Abstract
PROBLEM
CXCL6 is a potent pro-inflammatory neutrophil chemoattractant and activator whose activity during pregnancy is not well-established. The purpose of this study was to determine if CXCL6 is present in amniotic fluid (AF) and if CXCL6 concentrations in AF change with labor (preterm and term) or intra-amniotic infection/inflammation (IAI).
METHOD OF STUDY
A cross-sectional study was conducted with the following groups: 1) mid-trimester (n=65); 2) term no labor (n=20); 3) term labor (n=44); 4) patients with PTL with subsequent term delivery (n=57); 5) preterm labor (PTL) without IAI who delivered preterm (n=47); and 6) PTL with IAI (n=62). AF CXCL6 concentrations were determined by ELISA.
RESULTS
CXCL6 was present in all term samples, but undetectable in 64/65 mid-trimester specimens. Patients with PTL and IAI had a significantly higher median CXCL6 AF concentration than those with PTL without IAI [228.9 pg/ml (0.0–8344.8) vs. 55.7 pg/ml (0–454.4); p<0.05] and those with PTL and term delivery [41.5 pg/ml (0–279.0); p<0.05]. Median AF CXCL6 concentration did not change with spontaneous term labor [term no labor: 81.1 pg/ml (8.5–201.7) vs. term labor: 75.2 pg/ml (6.7–378.7): p=0.74].
CONCLUSIONS
1) CXCL6 is detectable in AF and its concentration increases with gestational age; 2) IAI results in increased CXCL6 AF concentrations, suggesting that CXCL6 plays a role in the deployment of an inflammatory response; 3) In contrast to related chemokines, specifically IL-8, AF CXCL6 does not appear to be involved in spontaneous term parturition. These observations are novel, and suggest a role for CXCL6 in the innate immune response to microbial invasion of the amniotic cavity.
Keywords: amniotic fluid, chemokine, CXCL6, intra-amniotic inflammation/infection, pregnancy, preterm labor
Introduction
In normal pregnancy, the amniotic cavity is traditionally regarded as a sterile compartment. Alteration of this state due to microbial invasion of the amniotic cavity and/or inflammation has been causally linked to preterm parturition,1;2 and infection mediated preterm labor remains the most eloquently described mechanism of this pathologic process. Amniotic fluid is considered to play a role in the maintenance of a sterile environment as abundant anti-microbial peptides-including human defensins-have been identified as constituents of amniotic fluid.3–5 Additional first-line mediators against intra-amniotic inflammation and infection include the cervical mucus plug6–10 and deployment of constituents of innate immunity, such as neutrophils, macrophages and natural killer (NK) cells, by the decidua, chorion, amnion,11;12 and trophoblast.13 However, in patients with intra-amniotic inflammation/infection, significantly increased amniotic fluid concentrations of potent pro-inflammatory cytokines (interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-alpha) and chemokines (IL-8, growth-regulated oncogene (GRO)-α, macrophage inhibitory protein (MIP)-1 alpha) have been identified in comparison to normal pregnancies.14–26
Chemokines are a large family of chemotactic cytokines comprised of small heparin-binding proteins which play an integral role in innate and adaptive host response, immune homeostasis, as well as angiogenesis.27;28 Based upon the position of conserved cysteine residues, chemokines are classified into 4 subfamilies (C,CC,CXC,CX3C).29 The CXC family is further classified according to the presence or absence of the ELR motif (glutamate-leucine-arginine). ELR positive (ELR+) CXC chemokines exert potent neutrophil chemotactic and stimulatory effects and promote angiogenesis, as opposed to ELR negative (ELR−) chemokines which recruit monocytes and macrophages while exhibiting potent angiostatic effects.28
CXCL6 (granulocyte chemotactic protein-2) is a member of the ELR+ CXC family which includes IL-8, the growth-regulated oncogenes (GRO-α,-β-γ), and epithelial neutrophil-activating peptide (ENA)-78.30 While CXCL6 and IL-8 share the highest functional homology31 amongst CXC chemokines, they exhibit disparate regulatory effects on recruited neutrophil responses. In an in vitro model, Williams et al32 report that while IL-8 and CXCL6 both dampen TNF-α induced oxidant production, only CXCL6 inhibits the oxidative response stimulated by complement factor C5a or bacterial cell wall peptide N-formyl-methionyl-leucyl-phenylalanine. First identified in a human osteosarcoma cell line,33 CXCL6 has been implicated in the pathogenesis and disease progression of inflammatory bowel disease,34 gastrointestinal malignancies,35 lung cancer,36 and endometriosis.37 In the chorioamniotic membranes of normal term pregnancies, an acute inflammatory gene expression pattern has been described with spontaneous term labor.38 Of interest, CXCL6 expression was significantly increased in the chorioamniotic membranes from normal pregnancies in labor, as compared to those of women not in labor.38
While the related CXC chemokines IL-8 and GRO-α are established physiologic constituents of amniotic fluid,19–22 the identification and role of CXCL6 in the innate immune response of the amniotic cavity has not been described. The objectives of this study were to determine whether: 1) CXCL6 is detectable in human amniotic fluid throughout gestation; 2) the amniotic fluid concentration of CXCL6 changes with spontaneous term labor; and 3) the presence of intra-amniotic inflammation/infection is associated with changes in the amniotic fluid concentration of CXCL6.
