Abstract
In rodents, where chemical signals play a particularly important role in determining intraspecies interactions including social dominance and intersexual relationships, various studies have shown that behavior is sensitive to conspecific odor cues. Mice use urinary scent marks for communication with individual conspecifics in many social contexts. Urinary scent involves genetic information about individuals such as species, sex, and individual identity as well as metabolic information such as social dominance, and reproductive and health status, which are mediated by chemical proteins in scent marks including the major histocompatibility complex and the major urinary proteins. The odor of the predator which can be considered to be a threatening signal for the prey also modulate mouse behavior in which scent marking is suppressed in response to the cat odor exposure in mice. These odorant chemicals are detected and recognized through two olfactory bulbs, the role of which in detection of chemosignals with biological relevant appears to be differential, but partly overlapped. Mice deposit scent marks toward conspecifics to maintain their social relationships, and inhibit scent marking in a context where natural predator, cat odor is contained. This suppression of scent marking is long-lasting (for at least 7 days) and context-dependent, while the odorant signaling to conspecifics tends to appear frequently (over 24 hrs but less than 7 days intervals) depending on the familiarity of each signal-recipient. It has been discussed that scent marking is a communicative behavior associated with territoriality toward conspecifics, indicating that the social signaling within species are sensitive to predator odor cues in terms of vulnerability to predation risk.
Keywords: Urine marking, predatory odor, predation risk, social memory, chemosignal, mice
1. Introduction
Animals exhibit a variety of adaptive behaviors for communicating with conspecifics and detecting and defending themselves from predators (Brown & MacDonald, 1985; Kats & Dill, 1998). In a variety of mammals, odors may permit detection and recognition of individuals of the same and different species (Brown & MacDonald, 1985; Haplin, 1986; Staples et al., 2008). In addition to the use of chemosignals in the formation and maintenance of social relationships with conspecifics (Hurst, 1990a,b, 1993) prey may use predator odors to detect and subsequently avoid predation (Apfelbach et al., 2005; Blanchard et al., 1989a; Kats & Dill, 1998), whereas predators may use prey odors to detect and locate their meals (Koivula & Korpimäki, 2001; Rosell & Sanda, 2006). In the former case, mutual benefits may ensue, but in the latter cases, the odor-recipient is benefited by the interchange while the odor-donor is not.
Here, we briefly review social communication via odorants with a focus on scent marking behavior in mice. We also consider functional differences in response to interspecific and heterospecific (predator-prey) chemosignals; specifically the temporal characteristics of social memory formation and duration concerning conspecifics and predators.
2. Social odorant communication
Olfaction is a major modality through which animals may detected and possibly identify, other animals. As early mammals were likely nocturnal, olfaction, along with audition and tactile senses, were particularly important modalities of intraspecific communication as well as predator detection (Brown, 1979; Eisenberg, 1981). Auditory signals exchanged with conspecifics or alarm cries of conspecifics are particularly important for group-living animals (Warkentin et al., 2001; Litvin et al., 2007). Rapid onset – rapid off set auditory signals are particularly useful in acute emergencies, as olfactory signals typically have a delay between signal emission and reception (Eisenberg & Kleiman, 1972); however, the latter remain for longer durations. As a result, odors may provide information to a wider range of recipients, conspecific as well as allospecific, concerning locations of animals that are no longer present (Blanchard et al., 2003a; Brown & MacDonald, 1985; Hurst & Beynon, 2004). Most commonly used laboratory rodent species are macrosomatic, using odors as a major mode of social and nonsocial detection and potentially recognition. Odor-based interactions have recently come to occupy an increasingly important place in laboratory work on social, sexual, and antipredator behaviors.
2.1. Intraspecies communication
Mice and other rodents use odors in a number of social, agonistic, and defensive contexts. These odors may provide information about other animals including species, sex, and individual identity, using this information to adjust their behavior in subsequent interactions (Bowers & Alexander, 1967; Brown & MacDonald, 1985; Halpin, 1986). Scent marks deposited in the environment may communicate information on territory ownership when the owner is absent (Hurst, 1987, 1989), social status (Hurst, 1993; Jones & Nowell, 1973a, 1974); reproductive, health, and nutritional status (Barnard & Fitzsimmons, 1988; Brown & MacDonald, 1985; Brown & Schellinck, 1995; Kavaliers et al., 2005; Mossman & Drickamer, 1996); and enable recognition of individuals (Bowers & Alexander, 1967). The informational content of odors may be maintained for over 24 hours in the marker’s absense (e.g., Hurst & Beynon, 2004) enabling a protracted interaction between individuals, compared to vocal or tactile communication (Hurst et al., 1998).
2.1.1. Male to male communication: Dominance relationships
Mice are territorial (Crowcroft & Rowe, 1963; Anderson & Hill, 1965), and urinary scent marking serves to indicate territorial boundaries (Humphries et al., 1999; Ralls, 1971). Scent marking and the counter-marking of the scent marks of other males are important components of dominance advertisement among male house mice and strongly influence their aggressive interactions (Hurst, 1993; Mugford & Nowell, 1970). In male mice, fresh scent marking propounds the mouse’s ability to dominate an area (Hurst & Rich, 1999) and to maintain the territory against male intruders (Gosling et al., 2000; Jones & Nowell, 1973b, 1989). Accordingly, dominant male mice make more urine marks, whereas subordinate males urinate in fewer locations (Desjardins et al., 1973; Hurst, 1990b), such that the number of scent marks can predict both aggression scores and social dominance status in mice (Drickamer, 2001). Subordinate males, in contrast, suppress such competitive signaling and avoid challenges (Desjardins et al., 1973), thereby reducing detection and attack.
The chemical signals in scent marks also modulate aggressive behavior in recipient mice (Lacey et al., 2007; Mucignat-Caretta et al., 2004). Resident male mice attack intact adult males, but not castrated male intruders or females (Mugford & Nowell, 1970). When the castrated male or the female (but not pups; Mucignat-Caretta et al., 2004) is sprayed with intact male urine, the resident male may attack them (Mugford & Nowell, 1970; Novotny et al., 1985). This suggests that chemicals present in male urine constitute an extremely important aggression-inducing signal (Mucignat-Caretta & Caretta, 1999).
Specificity of information available in scent marks is shown in studies indicating that dominant male mice countermark the scent marks from other males but show no such response to their own scent marks or to these from males genetically identical to themselves (Hurst, 1990b; Nevison et al., 2000). This countermarking response is an important part of male competitive advertisement to other males and to females (Hurst, 1993; Hurst & Rich, 1999). Although dominant males countermarked the urine marks of another when they could contact the mark, they failed to do this when contact was prevented by a sheet of nitrocellulose (Nevison et al., 2003), suggesting that non-volatile components of the scent mark are involved in this recognition (Hurst et al., 2005; Humphries et al., 1999).
Territorial urine marking is an androgen-dependent behavior influenced by dominance status (Desjardins et al., 1973; Kimura & Hagiwara, 1985, Ralls, 1971). Both testosterone propionate (TP) and estradiol benzoate (EB) were effective in restoring scent marking of castrated males (Kimura & Hagiwara, 1985). With some exceptions (CF-1 mice; Coquelin, 1992; Maruniak et al., 1975) intact female mice show low levels of marking that are further depressed by ovariectomy (Kimura & Hagiwara, 1985). Sexual experience and estrus cycle did not alter the frequency of scent marking in female mice (Maruniak et al., 1975), but TP and 5-α-dihydrotestosterone enhanced marking in ovariectomized females while EB restored the marking to the pre-ovariectomy level (Kimura & Hagiwara, 1985). Neonatally androgenized females showed much higher levels of scent marking than normal females (Kimura & Hagiwara, 1985). These findings suggest that sexual dimorphism in scent marking in mice is primarily determined by the hormonal (testosterone) environment during early postnatal age, with additional effects of hormones later in life.
2.1.2. Male to female communication: Reproductive relationships
Ultrasonic courtship vocalizations to females or female odors constitute another androgen-dependent reproductive behavior influenced by dominance status (Nyby et al., 1976, 1977). Dominant males vocalize more than subordinate males (Nyby et al., 1976) while castration decreases the frequency of vocalization to females as well as scent marking to males (Lumley et al., 1999; Nyby et al., 1992). TP replacement restores these behaviors to precastration level (Sipos & Nyby, 1998). However, courtship vocalizations and scent marking to females may be regulated by different mechanisms (Nyby et al., 1992). In castrated males, intracranial implants of testosterone into the medial preoptic hypothalamus, an important regulatory site for male sexual behavior (Meisel & Sachs, 1994), were sufficient to restore courtship vocalizations to female urine but only partly restore scent marking in response to female urine (Matochik et al., 1994; Sipos & Nyby, 1998). Repeated defeats suppress androgen levels in subordinate mice (Bronson & Desjardins, 1974). Following such defeats, DBA/2 males displayed prolonged inhibition (over 4 weeks) of territorial scent marking to a matched pair male, but only a short-term decrement (less than 24 hrs) of courtship vocalizations to female odor (Lumley et al., 1999). These findings suggest that courtship vocalization and scent marking have different functions in social communication, although both behaviors are androgen-dependent (Lumley et al., 1999; Sipos & Nyby, 1998). While courtship vocalizations may be considered to be an urgent response to a potential mate regardless of the context, scent marking is likely to be context-dependent and perhaps to incur a greater risk of aggressive intention from other males in the vicinity, even when females are not present.
Odorant signals may also function as a primer that alters the endocrine state of the recipient animal (for review, Koyama, 2004). Male mice scent mark to adult females more than to juvenile males or females (Arakawa et al., 2007), regardless of estrous stage in the stimulus females (Coquelin, 1992; Brown, 1988). Male scent marks may induce estrus, accelerate the onset of puberty, and synchronize estrous cycles in females (Vandenbergh, 1969; Whitten, 1956, 1957, 1958), with odors of dominant males exerting a stronger influence on accelerating the onset of puberty in females than odors of subordinates (Lombardi & Vandenbergh, 1977). However, female odors produce a delay in the onset of puberty and suppression of estrus in females (Drickamer & Hoover, 1979; Drickamer, 1982, 1983; Lee & van der Boot, 1955, Vandenbergh, 1994). Luteinizing hormone (LH) and prolactin are two hormones important for regulating these effects (Dulac & Torello, 2003; Haplern, 1987), and female mice are likely to respond to males odor with an increase in LH and reduction in prolactin: Male mice display an increase in prolactin as a response to female odor (Keverne & de la Riva, 1982).
