oncol. 2002;20:891–6. [PubMed] [Google Scholar]
- 30.Kim DH, Kim EJ, Kalota A, Gewirtz AA, Glickson J, Shogen H, Lee I. Possible mechanisms of improved response by cytotoxic RNase, Onconase, on 549 human lung cancer xenografts of nude mice. Adv Exp Biol Med. 2007;599:53–9. doi: 10.1007/978-0-387-71764-7_8. [DOI] [PubMed] [Google Scholar]
- 31.Halicka HD, Murakami T, Papageorgio CN, Mittelman A, Mikulski SM, Shogen K, Darzynkiewicz Z. Induction of differentiation of leukemic (HL-60) or prostate cancer (LNCaP, JCA-1) cells potentiates frequency of apoptosis triggered by onconase. Cell Prolif. 2000;33:407–17. doi: 10.1046/j.1365-2184.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halicka HD, Ardelt B, Shogen K, Darzynkiewicz Z. Mild hyperthermia predisposes tumor cells to undergo apoptosis upon treatment with onconase. Int J Oncol. 2007;30:841–7. [PubMed] [Google Scholar]
- 33.Van Antwerp DJ, Martin SJ, Kafri T, Green DG, Verma IM. Suppression of TNFα-induced apoptosios by NFκB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 34.Wang CY, Mayo MM, Baldwin AS., Jr TNF and cancer therapy-induced apoptosis: potentiation by inhibition of NFκB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 35.Begg AA, Baltimore D. An essential role for NFκB in preventing TNFα induced cell death. Science. 1996;274:782–4. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 36.Furusawa S, Wu J. The effects of biscoclaurine alkaloid cepharanthine on mammalian cells: implications for cancer, shock and inflammatory diseases. Life Sciences. 2007;80:1073–9. doi: 10.1016/j.lfs.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Morita K, Nakamura M, Nagamachi M, Kishi T, Miyachi Y. Seventeen cases of alopecia areata: combination of SADBE topical immunotherapy with other therapies. J Dermatol. 2002;29:661–4. doi: 10.1111/j.1346-8138.2002.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 38.Kimoto T, Suemitsu K, Nakayama H, Komori E, Ohtani M, Ando S. Therapeutic experience of venomous snakebites by the Japanese viper (Agkistrodon halys blomhoffii) with low dose of antivenin: report of 43 consecutive cases. Nippon Geka Hokan. 1997;66:71–7. [PubMed] [Google Scholar]
- 39.Ohta T, Morita K. Effect of cepharanthin on radiotherapy induced leucopenia. Rinsho Hoshasen. 1990;35:471–4. [PubMed] [Google Scholar]
- 40.Chea A, Hout S, Bun SS, Tabatadze N, Gasquet M, Azas N, Elias R, Balansard G. Antimalarial activity of alkaloids isolated from Stephania rotunda. J Ethnopharmacol. 2007;12:132–7. doi: 10.1016/j.jep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Goto M, Zeller WP, Hurley RM. Cepharanthine (biscoclaurine alkaloid) treatment in endotoxic shock of suckling rats. J Pharm Pharmacol. 1991;43:589–91. doi: 10.1111/j.2042-7158.1991.tb03542.x. [DOI] [PubMed] [Google Scholar]
- 42.Sato T, Ohnishi ST. In vitro anti-sickling effect on cepharanthine. Eur J Pharmacol. 1982;83:91–5. doi: 10.1016/0014-2999(82)90289-8. [DOI] [PubMed] [Google Scholar]
- 43.Shiraishi N, Arima T, Aono K, Inouye B, Morimoto Y, Utsumi K. Inhibition by biscoclaurine alkaloid of lipid peroxidation in biological membranes. Physiol Chem Physics. 1980;12:299–305. [PubMed] [Google Scholar]
- 44.Nakamura K, Tsuchiya S, Sugimoto Y, Sugimura Y, Yamada Y. Histamine release inhibition activity of bisbenzylisoquinoline alkaloids. Planta Medica. 1992;58:505–8. doi: 10.1055/s-2006-961536. [DOI] [PubMed] [Google Scholar]
- 45.Kohno H, Inoue H, Seyama Y, Yamashita S, Akasu M. Mode of the anti-allergic action of cepharanthine on an experimental model of allergic rhinitis. Nippon Yakurigaku Zasshi. 1987;90:205–11. doi: 10.1254/fpj.90.205. [DOI] [PubMed] [Google Scholar]
- 46.Fujimura T, Shibata H, Maekawa I, Furusawa S, Kawauchi H, Sasaki K, Takayanagi Y. Reversal of resistance to doxorubicin with cepharanthine in murine P388 leukemia cells. Japan J Pharmacol. 1990;54:464–7. doi: 10.1254/jjp.54.464. [DOI] [PubMed] [Google Scholar]
- 47.Sato T, Morita I, Fujita H, Ono M, Kimishima A, Tomiyama J, Murota S. Pharmacological characterization of cepharanthin in chronic idiopathic thrombocytopenic purpura. Platelets. 2001;12:156–62. doi: 10.1080/09537100120039334. [DOI] [PubMed] [Google Scholar]
- 48.Harada K, Supriatno Yamamoto S, Kawaguchi S, Yoshida H, Sato M. Cepharanthine exerts antitumor activity on oral squamous cell carcinoma cell lines by induction of p27Kip1. Anticancer Res. 2003;23:1441–8. [PubMed] [Google Scholar]
- 49.Yasukawa K, Takido M, Takeuchi M, Akasu M, Nakagawa S. Cepharanthine inhibits two-stage tumor promotion by 12-O-tetradecanoylphorbol 13-acetate and mezerein on skin tumor formation in mice initiated with 7, 12-dimethylbenz[á] anthracene. J Cancer Res Clin Oncol. 1991;117:421–4. doi: 10.1007/BF01612761. [DOI] [PubMed] [Google Scholar]