Methods and Materials
Study Population
A cross-sectional study was conducted by way of a search of our clinical database and bank of biological specimens. This study included women in the following groups: 1) mid-trimester of pregnancy (14–18 weeks of gestation) that underwent genetic amniocentesis followed by uncomplicated term delivery (n=65); 2) normal pregnant women with a term gestation (≥37 weeks) in spontaneous labor (n=44) or without labor (n=20); and 3) women with spontaneous preterm labor (PTL) and intact membranes (n=135). The PTL group was further classified into the following 3 categories: 1) women with PTL who delivered at term (n=57); 2) preterm delivery without intra-amniotic infection/inflammation (IAI; n=47); and 3) preterm delivery with IAI (n=62). Patients with fetal anomalies, multiple gestation, or preterm prelabor rupture of membranes were excluded.
Definitions
Normal pregnancy was defined as no obstetrical, medical, or surgical complications of pregnancy, and uncomplicated delivery of a term neonate with a birthweight greater than 2500 grams. Labor was diagnosed in the presence of spontaneous regular uterine contractions occurring at a frequency of 2 every 10 minutes accompanied by cervical change requiring hospital admission. Preterm delivery was defined as delivery at <37 weeks gestation. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was diagnosed when the amniotic fluid Interleukin-6 (IL-6) concentration was ≥ 2.6 pg/ml.39
Sample Collection
Transabdominal amniocentesis was performed under ultrasonographic guidance for chromosomal analysis in mid-trimester patients who delivered normal neonates at term, as well as to determine fetal lung maturity in patients approaching term. Women being assessed for PTL underwent amniocentesis to determine the microbial status of the amniotic cavity. All patients had intact membranes at the time of fluid collection. Following retrieval, amniotic fluid specimens were transported to the laboratory in a sterile capped syringe and underwent gram stain and culture for aerobic/anaerobic bacteria and genital Mycoplasmas. White blood cell (WBC) count and glucose concentration were also performed. The results of these tests were used for subsequent clinical management. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°, and the supernatant was aliquoted and stored at −70°.
All women provided written informed consent prior to the collection of amniotic fluid samples. The collection and utilization of samples for research purposes was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS, Bethesda, Maryland), as well as the Human Investigation Committees of Wayne State University (Detroit, Michigan), the Sóero del Rίo Hospital (Puente Alto, Chile), and the Pennsylvania Hospital (Philadelphia, Pennsylvania). Many of these samples have been used to study the biology of inflammation, hemostasis, growth factor concentrations, and angiogenesis regulation in both normal pregnant women, and those with complicated pregnancies.