Unfamiliar male odors can interrupt the establishment of pregnancy in females (Bruce, 1959; Kumar & Dominic, 1993; Parkes & Bruce, 1961), providing a functional test of familiarity. Females appear to form an olfactory memory of the stud male in the accessory olfactory bulb shortly after mating (Brennan et al., 1990; Brennan & Peele, 2003). This is mediated by prolactin and LH, to stimulate neuronal production in the olfactory bulb and hippocampus, respectively (Mak et al., 2007). If exposed to the scent of an unfamiliar male from a different strain within five days of mating, prolactin release is disrupted and the embryos fail to implant. This test has been applied to assess the olfactory signature used to recognize the stud male (Peele et al., 2003; Yamazaki et al., 1983).
2.2. Interspecies odorant signaling: predator-prey interaction
Animals discriminate between chemosignals from their own species and those from other species (heterospecifics) (Meredith & Westberry, 2004; Wyatt, 2003). In general, the adaptive advantages of within-species signaling may be countered by disadvantages with reference to nonconspecifics. Odor-based and other signals to conspecifics can have a negative effect by advertising an individual’s presence and location to predators (Koivula & Korpimäki, 2001; Roberts et al, 2001). Conversely, odors of predators may warn prey of their presence (Dickman, 1992; Merkens et al., 1991). Thus, rodents display aversion and avoidance responses to many of the odors of predators. This has been repeatedly demonstrated for the fur/skin odor of the domestic cat, which also supports rapid (1-trial) aversive conditioning, as an unconditioned stimulus (e.g., Blanchard et al., 1990a,b; Dielenberg & McGregor, 2001). Male and female mice also show a tendency to avoid cat odor (urinary and faecal odors) in a Y-maze choice situation (Kavaliers et al., 1994, 2001).
Predator odors may have major effects on within-species territorial scent marking (Roberts et al., 2001). High scent marking male laboratory mice approach competitors’ scent marks more quickly and spend more time in countermarking than those with a low scent marking frequency. The urine of ferrets (Mustela putorius furo) reduced approaches to the competitor’s marks, for high- but not low-marking males, suggesting that a high frequency of scent marking involves an inherent cost for predation risk. The odor of a non-predatory naked mole-rat (Heterocephalus glaber) failed to elicit this response, indicating that it is a response to predator odor, not just to an unfamiliar odor. Similar discriminations have been shown in meadow voles (Microtus pennsylvanicus) in tests between odors of weasels (Mustela erminea) and guinea pigs (Parsons & Bondrup-Nielsen, 1995). Reductions of scent marking following predator odor may reduce the risk of predation but also the benefits associated with signaling (Belwood & Morris, 1997; Wolff, 2004). Roberts et al. (2001) have suggested that, from a resource competition perspective, predation risk may enable low-markers to increase their scent marking investment, for improved access to resources. In such cases, scent marking may have greater benefits in the form of territory, reproductive competition, or sexual advertisement, than the cost of increased predation risk (Wolff, 2004).
3. Chemical signals in urinary scent marks
Evolved odor signals involve some components that are genetically determined (Boyse et al., 1987) and not susceptible to disruption by metabolic and environmental influences. However, environmental factors, such as food type, bacterial gut flora, and social stress, (Schellinck et al., 1992; Yamazaki et al., 1999, 2002) as well as parasited status (Kavaliers et al., 2005; Zala et al., 2003) also induce changes in volatile odors.
3.1. Odor chemicals between conspecifics
To provide relatively unambiguous information on identity, odor signals need to be sufficiently polymorphic so that each individual has a low probability of shared signatures in the local population (Hurst & Beynon, 2004). Among inbred mice, there may be difficulties in discrimination between individuals, and in assessment of familiarity and/or kinship (Barnard et al., 1991; Nevison et al., 2000). Indeed, individuals of highly inbred strains are unable to discriminate between each others’ volatile urinary odors when kept under identical conditions (Arakawa et al., 2008; Nevison et al., 2000; Yamaguchi et al., 1981).
3.1.1. The major histocompatibility complex associated chemosignal in scent marking
There are at least two chemical components of urinary scent that provide genetically-determined individual signatures in chemosignals (Brennan & Kendrick, 2006). The major histocompatibility complex (MHC) encodes highly polymorphic glycoproteins involved in self- non-self recognition in the immune system (Beauchamp & Yamazaki, 2003; Brown, 1995; Yamazaki et al., 1976). MHC-associated odors in mice are produced through a complex molecular mixture of volatile metabolites bound and released by urinary proteins (Singer et al., 1993, 1997). MHC associated urinary odors are used by mice in mate choice, discriminating kin from non-kin, and recognizing familiar individuals (e.g. Hurst, 1990a,b; Manning et al., 1992; Potts et al., 1994; Yamazaki et al., 1976, 1979). Yamazaki et al. (1976, 1988) demonstrated that female mice of congenic strains differing only in their MHC genotype tend to choose to mate with MHC type-different males (Jordan & Bruford, 1998). The maternal recognition of pups is also affected by MHC genotype (Brennan & Zufall, 2006; Yamazaki et al., 2000). Maternal mice preferred to retrieve pups of the same MHC types as themselves, while mouse pups were found to prefer nest odor of the maternal MHC type.
MHC genotype may play a significant role in the phenomenon of pregnancy block (PB). PB can be induced by scent from an unfamiliar strain that differs from the stud male only at the MHC (Yamazaki et al., 1983). Although many studies indicate that PB is a specific response to contact with androgen-dependent scents from an intact unfamiliar male (Bruce, 1960; Hoppe, 1975), Yamazaki et al., (1983) reported that urinary scent from unfamiliar females as well as males differing in MHC characteristics can induce PB (Yamazaki et al., 1983). Findings are inconsistent as to whether PB requires direct contact with urine odors, as opposed to airborne volatiles (Brennan & Peele, 2003; Dominic, 1966; Rajendren & Dominic, 1984; Yamazaki et al., 1983). Although there is no evidence that MHC proteins are able to bind volatiles, MHC genotypes have been shown to influence the profile of volatile fatty acids in urine (Schaefer et al., 2002). Responsivity to airborne volatiles associated with mice of unfamiliar MHC genotypes may reflect a generalized stress response to unfamiliar mouse odors, rather than activation of specific pathways involved in individual recognition of the stud male.
3.1.2. The major urinary proteins
Major urinary proteins (MUPs) genes (Beyon & Hurst, 2003; Hurst et al., 2001) are largely expressed in the liver under stimulation by androgens and their products are released in the urine by filtration from blood serum, providing long-lasting nonvolatile odor (Bacchini et al., 1992; Brenan & Keverene, 2004). Urinary MUPs are expressed at high concentrations by adult mice of both sexes (Beynon et al., 2002; Payne et al., 2001), although males invest more than females in urine marks (Hurst, 1990c; Maruniak et al., 1975) and MUP production (Beynon et al., 2002; Stopka et al., 2007). Individual mice express many different MUP patterns (Hurst et al., 2001), which may be essential in allowing mice to distinguish another mouse’s urine mark from their own (Hurst et al., 2001; Nevison et al., 2003).
Individuals of highly inbred strains have the same MUP patterns (Nevison et al., 2000; Yamaguchi et al., 1981). Male mice show countermarking when confronting urine odor from other males, but not their own, identical, odor (Hurst, 1993; Humprhies et al., 1999; Nevison et al., 2000). Changing the MUP profile of the scent marks by the addition of artificially produced MUPs increased countermarking by the scent donor, indicating dependence on MUP profile rather than MHC genotype (Hurst et al., 2001, 2005).
MUPs bind small volatile urinary chemosignals that are slowly released in a testosterone- dependent fashion (Bacchini et al., 1992; Cavaggioni & Mucignat-Caretta, 2000), advertising the presence of a reproductively capable male (Novotny, 2003). Spraying of MUPs compounds on castrated males, or females, but not pups, can elicit aggressive behavior toward the stimulus by male mice (Mucignat-Caretta & Caretta, 1999; Mucignat-Caretta et al., 2004; Novotny et al., 1985). Female mice may also use MUPs to advertise reproductive state by varying the concentration of MUPs during their estrous cycle (Stopka et al., 2007). MUPs may regulate the reproductive state of females including accelerating puberty (Novotny et al., 1999) and inducing estrus cycles (Jemiolo et al., 1986).
Urinary chemosignals represent a complex compound that includes MHC-dependent volatile and non-volatile, and MUP-dependent non-volatile cues, each of which may be capable of signaling individual identity in different contexts. It is possible that these functions could be differentiated by behavioral test paradigms; MHC-based recognition has been described in the context of mate choice and parent-offspring interactions influenced by kin relationships, while MUP derived compounds in urinary scent have been reported to be associated with recognition between individual males and among male-female pairs, especially in scent countermarking situations.
3.2. Detection of odorant signaling
3.2.1. Two olfactory bulbs
Odorants may be registered by two distinct chemosensory systems originating in the nose: the main olfactory system, located in the dorsal posterior aspect of the nasal cavity, and the vomeronasal organ (VNO), located in the inferior aspect of the nasal cavity (Greer, 1991; Matsunami & Buck, 1997; Rodriguez et al., 2002).
Receptors of the main olfactory system, located in the olfactory epithelium, can detect thousands of different volatile odor molecules. These receptors encode olfactory signals to the main olfactory bulbs (MOB), while the VNO sends its axons to the accessory olfactory bulb (AOB) an anatomically independent region in the posterior part of the olfactory bulb. The AOB and MOB, in turn, give rise to separate pathways that terminate in generally separate but sometimes overlapping areas of the basal telencephalon (Pro-Sistiaga et al, 2007). From the amygdala, vomeronasal pathways project to the medial preoptic area and the ventromedial nucleus of the hypothalamus (Rodriguez et al., 2002; Scalia & Winans, 1975).
3.2.2. Function of two olfactory organs
The roles of the two systems in detection of chemical signals with biological relevance appear to be partially overlapping (Restrepo et al., 2004). The MOB detects general odorants that provide information about the environment (Dulac & Torello, 2003; Lin et al, 2006). The VMO is particularly involved in the response to pheromones (Dulac & Torello, 2003; Halpern & Martínez-Morcos, 2003; Wysocki et al., 1982); and, likely, kairomones (Staples et al. 2007) mediating some particularly adaptive responses to the odors of other species such as predators; e.g., the vomeronasal receptor neurons are narrowly tuned, responding best to a more limited group of molecules.