- 50.Kogure K, Goto S, Abe K, Ohiwa C, Akasu M, Terada H. Potent antiperoxidation activity of the bisbenzylisoquinoline alkaloid cepharanthine: the amine moiety is responsible for its pH-dependent radical scavenge activity. Biochim Biophys Acta. 1999;1426:133–42. doi: 10.1016/s0304-4165(98)00146-9. [DOI] [PubMed] [Google Scholar]
- 51.Kogure K, Tsuchiya K, Abe K, Akasu M, Tamaki T, Fukuzawa K, Terada H. Direct radical scavenging by the bisbenzylisoquinoline alkaloid cepharanthine. Biochim Biophys Acta. 2003;1622:1–5. doi: 10.1016/s0304-4165(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 52.Oyaizu H, Adachi Y, Yasumizu R, Ono M, Ikebukuru K, Fukuhara S, Ikahara S. Protection of T cells from radiation-induced apoptosis by Cepharantin. Int J Immunopharmacol. 2001;1:2091–9. doi: 10.1016/s1567-5769(01)00127-8. [DOI] [PubMed] [Google Scholar]
- 53.Halicka HD, Ita M, Tanaka T, Kurose A, Darzynkiewicz Z. The biscoclaurine alkaloid cepharanthine protects DNA in TK6 lymphoblastoid cells from constitutive oxidative damage. Pharmacol Rep. 2008;60:93–100. [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports the aging-related processes. Cytometry A. 2007;71:905–14. doi: 10.1002/cyto.a.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamatami T, Azuma M, Motegi K, Takamaru N, Kawashima Y, Bando T. Cepharanthin-enhanced radiosensitivity through the inhibition of radiation-induced nuclear factor-kappaB activity in human oral carcinoma calls. Int J Oncol. 2007;31:761–8. [PubMed] [Google Scholar]
- 56.Ardelt B, Juan G, Burfeind P, Salomon T, Wu JM, Hsieh TC, Li X, Sperry R, Pozarowski P, Shogen K, Ardelt W, Darzynkiewicz Z. Onconase, an anti-tumor ribonuclease suppresses oxidative stress. Int J Oncol. 2007;31:663–9. doi: 10.3892/ijo.31.3.663. [DOI] [PubMed] [Google Scholar]
- 57.Jia YT, Ma B, Wei W, Xu Y, Wang Y, Tang HT, Xia ZF. Sustained activation of nuclear factorκB by reactive oxygen species is involved in the pathogenesis of stress-induces gastric damage in rats. Crit Care Med. 2007;35:1582–89. doi: 10.1097/01.CCM.0000266824.82280.17. [DOI] [PubMed] [Google Scholar]
- 58.Vile GF, Tanef-Ilitschew A, Tyrrel RM. Activation of NFkappaB in human skin fibroblasts by the oxidative stress generated by UVA radiation. Photochem Photobiol. 1995;62:463–8. doi: 10.1111/j.1751-1097.1995.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 59.Barr J, Sharma CS, Sarkar S, Wise K, Dong L, Peryakaruppan A, Ramesh GT. Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol Cell Biochem. 2007;297:93–9. doi: 10.1007/s11010-006-9333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, Bours V. Multiple redox regulation in NFkappaB transcription factor activation. Biol Chem. 1997;378:1237–45. [PubMed] [Google Scholar]
- 61.Gorczyca W, Bruno S, Darzynkiewicz RJ, Gong J, Darzynkiewicz Z. DNA strand breaks occurring during apoptosis: Their early in situ detection by the terminal deoxynucleotidyl transferase and nick translation assays and prevention by serine protease inhibitors. Int J Oncol. 1992;1:639–48. doi: 10.3892/ijo.1.6.639. [DOI] [PubMed] [Google Scholar]
- 62.Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of apoptosis by cytometry using TUNEL assay. Methods. 2008;44:250–4. doi: 10.1016/j.ymeth.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chou TC, Talalay P. Quantitative analysis of the dose-effect relationship: the combined effects of multiple drugs or enzyme inhibitors. Adv Enz Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 64.Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67:11111–6. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He X, He L, Hannon GJ. The guardian's little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–105. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 66.Li S, Blackburn EH. Expression and suppression of human telomerase RNA. Cold Spring Harb Symp Quant Biol. 2006;71:211–5. doi: 10.1101/sqb.2006.71.009. [DOI] [PubMed] [Google Scholar]
- 67.Greider CW. Telomerase RNA levels limit the telomere length equilibrium. Cold Spring Harb Symp Quant Biol. 2006;71:225–9. doi: 10.1101/sqb.2006.71.063. [DOI] [PubMed] [Google Scholar]
- 68.Baldwin AS., Jr The NFκB and IκB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 69.Darzynkiewicz Z, Carter SP, Old LJ. Effect of recombinant tumor necrosis factor on HL-60 cells: cell cycle specificity and synergism with actinomycin. D J Cell Physiol. 1987;130:328–35. doi: 10.1002/jcp.1041300304. [DOI] [PubMed] [Google Scholar]
- 70.Zingarelli N. Nuclear factorκB. Crit Care Med. 2005;33:414–6. [Google Scholar]