CXCL6 immunoassays in amniotic fluid
Human amniotic fluid CXCL6 concentrations were determined using specific and sensitive enzyme-linked immunoassays obtained from R&D Systems, Inc. (Minneapolis, MN, USA). Immunoassays were validated for use in the evaluation of human amniotic fluid in our laboratory prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in this assay system. Briefly, standards and unknown amniotic fluid samples were incubated in duplicate wells of the micro titer plates pre-coated with a monoclonal antibody specific for CXCL6. During this incubation, any CXCL6 present in the standards or amniotic fluid samples was bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for CXCL6 was added to the wells. After incubation and washing to remove excess and unbound materials, a substrate solution containing equal amounts of a stabilized chromogen (TMB, tetramethylbenzidine) and stabilized hydrogen peroxide was added to the wells of the micro titer plate. This initiated color development in proportion to the amount of antigen bound in the initial step of the assay. Color development was stopped with the addition of an acid solution and the color intensity was read using a programmable micro titer plate spectrophotometer (SpectraMax M2 micro plate workstation, Molecular Devices, Sunnyvale, CA). The concentrations of CXCL6 in amniotic fluid samples were determined by interpolation from individual standard curves composed of purified human CXCL6. The calculated inter- and intra-assay coefficients of variation for CXCL6 immunoassays in our laboratory were 4.3% and 5.4%, respectively. The lower limit of detection of the CXCL6 immunoassay was calculated to be 6.6 pg/ml.
Statistical Analysis
The Kolmogorov-Smirnov test was used to determine normality of data distribution. Comparisons of proportions between groups were performed using Chi-square or Fisher’s exact test for categorical variables. Mann-Whitney U and Kruskal-Wallis tests were employed for continuous variables and Spearman rho was utilized to investigate correlations. A p-value of <0.05 was considered statistically significant. The statistical package used was SPSS v.12.0 (SPSS Inc., Chicago, IL, USA).
Results
Two hundred and ninety-five women were included in this study. Table I and Table II display the demographic and clinical characteristics of the study groups.
Table I.
Demographic and clinical characteristics of the normal pregnancy groups
| Mid-trimester (n=65) |
p* | Term not in labor (n=20) |
Term in labor (n=44) |
p† | |
|---|---|---|---|---|---|
| Maternal age (years) | 37±3.4 | <0.001 | 28.5±6.8 | 23±5.2 | 0.03 |
| (24–42) | (17–40) | (16–37) | |||
| Parity | 1±0.7 | NS | 1±2.0 | 1±1.1 | NS |
| (0–3) | (0–7) | (0–3) | |||
| Gestational age at | 16.0±0.9 | <0.001 | 39.2±1.2 | 39±1.3 | NS |
| amniocentesis (weeks) | (14–18) | (38–42) | (37–42) | ||
| Gestational age at | 39±1.2 | NS | 39.3±1.2 | 39±1.3 | NS |
| delivery (weeks) | (37–41) | (38–42) | (37–42) | ||
| Birthweight (grams) | 3345±352 | NS | 3405±456 | 3220±388 | NS |
| (2809–4180) | (2810–4530) | (2540–4440) |
Values are expressed as a median ± standard deviation (range).
NS: not significant.
p*: Comparison between patients in the mid-trimester and those at term not in labor.
p†: Comparison between patients at term not in labor and those at term in labor.
Table II.
Demographic and clinical characteristics of the preterm labor groups
| PTL with Term Delivery (n=57) |
p¥ | PTD without IAI (n=47) |
p* | PTD with IAI (n=62) |
p† | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 23 | NS | 22±5.1 | NS | 22 | NS |
| (15–39) | (16–35) | (14–44) | ||||
| Parity | 0±0.8 | NS | 0±1.9 | NS | 0±1.3 | NS |
| (0–3) | (0–8) | (0–5) | ||||
| Gestational age at | 30.4±3.8 | NS | 31.1±3.2 | <0.05 | 26.4±3.7 | <0.05 |
| amniocentesis (weeks) | (20.7–33.2) | (22.4–33.1) | (22.6–33.7) | |||
| Gestational age at | 38.7±1.2 | <0.05 | 34.6±2.3 | <0.05 | 28.0±3.9 | <0.05 |
| delivery (weeks) | (37.1–42) | (26.6–36.4) | (23.6–36.9) | |||
| Birthweight (grams) | 2948±449 | <0.05 | 2125±544 | <0.05 | 1060±653 | <0.05 |
| (2410–4080) | (800–2860) | (520–2740) |
Values are expressed as a median ± standard deviation (range).