Bilateral removal of the olfactory bulb, which abolishes input from both systems, eliminates both mating behavior (Bean, 1982; Keller et al., 2006) and agonistic behavior (Bean, 1982; Ropartz, 1968) in male mice. Nasal perfusion of zinc sulfate, disrupting the main olfactory bulb but leaving the accessory system functionally intact (Power & Winans, 1975), has only a minor effect on intermale aggression in mice (Bean, 1982; Connor, 1972), but completely disrupts mating behavior in male mice regardless of their sexual experience (Keller et al., 2006). Removal of the VNO alone does not affect mating behavior (Bean, 1982; Keller et al., 2006), but VNO removal markedly reduces scent marking responses and aggressive behavior to other male mice (Maruniak et al., 1986; Pankevich et al., 2004) as well as aggressive behavior of lactating female mice (Bean & Wysocki, 1989). After VNO removal, male mice are able to mate with females but do not show preference to estrous females (Pankevich et al., 2004). These findings suggest that normal male aggressive and scent marking behaviors toward other males are dependent on the presence of an intact vomeronasal system for their expression, while normal male-female sexual interactions require the presence of an intact main olfactory system. However, removal of the VNO does not appear to disrupt responsivity to general environmental stimuli, such as food buried under cage shavings (Wysocki et al., 1982).
Additional support from this view comes from studies of the TRP2 ion channel, a critical part of the vomeronasal system signal transduction pathway. Male mice with a genetic ablation of the trp2 gene engage in sexual behavior with conspecifics of both sexes and fail to display male-male aggression (Leypold et al., 2002; Stowers et al., 2002). Trp2 −/− males mount and emit ultrasounds to castrated male mice scented with intact male urine, a response that normally would be expected toward females. Since TRP2 is found exclusively in the vomeronasal epithelium especially in the vomeronasal V2 category of receptor neurons (Chamero et al., 2007), these results strongly support the idea that the vomeronasal system is critical for male-specific behavior in response to sensory cues for sex discrimination (Leypold et al., 2002; Stowers et al., 2002).
3.2.3. Detection of predatory odor
Several lines of evidence suggest that predator odors are mediated by the AOB rather than the MOB (McGregor et al., 2004; Apfelbach et al., 2005; Staples, 2008). Laboratory rats exposed to cat odor in a confined environment showed strong activation of the granular, glomerular and mitral cell layers of the AOB, but little activation in the MOB (McGregor et al., 2004). The AOB projects to the medial amygdala as well as the bed nucleus of the stria terminalis (Dielenberg et al., 2001; Pro-Sistiaga et al, 2007), providing input to a medial hypothalamic circuit that plays a key role in defensive responses to predators and their odors (Blanchard et al., 2003b; Canteras et al., 1997; Canteras, 2002). The synthetic fox feces-derived odor, trimethylthiazoline (TMT) (Vernet-Maury 1980; Vernet-Maury et al., 1984) does not appear to activate the AOB, instead producing activation of the MOB (Fendt et al., 2005). TMT is found to strongly activate the central nucleus of the amygdala and paraventricular nucleus of the hypothalamus, while cat odor has no such effects (Day et al., 2004; Fendt et al., 2005). These findings suggest that cat fur/skin odor is a VNO-mediated kairomone (Apfelback et al., 2005), whereas TMT odor, although appearing to activate fear and aversion-related circuitry in the limbic system, is not (Day et al., 2004: Stables et al, 2007), a difference that may be influential in the patterns of defensive responses to these two predator odors when the two are tested under identical circumstances. Specifically, McGregor et al. (2002) suggested that TMT is a noxious, stimulus for rats and mice (as for humans!), a view that is compatible with findings (Endres & Fendt, 2007) that following 7 exposures, rats developed a conditioned place aversion for a chamber associated with TMT. In contrast, a single, brief, cat odor exposure reliably produces Pavlovian or associative conditioning to the exposure context, while TMT does not (Blanchard et al., 2003c; McGregor et al., 2002). These findings suggest that VNO involvement may be a factor in the specific and very rapid form of aversive conditioning seen with a cat fur/skin odor unconditioned stimulus.
4. Scent marking as communication with conspecifics
As noted earlier, mice deposit scent marks under circumstances suggesting that these function to communicate with a present or potentially present conspecifics. Male mice deposit more scent marks to adult females or their odors than toward juvenile females or juvenile males (Arakawa et al., 2007; Coquelin, 1992), suggesting male advertisement in a sexual context. Additionally, dominant but not subordinate males countermark when they find urine odor from genetically different males in their patrolling area or territory, but do not mark if they cannot detect any odor from conspecifics (Hurst, 1993; Hurst & Beynon, 2004; Rich & Hurst, 1999), or if the only odor is that of a familiar same-sex cagemate (Arakawa et al., 2007). These findings fit together very well in suggesting that in male mice scent marking can represent claims to ownership of a territory, along with the sexual opportunities that ownership may facilitate, with countermarking representing a challenge to the scent-mark mediated claims of another adult male; an interpretation congruent with findings that social defeat reduced both marking to a chamber, and ultrasonic vocalizations to a female mouse, albeit with different post-defeat durations of inhibition for the two behaviors (Lumley et al, 1999).
Habituation effects are consonant with these views: When repeatedly exposed to a chamber or a 15 conspecific, C57BL/6J males showed marked habituation to both, as expressed by decreased scent marking over trials (Arakawa et al., 2008). In terms of evolutionary adaptiveness of scent marking, these habituation effects may reflect reduced value of marking to familiar conspecifics, such that habituation may serve as an index of social memory in mice. In addition, however, persistent marking may incur an enhanced risk of predation to the marker (Robert et al., 2001; Rosell & Sanda, 2006), a risk that should be independently manipulable by cues suggesting predator presence.
To assess these relationships, we measured scent marking responses of male mice to a conspecific male, as influenced by cat odor, a cue suggesting the presence of a predator, to investigate whether odor from conspecifics and predators appear to mediate different functional mechanisms of odorant communication. Specifically, long-lasting effects of conspecific and predator odor exposure on scent marking responses were investigated in the context of social memory concerning conspecifics and predator odors.
4.1. Scent marking to conspecific and predator odors: Experiments
4.1.1. Materials and methods
Animals and rearing condition
Fifty nine male C57BL/6J (C57) mice (25–30 g), 16–18 weeks of age, were used as the subjects (32 males for experiment 1 and 27 males for experiment 2). They were bred from stock obtained from the Jackson Laboratory (Bar harbor, ME). All subjects were weaned at 23–25 days of age, and then housed in groups of 2–3 same sex animals in standard polypropylene cages, 26.5 × 17 × 11.5 (height) cm, under 12L:12D cycle (lighting on 06:00) in a temperature- (22±2 °C) and humidity- (60 %) controlled room at the University of Hawaii Laboratory Animal Services. They were housed individually in the polypropylene cages at least for 1 week prior to testing, and then they were randomly assigned into one of four (experiment 1) or three (experiment 2) groups. Sixteen male CD-1 mice, 14 weeks of age, were obtained from Charles River Laboratories (Wilmington, MA), were used as the stimulus animals. They were housed individually in polypropylene cages at least for 1 week prior to the test.
All animals were allowed free access to food and water in their home cage. All protocols and animal handling and treatment were approved by the Institutional Animal Care and Use Committee at the University of Hawaii.
Apparatus
In both experiments, scent mark tests were conducted in a clean 46 × 24 × 21 cm Polycarbonate cage, placed upside-down on a rough paper (457 × 365 mm, Rough Newsprint paper, Bienfang) substrate. The cage was divided into two equal-sized compartments by a wire mesh screen that prevented direct physical contact between subject and stimulus, but allowed olfactory, visual, and auditory cues to be received.
In Experiment 2, a different cage was used to provide the novel context. This was a triangle-shaped opaque plastic cage, 47.4 × 36 × 18 (height) cm, with a clear Plexiglas top, one side of which was a wire mesh wall.
Test procedure
All test trials were conducted during the light phase of the light/dark cycle under dimly lit conditions. The subjects were moved from the holding room to the experiment room in their homecages 20 min before the beginning of the test. At the end of the each 20 min trial, the animals were returned to their home cage and moved back to the holding room. Between trials, the apparatus was cleaned with 15% alcohol, dried with paper towels, and given a fresh paper substrate. Scent marking was evaluated on each trial.
Experiment 1
The goal of this experiment was to investigate habituation of scent marking with repeated exposure to the same conspecific and to determine the duration of social memory as reflected in such habituation.
Subjects were divided into four groups; receiving the same or a different stimulus mouse on the test day, after an interval of 24 hr, or 7 days (N = 8/group). Each stimulus mouse was confronted with an initially novel CD-1 male in the test chamber, for 20 min, on each of four daily trials. On the fifth trial, animals in the SAME groups were exposed to the same CD-1 male following an interval of 24 hrs or 7 days, while animals in the DIFFERENT groups were confronted by a novel CD-1 male, after the same intervals.
Experiment 2
The aim of this experiment was to investigate the short- and long-term impact of exposure to cat fur/odor, in the homecage or in the test situation, on scent marking behavior in isolate C57 mice. In this experiment a HOMECAGE ODOR group was exposed to cat fur/skin odor in the home cage, whereas a TEST ODOR group encountered cat fur/skin odor in the test situation while CONTROL mice received no cat odor exposure. Tests 7 days later (D8) evaluated the durations of cat odor-induced changes in scent marking, and an additional test (D9) in a different chamber, determined the situational specificity of cat-odor effects on scent marking responses.
On D1, twenty minute before the beginning of the test, subjects were moved from the holding room to the experimental room in their homecages. Animals in the TEST ODOR and CONTROL groups were left undisturbed for 20 min, while those in the HOMECAGE ODOR group had a cloth-wrapped plastic block (9 × 9 × 2 cm) placed on top of the wire mesh lid of their cage, for a similar duration. This block had been rubbed for 5 min against the fur of a laboratory-housed domestic cat and then stored in a Ziploc plastic bag until used as the cat-odor stimulus. During testing, each subject was placed in one compartment of the test chamber for 20 min, with a cloth block placed on the cage lid of the compartment on the other side of the wire mesh barrier. For the TEST ODOR group, this cloth had been rubbed with cat fur/skin, but was without odor for subjects in the HOMECAGE ODOR and CONTROL groups. At the end of the period, the cage lid was changed and all animals were returned to their holding rooms. Behaviors were recorded using an over-head video camera. On D2 and D8, each subject was placed in one compartment of the test chamber cage for 20 min. A no-odor cloth block was located in the other compartment. On D9, all subjects were evaluated in the novel, triangle-shaped test chamber.