PTL: preterm labor; PTD: preterm delivery; IAI: intra-amniotic infection/inflammation
NS: not significant.
p¥: Comparison between patients with an episode of PTL who subsequently delivered at term and patients with spontaneous preterm delivery without IAI.
p*: Comparison between patients with spontaneous preterm labor without IAI and those with spontaneous preterm labor with IAI.
p†: Comparison between patients with preterm delivery with IAI compared to those with an episode of PTL who subsequently delivered at term.
Detection of CXCL6 in amniotic fluid
CXCL6 was detectable in 77% (227/295) of all amniotic fluid specimens. However, while CXCL6 was detected in 100% of amniotic fluid samples obtained from women at term, and in 98% (162/166) of specimens from the preterm groups, 98% (64/65) of mid-trimester specimens had a CXCL6 concentration below the limit of detection.
Amniotic fluid CXCL6, gestational age, and term labor
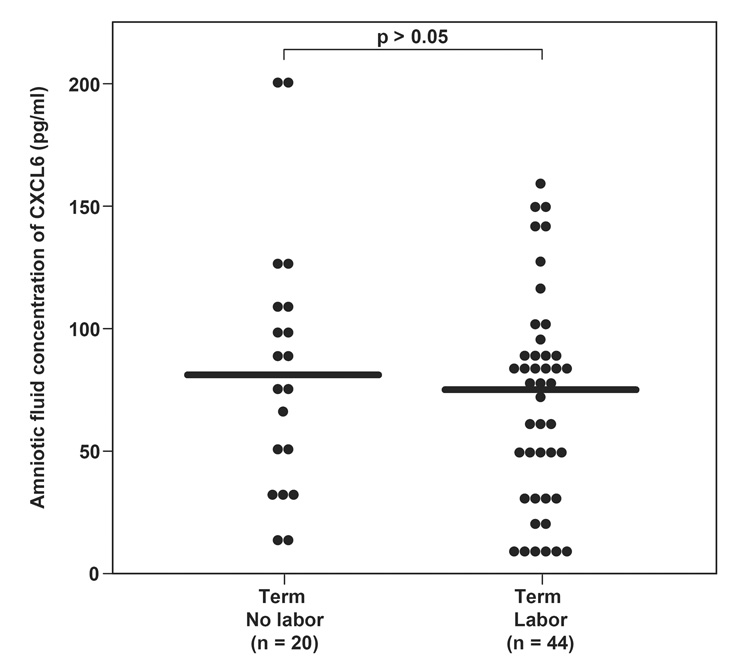
The median concentration of CXCL6 in amniotic fluid was significantly higher at term when compared to the mid-trimester [mid-trimester: median 0.0 pg/ml (0–7.7) vs. term not in labor: median 81.1 pg/ml (8.5–201.7; p<0.001)] and had a significant positive correlation with gestational age (r=0.8; p<0.001). However, spontaneous labor at term did not significantly change the median concentration of CXCL6 in amniotic fluid [term not in labor: median 81.1 pg/ml (range: 8.5–201.7) vs. term in labor: median 75.2 (range: 6.7–378.7); p=0.74, Figure I].
Figure I.
Amniotic fluid (AF) concentrations of CXCL6 in normal pregnant women at term not in labor, and in those at term in labor. There was no difference in AF CXCL6 median concentration between women at term not in labor and those in labor [median 81.1 pg/ml (range 8.5–201.7) vs. median 75.2 pg/ml (range 6.73–378.7), respectively; p=0.74].
Amniotic fluid concentration of CXCL6 in women with preterm labor
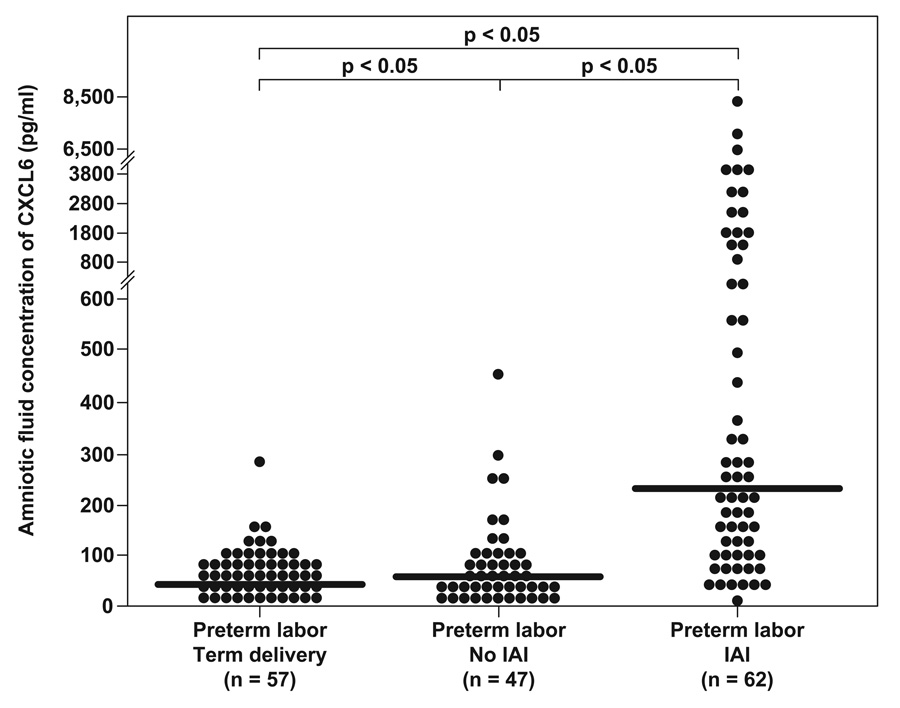
Among women with preterm labor, those who delivered preterm with intra-amniotic infection/inflammation had a significantly higher median amniotic fluid concentration of CXCL6 than women with preterm labor and intact membranes without intra-amniotic infection/inflammation who delivered a preterm neonate [PTL with IAI: median 228.9 pg/ml (range: 0–8344.8) vs. PTL without IAI: median 55.7 pg/ml (range: 0–454.4; p<0.05)], and those with an episode of preterm labor who subsequently delivered at term [PTL with term delivery: median 41.5 pg/ml (range: 0–279); p<0.05)].
In addition, the median amniotic fluid concentration of CXCL6 was significantly higher in women with spontaneous preterm labor and delivery without intra-amniotic infection/inflammation when compared to patients experiencing an episode of PTL followed by a term delivery (p<0.05; Figure II).
Figure II.
Amniotic fluid (AF) concentrations of CXCL6 in women with preterm labor and intact membranes (PTL). The median AF concentrations of CXCL6 were higher in patients without intra-amniotic inflammation (IAI) and intact membranes who had PTL with a spontaneous preterm delivery compared to those who subsequently delivered at term (PTL with term delivery: median 41.5 pg/ml, range 0–279 vs. PTL no IAI: median 55.7 pg/ml, range 0–454.4; p<0.05). However, CXCL6 median AF concentrations were significantly higher in women with PTL and IAI than both women with PTL without evidence of IAI (PTL with IAI: median 228.9 pg/ml, range 0–8344.8; p<0.05) and those with an episode of preterm labor who deliver at term (p<0.05).
Discussion
Principle findings of this study
1) CXCL6 was detectable in human amniotic fluid; 2) the amniotic fluid concentration of CXCL6 was significantly different between patients in the mid-trimester and at term. Indeed, CXCL6 concentrations were below the limit of detection in 98% of specimens obtained in the mid-trimester; 3) the concentration of CXCL6 in amniotic fluid from women at term did not significantly change in the presence of labor; and 4) median CXCL6 concentrations in amniotic fluid were significantly higher in patients delivering preterm with intra-amniotic infection/inflammation than in women delivering preterm without intra-amniotic infection/inflammation, or in those with an episode of preterm labor who deliver at term. In addition, spontaneous preterm delivery without intra-amniotic infection/inflammation was associated with a higher median CXCL6 amniotic fluid concentration than patients with an episode of PTL who delivered at term.
What is CXCL6?