Scoring scent marking
Mouse urine was fixed by Ninhydrin spray (LC-NIN-16, Criminal Research Products, LLC) (Fig. 1). After drying for 24 hrs, the number of scent marks was measured by placing a transparent grid sheet over the substrate paper and counting the total number of grids (each 10 × 10 mm) containing scent marks (maximum: 552 squares). Pools of urine larger than four square grids that formed a larger quadrant were not included in this count. Four squares in a row, however, were included. Numbers of urine pools and feces were also recorded.
Fig. 1.
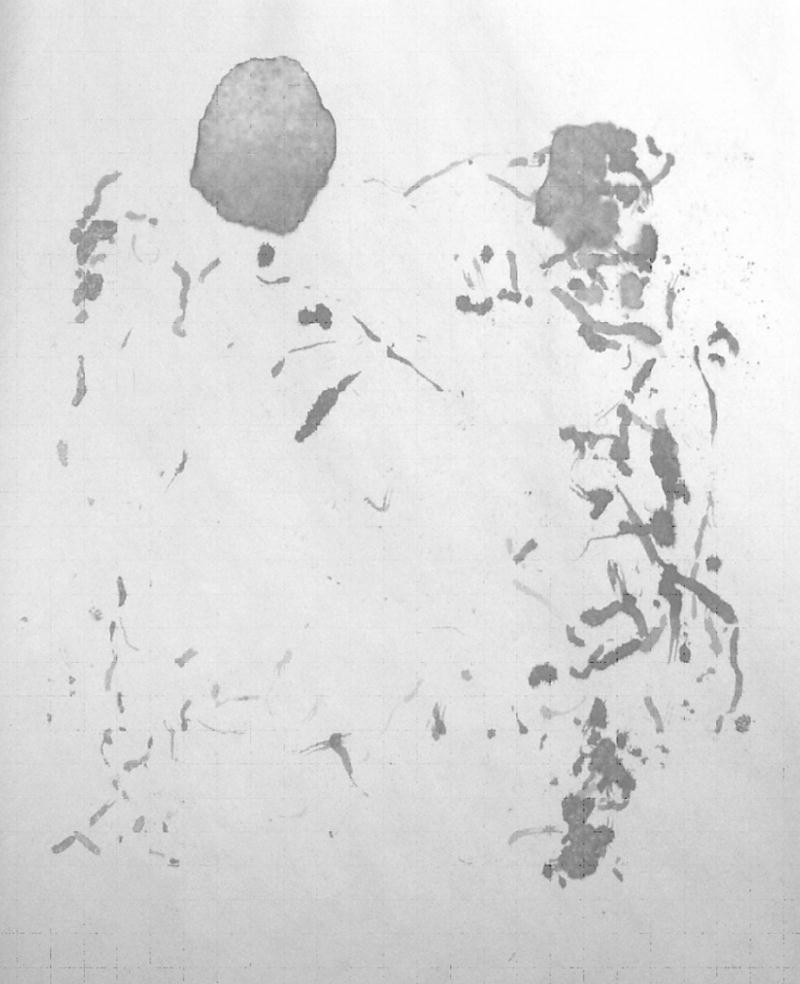
Scent marks of C57 male mice fixed and colored by ninhydrin spray. A C57 male was introduced into a rectangular cage which was placed upside-down on a rough newsprint paper for 20 minute. Following removal of the mouse, the ethanol with ninhydrin was sprayed on the paper sheet and the sheet was left to be dried for 24 hours in room temperature. A large round shaped urine spot on an upper left portion of the sheet was counted as a urine pool.
Behavioral indices
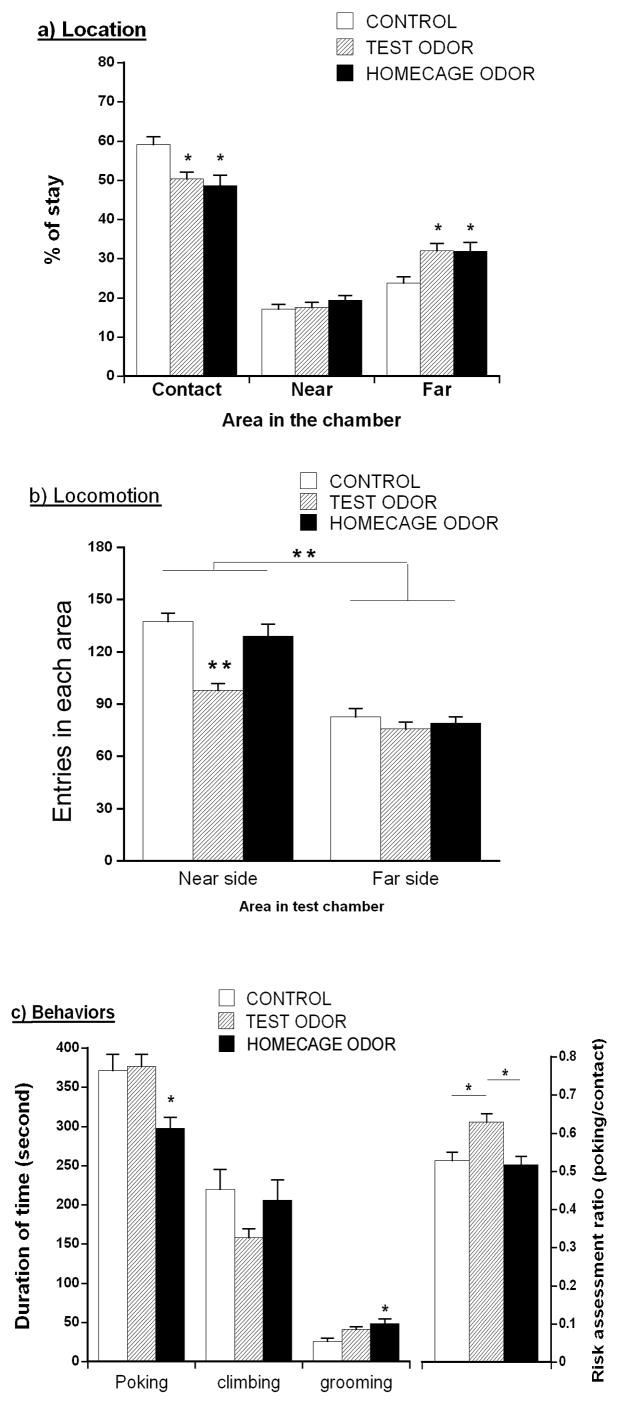
In experiment 2, on D1 (first test exposure) subjects’ behaviors were recorded by a DVD recorder, and subsequently analyzed. The floor of the test chamber was divided into four equal blocks (each 12 × 6 cm) by two perpendicular lines, and the number of entries into each block of the chamber area was counted as a measure of locomotion. To assess proximity to the scent block, durations in three locations were measured: “contact” was location in an area closer than 4 cm to the wire mesh barrier; “near” was in the remainder of the two blocks nearest the mesh, i.e. location in a 24 × 8 cm area just behind the ‘contact” area; “far” was location in the 24 × 12 area most distant from the mesh barrier. Durations of sniffing of the mesh barrier, climbing of the mesh barrier, and grooming were measured.
Statistical analysis
Data were analyzed by two-way analyses of variance (ANOVA) with a between-subject factor of groups (same or different for experiment 1, and HOMECAGE ODOR, TEST ODOR, or CONTROL for experiment 2) and a within-subject factor of trials (trials 1-5) for experiment 1 or day (D1, D2, D8 or D9) for experiment 2. In experiment 2, the number of crossings in each area of the chamber (locomotion) was analyzed by a with a within-subject factor of chamber area (near or far areas) and between-subject factor of group (HOMECAGE ODOR, TEST ODOR, or CONTROL). The duration of stay in each area and of each behavior (poking, climbing, and grooming) and risk assessment ratio were analyzed by a one-way ANOVA between groups. Post hoc comparisons used the Tukey’s HSD test for between-subject factors and Bonferroni test for within-subject factors. A probability level of p<.05 was adopted as the level of statistical significance for all analyses.
4.1.2. Results
Experiment 1: Impact of repeated exposure and change of stimulus animal on scent marking response in C57 males
Fig. 2 depicts the number of squares with scent marks for C57 males toward an initially unfamiliar CD-1 male for the first 4 trials and then toward a same (SAME) or a novel (DIFFERENT) CD-1 male on trial 5. Two-way ANOVA on these scores found a significant main effect of trial, F(4,56) = 15.788, p<.001, but not of group, F(1,14) = 0.217. The interaction between trial and group was significant, F(4,56) = 3.229, p<.019 and subsequent analyses indicated that C57 males showed high levels of marking on the first trial which decreased during 4 exposures to the same CD-1 male, with significantly reduced marking on trials 3 and 4 compared to trial 1. On trial 5 (Fig. 2a), C57 males exposed to the familiar CD-1 male showed less marking compared to those exposed to a novel CD-1 male (p<.01). Marking to the novel, but not the familiar, male on trial 5 was also significantly higher than to the habituated male on trial 4 (p<.01).
Fig. 2.