ELR+ CXC chemokines such as CXCL6 play key roles in the recruitment and activation of neutrophils. The structure of CXCL6 is >75% homologous to ENA-78,40 but is unique in that it binds with high-affinity to both CXCR1 and CXCR2.41– 43 Of the ELR+ CXC chemokines, only CXCL6 and IL-8 induce immune responses via both the CXCR1 and CXCR2 receptors.42;43 Both receptors are expressed on components of innate immunity including neutrophils, monocytes, mast cells, and NK cells. Recently, CXCR1 expression has been described on effector CD8+ T cells, suggesting a role for its ligands in adaptive immunity.44 Neither CXCR1 nor CXCR2 have been localized on B cells or eosinophils.45–48
Of interest, although CXCL6 was first discovered in a malignant cell-line, tumor cells are poor producers of this chemokine. High levels of CXCL6 mRNA have been identified in the heart, lung, liver, and pancreas, while only weak expression has been found in the brain, kidney, and placenta.31 The CXCL6 gene has been mapped to chromosome 4q12-q13 and is included in a tight cluster with related ELR+CXC genes. Indeed, Modi et al49 provide evidence supporting the role of tandem gene duplication in the evolution of CXC chemokines. CXCL6 is produced in the highest concentrations by mesenchymal cells, including fibroblasts and both micro and macrovascular endothelial cells.34;50 IL-1β is the most effective inducer of CXCL6 production, while its expression is down-regulated by interferon-γ.50;51 Of interest, differential regulation of CXCL6 and IL-8 secretion by mononuclear lymphocytes and granulocytes has been observed. Wuyts et al50 describe detectable production of CXCL6 only from differentiated or recruited hematopoietic cells whereas naive peripheral leukocytes failed to secrete detectable CXCL6 despite LPS stimulation.
CXCL6 in pathologic conditions
Chemokines have been shown to play a role in autoimmune disease, graft rejection, infection, allergy, neoplasia, and vascular disease.52 In states of chronic inflammation such as inflammatory bowel disease (characterized by unremitting leukocyte infiltration in affected tissues), CXCL6 expression is selectively upregulated in endothelial cells at sites of intestinal inflammation and ulceration.34 Furthermore, Rudack et al53 describe the pattern of chemokine expression in patients with chronic rhinosinusitis, a neutrophil-mediated condition. CXCL6 and GRO-α were the predominant chemokines produced by affected mucosa and epithelial cells. Indeed, 43% and 36% of neutrophil chemotaxis was inhibited with blockage of CXCL6 and GRO-α, respectively.
CXC chemokines have been implicated as the “link” between inflammation and angiogenesis, with deviations in this coordinated interplay resulting in an environment favoring tumorigenesis and metastasis.54 CXCL6 expression and activity has been well-documented in human malignancies such as lung cancer and gastrointestinal tumors. In vivo cell line experiments of small cell lung cancer have established a role for CXCL6 as an autocrine growth factor leading to both tumor progression and metastasis.36 Furthermore, the pro-angiogenic activity of this chemokine was enhanced by treatment with IL-1β and in conditions of hypoxia. Gijsbers et al35 describe both the production of CXCL6 at sites of gastrointestinal tumor neovascularization and the synergistic interplay of CXCL6 and CCL2 (monocyte chemotactic protein-1) induction, which results in a ten-fold increase in neutrophil infiltration at the tumor site. Furthermore, CXCL6 expression correlated with leukocyte infiltrate secretion of matrix metalloproteinase-9 (MMP-9), a potent matrix degradation mediator, thereby contributing to tumor expansion and metastasis.
Pathologic states derived from local inflammation and dysregulation of innate immunity have also been associated with increased concentrations of CXCL6. Suzumori et al37 compared the CXCL6 concentration in peritoneal fluid between symptomatic women with endometriosis and women undergoing surgical intervention for a cystadenoma. Women with endometriosis (in the proliferative phase) had a significantly higher median concentration of CXCL6 in peritoneal fluid than the control group, with a positive correlation between median CXCL6 concentration and the stage of disease. This data further suggests that the pathogenesis and progression of inflammation-linked pathology is associated with increased secretion of this pro-inflammatory and pro-angiogenic chemokine.
Chemokines and fetal development
Recent evidence has suggested a role for chemokines in human fetal development. Lu et al55 applied RT-PCR and immunohistochemistry techniques to evaluate the expression of the CXC receptors CXCR1,CXCR2,CXCR3,CXCR4, and stromal cell-derived factor 1α in non-hematopoietic tissues of normal human fetuses of early gestation. The investigators found that specific CXC receptor expression varied with gestational age. However, mRNA for receptors of CXCL6, CXCR1 and CXCR2, were constitutively expressed in all examined fetal tissues (brain, heart, intestine, kidney, bone marrow, liver) at all gestational ages examined (12–19 weeks). However, protein expression of CXCR1 was not as abundant as that of CXCR2. While CXCR2 protein was noted on glial cells, there was no staining for CXCR1 in the fetal brain. Given the differential gestational age and site expression of chemokine receptors during early gestation, it is possible that chemokines contribute to normal fetal development.
CXCL6 in normal pregnancy
In this study, we report the first identification of CXCL6 in human amniotic fluid and describe the change in median amniotic fluid CXCL6 concentration with gestational age. While not previously noted in the amniotic cavity, CXCL6 production has been demonstrated in cultured endometrial stromal cells in response to inflammatory mediators.56 Although the exact roles of chemokines in the female reproductive tract are incompletely described, they are proposed to include functions in physiologic events such as menstruation,57 implantation,58 and the maintenance of early pregnancy.59–61 The concentration of leukocytes in human endometrium is known to vary during the menstrual cycle, as well as during pregnancy. Starkey et al62 originally described that 50% of the lymphocyte population in first trimester decidua was composed of large granular lymphocytes (LGL). However, the concentration of these lymphocytes decreases as pregnancy progresses, with few LGLs detected in term decidual tissues. While the exact mechanisms of cell trafficking during pregnancy are unknown, the recruitment of these peripheral lymphocytes to the female reproductive tract is proposed to be mediated by chemokines. Liggins63 first described the role of inflammatory cells in cervical ripening in 1981. Since then, a multitude of studies have demonstrated that cervical dilatation is accompanied by an influx of neutrophils. Chemokines, in particular IL-8, have been identified as mediators of this recruitment.64–69 However, unlike the closely related IL-8, whose concentration in amniotic fluid increases with the onset of both term and preterm labor,19;22;70–73 median CXCL6 concentration in amniotic fluid did not differ with term labor, as shown herein. The differential secretion and sources of functionally-related chemokines in the amniotic cavity and their role in the common pathway of parturition warrants further investigation.
CXCL6 in preterm labor
Herein, we describe the novel finding of elevated concentrations of the chemokine CXCL6 in the amniotic fluid of women who deliver preterm with intra-amniotic infection/inflammation, in comparison to women with spontaneous preterm labor and intact membranes who deliver either preterm or at term without intra-amniotic infection/inflammation. Furthermore, we provide data demonstrating that spontaneous preterm delivery without intra-amniotic infection/inflammation is also associated with a higher amniotic fluid concentration of CXCL6 than preterm labor with subsequent term delivery. Comparison of subgroup demographic data did reveal a significant difference in gestational age at amniocentesis between the diagnostic groups; patients diagnosed with PTL and intra-amniotic infection/inflammation underwent amniocentesis at an earlier gestational age than the other diagnostic groups. However, given the increasing concentration of CXCL6 with advancing gestational age, we would expect the PTL with intra-amniotic infection/inflammation group to have a lower median concentration of CXCL6 in amniotic fluid than the PTL with term delivery group, which had a higher median gestational age at amniocentesis. Yet this was not the case, as patients with PTL and IAI had the highest median amniotic fluid CXCL6 concentration among all groups included. Therefore, we did not proceed with matching the subgroups for gestational age.