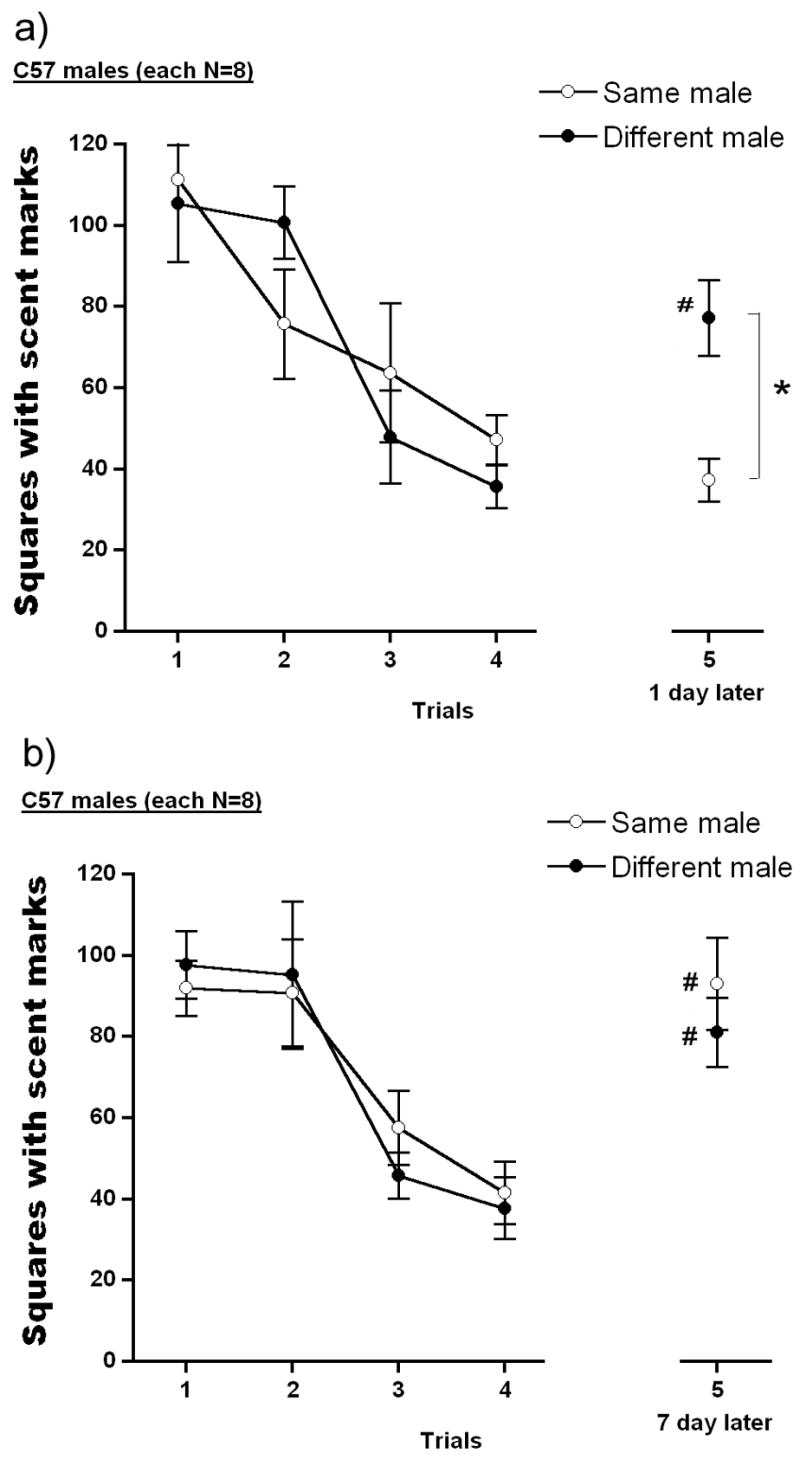
Number of squares with scent marks of C57 males which were confronted with the same, initially novel, CD-1 male during the first 4 trials, and then on 5th trial, exposed to the same CD-1 male (same male) or a different, novel CD-1 male (different male). The inter-trial interval was 24 hrs on the first 4 trials, and on 5th trial 24 hrs (upper panel) or 7 days (bottom panel). Data are expressed as mean ± S.E.M. Significant post hoc differences between groups; *p<.05, and between trials compared to trial 4, #p<.05..
However, when a 7 day interval separated trials 4 and 5 (Fig. 2b), C57 males showed recovery of scent marking to the familiar stimulus mouse. This was confirmed by ANOVA, which found a significant main effect of trials, F(4,56) = 16.226, p<.001, but not of group, F(1,14) = .107. The interaction between trials and group was not significant, F(4,56) = .441.
There were no significant differences in the total number of squares that had urine pools, or in the number of fecal boli, for each group in either the 24 hrs or the 7 days retention tests.
Experiment 2: Immediate and long-term impact of cat odor on scent marking behavior
Scent marking response
Fig. 3 indicates squares with scent marks for male C57 mice on D1– D9 for groups with or without cat fur/odor on D1. Two-way ANOVA conducted on these scores found significant main effects of group F(2,24) = 13.567, p<.001, but not of test days, F(3,72) = 0.116, n.s. The group × test day interaction was significant, F(6,72) = 2.275, p<.05. Subsequent analyses confirmed that mice exposed to cat odor in the test chamber (TEST ODOR) or in homecage prior to the test (HOMECAGE ODOR) showed significant reductions of scent marking compared to CONTROLS (D2, p<.05, and D1 and D8, p<.01, respectively). When the test chamber was changed to a different (triangle-shaped) cage on day 9, TEST ODOR mice showed similar levels of scent marking to CONTROLS, and both groups displayed significantly more scent marking than mice that had been exposed to cat odor in their homecage on day 1 (p<.05).
Fig. 3.
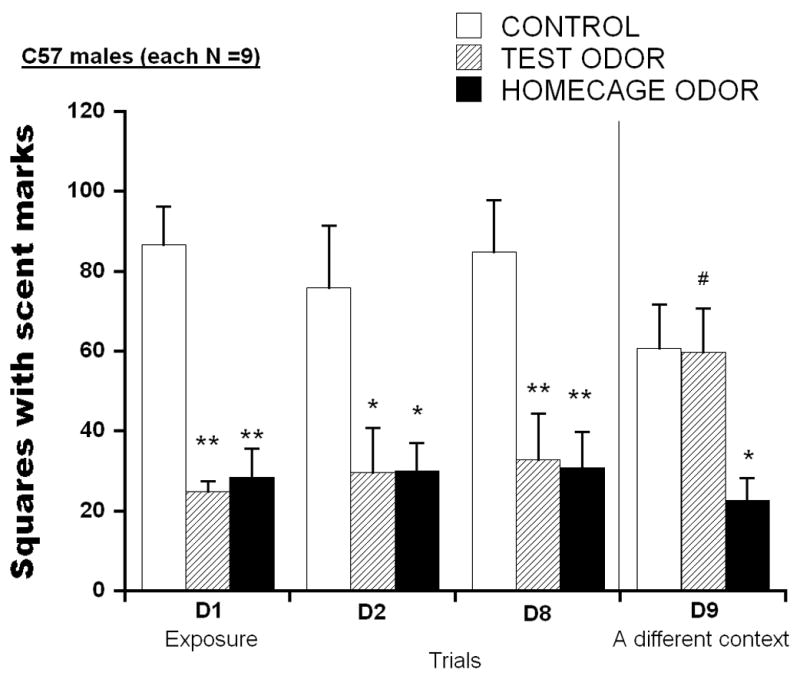
Number of squares with scent marks of male C57 mice. On day 1, they were placed into the test chamber with a wire mesh partition, on the opposite side of which a towel block without odor (CONTROL), or with cat fur/skin odor (TEST ODOR) was placed. A third group was exposed to cat odor in their home cage prior to the test (HOMECAGE ODOR). Twenty-four hours (D2) and 7 days later (D8), they were replaced into the identical chamber and confronted with a towel block without cat odor. On day 9 they were placed into a differently shaped (triangular) chamber with a towel block without cat odor on the opposite side of a wire mesh partition. Data are expressed as mean ± S.E.M. Significant post hoc differences between groups compared to CONTROL; *p<.05
There were no significant differences in the number of urine pools [group, F(2,24)=.715, days, F(3,72)=.082, and group × days interaction, F(6,72)= 1.048]. ANOVA on feces found a significant main effect of days, F(3,72)=2.775, p<.05, but not of group, F(2,24)= 1.464, or of interaction between days and group F(6,72)= 0.353. For all groups combined, the number of feces was fewer on day 9 than on day 8 (p<.05).
These results indicate that mice exposed to cat odor in either the test chamber or their home cage prior to the test showed reductions of scent marking for at least 7 days. When the test chamber was changed to a differently shaped chamber on day 9, mice exposed to cat odor in the test chamber displayed recovery of scent marking but those exposed to cat odor in their home cages did not.
The impact of cat odor exposure on other defensive behaviors
With regard to other defensive behaviors on day 1 when animals were exposed to cat odor, location and locomotor activity in the test chamber were analyzed. The percentage of time that animals spent in each area (contact, near, and far areas) was calculated as shown in Fig. 4 (top panel). A one-way ANOVA for time in each area found a significant effect of condition, F(2,24) = 6.311, p<.01; mice exposed to cat odor in the test chamber and those exposed in their home cages showed significantly decreased time in contact with the wire mesh compared to mice not exposed to cat odor (p<.05 or less). Similarly, ANOVA for group was significant for location in the far side of the chamber F(2,24) = 5.771, p<.01, with mice exposed to cat odor in the chamber or in their home cages staying on the far side of the chamber longer than mice without cat odor (p<.05 or less). However, there was no significant group difference in the percentage of time spent on the near side of the chamber F(2,24) = .835.
Fig. 4.
Behaviors of C57 males in the test chamber on D1 when confronted with a towel block containing cat fur/skin odor (TEST ODOR) or no odor (CONTROL), or exposed to cat odor in their home cage prior to the test (HOMECAGE ODOR). a) Location: Time in each location (Contact; contacted with wire mesh; near; or far from the towel block side). b) Locomotion: Number of crossings of the lines on the chamber floor. c) Behaviors: Sniffing or poking at the wire mesh; climbing on the wire mesh; grooming; or risk assessment ratio -- duration of sniffing or nose poking per contact period with the wire mesh. Data are expressed as mean ± S.E.M. Significant post hoc differences between groups compared to CONTROL; *p<.05.
The total number of entries into the blocks of the test chamber areas (2 near-side and 2 far-side blocks) is presented in Fig. 4 (middle panel). A two-way ANOVA conducted on these scores showed significant main effects of area, F(1,24) = 92.962, p<.001, and group, F(2,24) = 5.853, p<.01. The interaction between area and group was not significant, F(2,24) = 2.765, p<.10. Mice that were exposed to cat odor in the test chamber but not in their home cage, displayed decreased locomotion in the test chamber compared to mice exposed to the chamber without cat odor (p<.05).
Figure 4 depicts the total duration for each behavior; nose poking or sniffing at mesh barrier, climbing mesh barrier, and grooming, for each group of mice, as well as a risk assessment ratio (sniffing at barrier over total contact time. Group effects on sniffing at the barrier were significant F(2,24)= 6.123, p<.01, and mice exposed to cat odor in their home cages showed less sniffing compared to the other two groups (p<.05 or less). Group differences were not significant for the duration of climbing on the mesh F(2,24)= 1.609, but were significant for grooming F(2,24) = 5.457, p<.05: Mice exposed to cat odor in their home cage exhibited a longer duration of grooming in the test chamber than no-odor controls (p<.05). The risk assessment ratio was also significant for groups F(2,24) = 7.364, p<.01, with mice exposed to cat odor in the test chamber showing a higher risk assessment ratio compared to other two groups (p<.05).
These results indicate that mice exposed to cat odor in either the test chamber or their home cages prior to testing showed similar reductions in time near the wire mesh separating the subject compartment from the cat odor, whereas only those exposed to cat odor in the test chamber showed reduced locomotion, and an increased risk assessment ratio. However, mice exposed to cat odor in their home cages but not in the test situation showed reduced sniffing at the wire mesh and increased grooming. This may reflect that the stress of homecage odor exposure led to subsequent avoidance of highly salient novel stimuli (the wire mesh barrier) even in a novel situation.
4.2. Discussion: Expression and habituation of scent marking response to conspecific odor
The present experiments demonstrated that male C57 mice deposited substantial scent marks to an initially novel CD-1 male, with the number of marks decreasing over daily trials, indicating habituation to this CD-1 male. These C57 subjects showed a marked restoration of marking when confronted by a novel CD-1 male, indicating that they could differentiate animals on the basis of this previous exposure. However, when, following habituation to the same CD-1 male with consecutive 4 daily exposures, animals were exposed to the same CD-1 male 7 days later, they failed to show decrements in the deposition of scent marks.
There are two possible explanations for this finding. One is that social memory in this test paradigm cannot be maintained for 7 days due to limitations of mouse social memory systems. This view has some support in findings that rodent social memory evaluated as exploratory or aggressive behavior to an novel juvenile or ovariectmized female has a relatively short term duration (Winslow & Camacho, 1995; Sekiguchi et al., 1991; Thor & Holloway, 1981, 1982). However, one report using group-housed mice showed long-lasting, at least 7 days, social memory in this paradigm (Kogan et al., 2000).
Another possible explanation is that scent marking behavior is a communication tool for maintenance of social relationships that may be functional even if social memory for a specific individual is still remained. Male mice deposit scent marks in response to changes in the environment as well as opponents (Arakawa et al., 2007, 2008; Bronson & Desjardins, 1974), and tend to patrol and mark their territories frequently (Hurst, 1993). These factors reflect that scent marking represents the active emission of a signal, and not just an automatic response to the signal emitted by another mouse, and suggest that caution may be appropriate in terms of the ability of such tests to evaluate the duration of mouse social memory. This view, urging consideration of functional aspects of behavior changes used to evaluate social memory, receives indirect support from the literature on pregnancy blocks in female mice, in response to the odor of a novel male (Brennan et al., 1990; Yamazaki et al., 1983). In this context social memory may be acquired in a single trial, and endure for 30 days (Brennan et al., 1990; Kaba et al., 1988).
4.3. Immediate and residual defensive responses of mice to predator odor
Experiment 2 demonstrated that exposure of mouse subjects to cat fur/skin odor, either in the home cage or in the scent-marking situation, produced strong, long-lasting (7 days) reductions in scent marking behaviors of male mice. When the test chamber was changed to a novel, different shaped chamber on day 9, mice exposed to cat odor in the initial test chamber showed recovery of scent marking, while home cage exposed mice did not, suggesting that the suppressive effects of cat odor exposure were conditioned to the test situation for the former, but not the latter, group. This is in agreement with a plethora of findings that exposure to a threatening stimulus results in rapid conditioning of aversive emotional responses to the situation in which exposure occurs (Blanchard & Blanchard, 1969; Blanchard et al., 2001, 2003d; Bouton et al., 2006; Fanselow, 2000; Rudy et al., 2004). The present findings clearly indicate that mice are enable to maintain their memory of the context associated with cat fur/skin odor for at least 7 days.
Findings of long-lasting effects of cat fur/skin odor in the home cage on mouse behavior in the scent-marking test are amenable to two different interpretations. First, because the cat odor stimulus block was present in both the home cage and in the test situation, it is possible that these findings reflect a cue conditioning effect of the single home cage exposure. This view is compatible with earlier findings (Blanchard et al, 2001) of single trial conditioning of defensiveness to a cat odor block as indexed by enhanced defensiveness when the original odor context, but not the cue, was extinguished, in comparison to a group for which both cue and context were included in the extinction experience. The alternative view, that predator odor exposure can produce a lasting unconditioned increase in general defensiveness, is supported by findings (Hebb et al, 2003) that brief (2 to 10 min) exposure of mice to predator odor (rat-soiled bedding) can enhance acoustic startle several days later. This paradigm appears to have no obvious similarity between the exposure and test situations that might support conditioning. Adamec et al, 2006 also reported enhanced anxiety-like responses in an elevated plus maze for female, but not male, C57BL/6 mice following exposure to a room in which cats had previously confronted and come into contact with rodents. Thus, while one-trial cue conditioning to cat odor is certainly possible, it is not clear whether this provides the sole mechanism for the present home cage findings.
It might be noted that C57 mice may represent a particularly advantageous strain in terms of responsivity to cat odor, in that they, but not 129Sv mice, showed activity reductions in response to a cat fur/skin odor cloth (Raud et al, 2007). However, this specific difference may also reflect that the baseline activity levels of the 129Sv mice were also lower (Voilar et al., 2001), suggesting the possibility of a floor effect.
5. Conclusion
Odor discrimination, and urinary scent marks in unmarked situations or in response to the marks of a conspecific, have ethologically important roles in social communication among conspecifics in many rodent species (Brown, 1979; Eisenberg & Kleiman, 1972; Ralls, 1971). Social odor signals contain information about the individuals that deposit them, that may be of value to both the depositing individual and the conspecific recipients if that information (Hurst, 1993; Lacey & Hurst, 2005). Scent marking facilitates formation and maintenance of a territory, reproductive competition, and sexual advertisement (Brown, 1979; Wolff, 2004). However, the advantages of scent marking may trade off with a potential cost in terms of predation risk. The present studies indicate that mice show suppression of scent marking in environments containing predator odor, compatible with the prediction that predator odor exposure produces rapid conditioning of defensiveness to the exposure situation. However, these data showed equally strong and durable inhibition of marking following cat odor exposure in the home cage just prior to placement in a situation that would normally elicit scent marking. The latter finding is in agreement with a growing literature indicating that exposure to cat fur/skin odor elicits a durable aversive emotional response that may be dependent on either the exposure context or on specific cues associated with the odor.
These findings, of the interactions of responsivity to predator and conspecific odors, are of interest in terms of the complex control of communicatory behaviors in rodents. Findings on habituation of scent marking to a familiar conspecific add to a body of findings (Arakawa et al, 2007, 2008) suggesting that scent marking in mice may provide a useful model for research on the physiology and pathology of communication in this species, complementary to mouse social recognition paradigms (Crawley et al., 1999; Crawley, 2004; Moy et al., 2004, 2007) that have been used for a variety of models associated with neurocognitive disorders including autism (Qiu et al., 2006; Tueting et al., 2006), Fragile X syndrome (Mineur et al., 2006; Spencer et al., 2005), and Rett syndrome (Moretti et al., 2005; Shahbazian et al., 2002) (cf. Ferkin & Li, 2005). Present findings that conditioned or unconditioned emotional responses to predator odors are capable of reducing initial scent-mark signaling (i.e. scent marking in the absence of conspecific scent) additionally suggest the potential use of this paradigm as a probe to assay motivations associated with scent marking behaviors.
Acknowledgments
This study was supported by SNRP grant 5U54NS039406 to RJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Anderson PK, Hill JL. Mus musculus: Experimental induction of territory formation. Science. 1965;148:1753–1755. doi: 10.1126/science.148.3678.1753. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: Sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchini A, Gaetani E, Cavaggioni A. Pheromone binding proteins of the mouse, Mus musculus. Experientia. 1992;48:419–421. doi: 10.1007/BF01923448. [DOI] [PubMed] [Google Scholar]
- Barnard CJ, Fitzsimmons J. Kin recognition and mate choice in mice: the effects of kinship familiarity and social interference on intersexual interactions. Anim Behav. 1988;36:1078–1090. [Google Scholar]
- Barnard CJ, Hurst JL, Aldhous P. Of mice and kin: the functional significance of kin bias in social behaviour. Biol Rev. 1991;66:379–430. doi: 10.1111/j.1469-185x.1991.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Bean NJ. Modulation of agonistic behavior by the dual olfactory system in male mice. Physiol Behav. 1982;29:433–437. doi: 10.1016/0031-9384(82)90262-1. [DOI] [PubMed] [Google Scholar]
- Bean NJ, Wysocki CJ. Vomeronasal organ removal and female mouse aggression: the role of experience. Physiol Behav. 1989;45:875–882. doi: 10.1016/0031-9384(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Yamazaki K. Chemical signalling in mice. Biochem Soc Trans. 2003;31:147–151. doi: 10.1042/bst0310147. [DOI] [PubMed] [Google Scholar]
- Belwood J, Morris GK. Bat predation and its influence on calling behavior in neotropical katydids. Science. 1997;238:64–67. doi: 10.1126/science.238.4823.64. [DOI] [PubMed] [Google Scholar]
- Beynon RJ, Veggerby C, Payne CE, Robertson DH, Gaskell SJ, Humphries RE, Hurst JL. Polymorphism in major urinary proteins: molecular heterogeneity in a wild mouse population. J Chem Ecol. 2002;28:1429–1446. doi: 10.1023/a:1016252703836. [DOI] [PubMed] [Google Scholar]
- Beynon RJ, Hurst JL. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochem Soc Trans. 2003;31:142–146. doi: 10.1042/bst0310142. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Defensive reactions in the albino rat. Learn Motiv. 1971;21:351–362. [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K. Ethoexperimental approaches to the study of defensive behavior. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental Approaches to the Study of Behavior. Martinus Nijhoff; Dordrecht: 1989a. pp. 114–136. [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989b;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Rodgers RJ. Pharmacologial and neural control of anti-predator defense in the rat. Aggress Behav. 1990a;16:165–175. [Google Scholar]
- Blanchard RJ, Blanchard DC, Rodgers J, Weiss SM. The characterization and modelling of antipredator defensive behavior. Neurosci Biobehav Rev. 1990b;14:463–472. doi: 10.1016/s0149-7634(05)80069-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yang M, Li CI, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neurosci Biobehav Rev. 2001;25:587–595. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003a;436:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, Blanchard RJ. Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neursci Lett. 2003b;345:145–148. doi: 10.1016/s0304-3940(03)00415-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003c;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003d;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–1210. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- Boyse EA, Beauchamp GK, Yamazaki K. The genetics of body scent. Trens Genet. 1987;3:97–102. [Google Scholar]
- Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Peele P. Towards an understanding of the pregnancy-blocking urinary chemosignals of mice. Biochem Soc Trans. 2003;31:152–155. doi: 10.1042/bst0310152. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–R89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Phil Trans R Soc B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;16:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Desjardins C. Relationships between scent marking by male mice and the pheromone-induced secretion of the gonadotropic and ovarian hormones that accompany puberty in female mice. In: Montagna W, Sadler WA, editors. Reproductive behavior. Plenum Press; New York: 1974. pp. 157–178. [DOI] [PubMed] [Google Scholar]
- Brown RE. Mammalian social odours: a critical review. Adv Study Behav. 1979;10:103–162. [Google Scholar]
- Brown RE, MacDonald DW. Social odours in mammals. 1 & 2. Oxford: Clarendon Press; 1985. [Google Scholar]
- Brown RE. Individual odors of rats are discriminable independently of changes in gonadal hormone levels. Physiol Behav. 1988;43:359–363. doi: 10.1016/0031-9384(88)90199-0. [DOI] [PubMed] [Google Scholar]
- Brown RE. What is the role of the immune system in determining individually distinct body odours? Int J Immunopharmacol. 1995;17:655–661. doi: 10.1016/0192-0561(95)00052-4. [DOI] [PubMed] [Google Scholar]
- Brown RE, Schellinck HM. Effects of selective depletion of gut bacteria on the odours of individuality in rats. In: Apfelbach R, Müller-Schwarze D, Reutter K, Weiler E, editors. Chemical Signals in Vertebrates VII. Pergamon Press; New York: 1995. pp. 267–271. [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Bruce HM. A block to pregnancy in the mouse caused by proximity of strange males. J Reprod Fertil. 1960;1:96–103. doi: 10.1530/jrf.0.0010096. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Ribeiro Do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Cavaggioni A, Mucignat-Caretta C. Major urinary proteins, α2U-globulins and aphrodisin. Biochim Biophys Acta. 2000;1482:218–228. doi: 10.1016/s0167-4838(00)00149-7. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behavior. Nature. 2007;450:899–903. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Connor J. Olfactory control of aggressive and sexual behavior in the mouse (Mus musculus L.) Psychon Sci. 1972;27:1–3. [Google Scholar]
- Coquelin A. Urine-marking by female mice throughout their reproductive cycle. Horm Behav. 1992;26:255–271. doi: 10.1016/0018-506x(92)90046-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crowcroft P, Rowe FP. Social organization and territorial behaviour in the wild house mouse (Mus musculus L.) Proc Zool Soc Lond. 1963;140:517–531. [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dickman CR. Predation and habitat shift in house mouse, Mus domesticus. Ecology. 1992;73:313–322. [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Dominic CJ. Observations on the reproductive pheromones of mice: II neiroendcrine mechanisms involved in the olfactory block to pregnancy. J Reprod Fert. 1966;11:415–421. doi: 10.1530/jrf.0.0110415. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Hoover JE. Effect of urine from pregnant and lactating female house mice on sexual maturation of juvenile females. Dev Psychobiol. 1979;12:545–551. doi: 10.1002/dev.420120604. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Delay and acceleration of puberty in female mice by urinary chemosignals from other females. Dev Psychobiol. 1982;15:433–445. doi: 10.1002/dev.420150505. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Chemosignal effects on puberty in young female mice: urine from pregnant and lactating females. DevPsychobiol. 1983;16:207–217. doi: 10.1002/dev.420160307. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Urine marking and social dominance in male house mice (Mus musculus domesticus) Behav Proc. 2001;53:113–120. doi: 10.1016/s0376-6357(00)00152-2. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:1–13. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Eisenberg JF, Kleiman DG. Olfactory communication in mammals. Annu Rev Ecol Syst. 1972;3:1–32. [Google Scholar]
- Eisenberg JF. The Mammalian Radiations: An Analysis of Trends in Evolution, Adaptation, and Behavior. University of Chicago Press; Chicago: 1981. [Google Scholar]
- Endres T, Fendt M. Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline. Behav Neurosci. 2007;121:594–601. doi: 10.1037/0735-7044.121.3.594. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Li HZ. A battery of olfactory-based screens for phenotyping the social and sexual behaviors of mice. Physiol Behav. 2005;85:489–499. doi: 10.1016/j.physbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Gosling LM, Roberts SC, Thornton EA, Andrew MJ. Life history costs of olfactory status signaling in mice. Behav Ecol Sociobiol. 2000;48:328–332. [Google Scholar]
- Greer CA. Structural organization of the olfactory system. In: Getchell TV, Bartoshuk LM, Doty RL, Snow JB Jr, editors. Smell and taste in health and disease. Raven Press; New York: 1991. pp. 65–81. [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu Rev Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martínez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Halpin ZT. Individual odors among mammals: origins and functions. Adv Study Behav. 1986;16:39–70. [Google Scholar]
- Hebb AL, Zacharko RM, Dominguez H, Laforest S, Gauthier M, Levac C, Drolet G. Changes in brain cholecystokinin and anxiety-like behavior following exposure of mice to predator odor. Neuroscience. 2003;116:539–551. doi: 10.1016/s0306-4522(02)00710-8. [DOI] [PubMed] [Google Scholar]
- Hoppe PC. Genetic and endocrine studies of the pregnancy-blocking pheromone of mice. J Reprod Fert. 1975;45:109–115. doi: 10.1530/jrf.0.0450109. [DOI] [PubMed] [Google Scholar]
- Humphries RE, Robertson DHL, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mouse. Anim Behav. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- Hurst JL. The functions of urine marking in a free-living population of house mice, Mus domesticus. Rutty Anim Behav. 1987;35:1433–1442. [Google Scholar]
- Hurst JL. The complex network of olfactory communication in populations of wild house mice, Mus domesticus rutty: urine marking and investigation within family groups. Anim Behav. 1989;37:705–725. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice Mus domesticus rutty. I. Communication between males. Anim Behav. 1990a;40:209–222. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice Mus domesticus rutty. II. Communication between females. Anim Behav. 1990b;40:223–232. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. III. Communication between the sexes. Anim Behav. 1990c;40:233–243. [Google Scholar]
- Hurst JL. The priming effects of urine substrate marks on interactions between male house mice Mus musculus domesticus Schwarz and Schwarz. Anim Behaiv. 1993;45:55–81. [Google Scholar]
- Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim Behav. 1998;55:1289–1297. doi: 10.1006/anbe.1997.0650. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Rich TJ. Scent marks as competitive signals of mate quality. In: Johnston RE, Muller-Schwarze D, Sorensen P, editors. Advances in Chemical Signals in Vertebrates. Plenum Press; New York: 1999. pp. 209–225. [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signaling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Thom MD, Nevison CM, Humphries RE, Beynon RJ. MHC odours are not required or sufficient for recognition of individual scent owners. Proc R Soc B. 2005;272:715–724. doi: 10.1098/rspb.2004.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo B, Harvey S, Novotny M. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc Natl Acad Sci USA. 1986;83:4576–4579. doi: 10.1073/pnas.83.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Nowell NW. Aversive and aggression-promoting properties of urine from dominant and subordinate male mice. Anim Behav. 1973a;21:207–210. doi: 10.3758/bf03199141. [DOI] [PubMed] [Google Scholar]
- Jones RB, Nowell NW. The coagulating glands as a source of averise and aggression-inhibiting pheromone(s) in the male albino mouse. Physiol Behav. 1973b;10:221–223. doi: 10.1016/0031-9384(73)90031-0. [DOI] [PubMed] [Google Scholar]
- Jones RB, Nowell NW. A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Anim Learn Behav. 1974;2:141–144. doi: 10.3758/bf03199141. [DOI] [PubMed] [Google Scholar]
- Jones RB, Nowell NW. Aversive potency of urine from dominant and subordinate male laboratory mice (Mus musculus): resolution of a conflict. Aggress Behav. 1989;15:291–296. [Google Scholar]
- Jordan WC, Bruford MW. New perspectives on mate choice and the MHC. Heredity. 1998;81:127–133. doi: 10.1046/j.1365-2540.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- Kaba H, Rosser AE, Keverne EB. Hormonal enhancement of neurogenesis and its relationship to the duration of olfactory memory. Neuroscience. 1988;24:93–98. doi: 10.1016/0306-4522(88)90314-4. [DOI] [PubMed] [Google Scholar]
- Kats LB, Dill LM. The scent of death: Chemosensory assessment of predation risk by animals. EcoScience. 1998;5:361–394. [Google Scholar]
- Kavaliers M, Wiebe JP, Galea IAM. Reduction of predator odor-induced anxiety in mice by the neurosteroid 3α-hydroxy-4-pregnen-20-one (3αHP) Brain Res. 1994;645:325–329. doi: 10.1016/0006-8993(94)91667-5. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Colwell DD. Brief exposure to female odors “emboldens” male mice by reducing predator-induced behavioral and hormonal responses. Horm Behav. 2001;40:497–509. doi: 10.1006/hbeh.2001.1714. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Pfaff DW. Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci Biobehav Rev. 2005;29:1347–1359. doi: 10.1016/j.neubiorev.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate--lesioning of the main olfactory epithelium on sexual behavior in male mice. Chem Senses. 2006;31:753–762. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- Keverne EB, de la Riva C. Pheromones in mice: reciprocal interaction between the nose and brain. Nature. 1982;296:148–150. doi: 10.1038/296148a0. [DOI] [PubMed] [Google Scholar]
- Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Horm Behav. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Koivula M, Korpimäki E. Do scent marks increase predation risk of microtine rodents? Oikos. 2001;95:275–281. [Google Scholar]
- Koyama S. Primer effects by conspecific odors in house mice: a new perspecitive in the study of primer effects on reproductive activities. Horm Behav. 2004;46:303–310. doi: 10.1016/j.yhbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dominic CJ. Male-induced implantation failure (the Bruce effect) in mice: protective effect of familiar males on implantation. Physiol Behav. 1993;54:1169–1172. doi: 10.1016/0031-9384(93)90343-e. [DOI] [PubMed] [Google Scholar]
- Lacey JC, Hurst JL. The role of scent in inter-male aggression in house mice and laboratory mice. In: Mason RT, LeMaster MO, Müller-Schwarze D, editors. Chemical Signals in Vertebrates: 10. Springer; New York: 2005. pp. 209–215. [Google Scholar]
- Lacey JC, Beynon RJ, Hurst JL. The importance of exposure to other male scents in determining competitive behaviour among inbred male mice. Appl Anim Behav Sci. 2007;104:130–142. [Google Scholar]
- Lee S, van der Boot LM. Spontaneous pseudopregnancy in mice. Acta Physiol Pharmacol Neerl. 1955;4:442–443. [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav Brain Res. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Lombardi JR, Vandenbergh JG. Pheromonally induced sexual maturation in females: regulation by the social environment of the male. Science. 1977;196:545–546. doi: 10.1126/science.557838. [DOI] [PubMed] [Google Scholar]
- Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav. 1999;67:769–775. doi: 10.1016/s0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nature Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Manning CJ, Wakeland EK, Potts WK. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature. 1992;360:581–583. doi: 10.1038/360581a0. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Sipos ML, Nyby JG, Barfield RJ. Intracranial androgenic activation of male-typical behaviors in house mice: motivation versus performance. Behav Brain Res. 1994;60:141–149. doi: 10.1016/0166-4328(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in female house mice: effects of ovarian steroids, sex experience, and type of stimulus. Behav Biol. 1975;13:211–217. doi: 10.1016/s0091-6773(75)91920-3. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Wysocki CJ, Taylor JA. Mediation of male mouse urine marking and aggression by the vomeronasal organ. Physiol Behav. 1986;37:655–657. doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press, Ltd; New York: 1994. pp. 3–105. [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkens M, Harestad AS, Sullivan T. Cover and efficacy of predator-based repellents for Townsend’s vole, Microtus townsendii. J Chem Ecol. 1991;17:401–412. doi: 10.1007/BF00994341. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Gen. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Mossman CA, Drickamer LC. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J Comp Psychol. 1996;110:131–138. doi: 10.1037/0735-7036.110.2.131. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Caretta A. Urinary chemical cues affect light avoidance behaviour in male laboratory mice, Mus musculus. Anim Behav. 1999;57:765–769. doi: 10.1006/anbe.1998.1023. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Cavaggioni A, Caretta A. Male urinary chemosignals differentially affect aggressive behavior in male mice. J Chem Ecol. 2004;30:777–791. doi: 10.1023/b:joec.0000028431.29484.d7. [DOI] [PubMed] [Google Scholar]
- Mugford RA, Nowell NW. Pheromones and their effect on aggression in mice. Nature. 1970;22:967–968. doi: 10.1038/226967a0. [DOI] [PubMed] [Google Scholar]
- Nevison CM, Barnard CJ, Hurst JL, Beynon RJ. The consequences of inbreeding for recognizing competitors. Proc R Soc B. 2000;267:687–694. doi: 10.1098/rspb.2000.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevison CM, Armstrong S, Beynon RJ, Humphries RE, Hurst JL. The ownership signature in mouse scent marks is involatile. Proc R Soc B. 2003;270:1957–1963. doi: 10.1098/rspb.2003.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny M, Harvey S, Jemiolo B, Alberts J. Synthetic pheromones that promote inter-male aggression in mice. Proc Natl Acad Sci USA. 1985;82:2059–2061. doi: 10.1073/pnas.82.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny MV, Weidong M, Wiesler D, Zidek L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc R Soc B. 1999;266:2017–2022. doi: 10.1098/rspb.1999.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny MV. Pheromones, binding proteins and receptor responses in rodents. Biochem Soc Trans. 2003;31:117–122. doi: 10.1042/bst0310117. [DOI] [PubMed] [Google Scholar]
- Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behav Biol. 1976;18:285–289. doi: 10.1016/s0091-6773(76)92198-2. [DOI] [PubMed] [Google Scholar]
- Nyby J, Dizinno G, Whitney G. Sexual dimorphism in ultrasonic vocalizations of mice (Mus musculus): gonadal hormone regulation. J Comp Physiol Psychol. 1977;91:1424–1431. doi: 10.1037/h0077411. [DOI] [PubMed] [Google Scholar]
- Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus) Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes AS, Bruce HM. Olfactory stimuli in mammalian reproduction. Science. 1961;134:1049–1054. doi: 10.1126/science.134.3485.1049. [DOI] [PubMed] [Google Scholar]
- Parsons GJ, Bondrup-Nielsen S. Partial albinism in an island population of Meadow Voles, Microtus pennsylvanicus, from Nova Scotia. Canadian Field-Naturalist. 1995;109:263–264. [Google Scholar]
- Payne CE, Malone N, Humphries RE, Bradbrook C, Veggerby C, Beynon RJ, Hurst JL. Heterogeneity of major urinary proteins in house mice: population and sex differences. In: Marchelewska-Koj A, Muller-Schwarze D, Lepri J, editors. Chemical Signals in Vertebrates 9. Plenum Press; New York: 2001. pp. 233–240. [Google Scholar]
- Peele P, Salazar I, Mimmack M, Keverne EB, Brennan PA. Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur J Neurosci. 2003;18:622–628. doi: 10.1046/j.1460-9568.2003.02790.x. [DOI] [PubMed] [Google Scholar]
- Potts WK, Manning CJ, Wakeland EK. The role of infectious disease, inbreeding and mating preferences in maintaining MHC genetic diversity: an experimental test. Philos Trans R Soc Lond B Biol Sci. 1994;346:369–378. doi: 10.1098/rstb.1994.0154. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science. 1975;187:961–963. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Bañon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Pérula E, et al. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Dominic CJ. Role of the vomeronasal organ in the male-induced implantation failure (the Bruce effect) in mice. Arch Biol (Bruxelles) 1984;95:1–9. [Google Scholar]
- Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- Raud S, Sütt S, Plaas M, Luuk H, Innos J, Philips MA, Kõks S, Vasar E. Cat odor exposure induces distinct changes in the exploratory behavior and Wfs1 gene expression in C57BL/6 and 129Sv mice. Neursci Lett. 2007;426:87–90. doi: 10.1016/j.neulet.2007.08.052. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim Behav. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- Robert SC, Gosling LM, Thornton EA, McClung J. Scent-marking by male mice under the risk of predation. Behav Ecol. 2001;12:698–705. [Google Scholar]
- Rodriguez I, Del Punta K, Rothman A, Ishii T, Mombaerts P. Multiple new and isolated families within the mouse superfamilies within the mouse superfamily of V1r vomeronasal receptors. Nat Neurosci. 2002;5:134–140. doi: 10.1038/nn795. [DOI] [PubMed] [Google Scholar]
- Ropartz P. The relation between olfactory stimulation and aggressive behaviour in mice. Anim Behav. 1968;16:97–100. doi: 10.1016/0003-3472(68)90117-6. [DOI] [PubMed] [Google Scholar]
- Rosell F, Sanda I. Potential risks of olfactory signaling: the effect of predators on scent marking by beavers. Behav Ecol. 2006;17:897–904. [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinck HM, West AM, Brown RE. Rats can discriminate between the urine odors of genetically identical mice maintained on different diets. Physiol Behav. 1992;51:1079–1082. doi: 10.1016/0031-9384(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Sekiguchi R, Wolterink G, van Ree JM. Analysis of the influence of vasopressin neuropeptides on social recognition of rats. Eur Neuropsychopharmacol. 1991;1:123–126. doi: 10.1016/0924-977x(91)90713-5. [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Young JI, Yuva-Paylor LA, Spencer CM, Antalffy BA, Noebels JL, Armstrong DL, Paylor R, Zoghbi HY. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Singer AG, Tsuchiya H, Wellington JL, Beauchapm GK, Yamazaki K. Chemistry of odortypes in mice- fractionation and bioassay. J Chem Ecol. 1993;19:569–579. doi: 10.1007/BF00994326. [DOI] [PubMed] [Google Scholar]
- Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci USA. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos ML, Nyby JG. Intracranial androgenic activation of male-typical behaviours in house mice: concurrent stimulation of the medial preoptic area and medial nucleus of the amygdale. J Neuroendocrinol. 1998;10:577–586. doi: 10.1046/j.1365-2826.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Stopka P, Janotova K, Heyrovsky D. The advertisement role of major urinary proteins in mice. Physiol Behav. 2007;91:667–670. doi: 10.1016/j.physbeh.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Persistence of social investigatory behavior in the male rat: evidence for long-term memory of initial copulatory experience. Anim Learn Behav. 1981;9:561–565. [Google Scholar]
- Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96:1000–1006. [Google Scholar]
- Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–1077. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–660. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J. Pheromones and mammalian reproduction. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Raven Press; New York: 1994. pp. 343–359. [Google Scholar]
- Vernet-Maury E. Trimethyl-thiazoline in fox feces: a natural alarming substance for the rat. Olfactioin Taste. 1980;VII:407. [Google Scholar]
- Vernet-Maury E, Polak EH, Demael A. Structure-activity relationship of stress-inducing odorants in the rat. J Chem Ecol. 1984;10:1007–1018. doi: 10.1007/BF00987509. [DOI] [PubMed] [Google Scholar]
- Voikar V, Kõks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Warkentin KJ, Keeley ATH, Hare JF. Repetitive calls of juvenile Richardson’s ground quirrels (Spermophilus richardsonii) communicate response urgency. Can J Zool. 2001;79:569–573. [Google Scholar]
- Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol. 1956;13:399–404. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Effect of exteroceptive factors on the oestrous cycle of mice. Nature. 1957;180:1436. doi: 10.1038/1801436a0. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male; changes in the oestrous cycle determined by vaginal smears. J Endocrinol. 1958;17:307–313. doi: 10.1677/joe.0.0170307. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology (Berl) 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- Wolff JO. Scent marking by voles in response to predation risk: a field-laboratory validation. Behav Ecol. 2004;15:286–289. [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav. 1982;29:315–327. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L, Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA, Thomas L. Control of mating preferences in mice by genes in the major histocompatibility complex. J Exp Med. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Yamaguchi M, Baranoski L, Bard J, Boyse EA, Thomas L. Recognition among mice: evidence from the use of a Y-maze differentially scented by congenic mice of different major histocompatibility types. J Exp Med. 1979;150:755–760. doi: 10.1084/jem.150.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Wysocki CJ, Bard J, Thomas L, Boyse EA. Recognition of H-2 types in relation to the blocking of pregnancy in mice. Science. 1983;240:1331–1332. doi: 10.1126/science.6857281. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Kupniewski D, Bard J, Thomas L, Boyse EA. Familial imprinting determines H-2 selective mating preferences. Science. 1988;240:1331–1332. doi: 10.1126/science.3375818. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Singer A, Bard J, Boyse EA. Odortypes: their origin and composition. Proc Natl Acad Sci USA. 1999;96:1522–1525. doi: 10.1073/pnas.96.4.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Curran M, Bard J, Boyse EA. Parent-progeny recognition as a function of MHC odortype identity. Proc Natl Acad Sci USA. 2000;97:10500–10502. doi: 10.1073/pnas.180320997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Boyse EA, Bard J, Curran M, Kim D, Ross SR, Beauchamp GK. Presence of mouse mammary tumor virus specifically alters the body odors of mice. Proc Natl Acad Sci USA. 2002;99:5612–5615. doi: 10.1073/pnas.082093099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala SM, Potts WK, Penn DJ. Scent-marking displays provide honest signals of health and infection. Behav Ecol. 2003;15:338–344. [Google Scholar]