These above findings support previous work linking intra-uterine inflammation and preterm delivery. The relationship between the preterm parturition syndrome and inflammation is well-established. Indeed, intra-uterine inflammation/infection is the most thoroughly described, and strongly supported mechanism of preterm delivery.1;39;74;74–87 Twenty-five to 40% of preterm birth is attributed to microbial invasion of the uterine cavity,88;89 and a compelling body of evidence supports the central role of cytokines in preterm parturition.16;81;90–101 Furthermore, intra-uterine inflammation, diagnosed by increased amniotic fluid cytokine concentration, can assist in the identification of women destined to deliver preterm.15;17;18;102;103 Of interest, the elevation of inflammatory mediators in amniotic fluid is not mirrored in maternal serum,102 a finding that supports the existence of a location-specific immune response in the uterine cavity during pregnancy. Interleukin-1 was the first cytokine ascribed to play a role in inflammation-mediated preterm labor.99 It is also recognized as the most potent stimulator of CXCL6 chemoattractant activity.31;40;51 Mine et al56 described an IL-1α and IL-1β instigated increase of CXCL6 production in endometrial stromal cells by 137-fold and 111-fold, respectively, when compared with non-stimulated controls. These observations are consistent with our findings as amniotic fluid affected by inflammation contained significantly higher concentrations of CXCL6 compared to controls.
Conclusions
Herein, we describe the novel identification of CXCL6 as a physiological constituent of amniotic fluid. The median CXCL6 concentration in amniotic fluid did not change with the presence of labor at term, but is significantly increased in the amniotic fluid from patients with preterm labor and intact membranes complicated by intra-amniotic inflammation as well as those with spontaneous preterm delivery without intra-amniotic infection/inflammation. Further investigation regarding the interaction between components and pathways of the innate immune system in the amniotic cavity may provide insight into the role of chemokines in the mechanisms of both normal and abnormal human parturition.
Acknowledgement
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15 Suppl 2:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 3.King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate Immune Defences in the Human Uterus during Pregnancy. Placenta. 2007;28:1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, Sorokin Y. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Gomez R, Araneda H, Ramirez M, Cotton DB. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol. 1993;168:312. [Google Scholar]
- 7.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000;15:778–784. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 8.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 9.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 10.Hein M, Petersen AC, Helmig RB, Uldbjerg N, Reinholdt J. Immunoglobulin levels and phagocytes in the cervical mucus plug at term of pregnancy. Acta Obstet Gynecol Scand. 2005;84:734–742. doi: 10.1111/j.0001-6349.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 11.Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, Uldbjerg N, Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–229. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 12.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 13.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–183. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 19.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, Appelbaum PC. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–1303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, Goncalves LF, Gomez R. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996;35:23–29. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CD, Meaddough E, Aversa K, Copel JA. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol. 1998;178:428–432. doi: 10.1016/s0002-9378(98)70414-4. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 23.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol. 1996;87:94–98. doi: 10.1016/0029-7844(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 24.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 25.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–113. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 27.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van DJ, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 28.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van DJ, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 29.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 30.Proost P, Wuyts A, Conings R, Lenaerts JP, Billiau A, Opdenakker G, Van DJ. Human and bovine granulocyte chemotactic protein-2: complete amino acid sequence and functional characterization as chemokines. Biochemistry. 1993;32:10170–10177. doi: 10.1021/bi00089a037. [DOI] [PubMed] [Google Scholar]
- 31.Van DJ, Wuyts A, Froyen G, Van CE, Struyf S, Billiau A, Proost P, Wang JM, Opdenakker G. Granulocyte chemotactic protein-2 and related CXC chemokines: from gene regulation to receptor usage. J Leukoc Biol. 1997;62:563–569. doi: 10.1002/jlb.62.5.563. [DOI] [PubMed] [Google Scholar]
- 32.Williams MA, Cave CM, Quaid G, Solomkin JS. Chemokine regulation of neutrophil function in surgical inflammation. Arch Surg. 1999;134:1360–1366. doi: 10.1001/archsurg.134.12.1360. [DOI] [PubMed] [Google Scholar]
- 33.Proost P, De Wolf-Peeters C, Conings R, Opdenakker G, Billiau A, Van DJ. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10, and IL-8. J Immunol. 1993;150:1000–1010. [PubMed] [Google Scholar]
- 34.Gijsbers K, Van AG, Joossens S, Struyf S, Proost P, Rutgeerts P, Geboes K, Van DJ. CXCR1-binding chemokines in inflammatory bowel diseases: down-regulated IL-8/CXCL8 production by leukocytes in Crohn's disease and selective GCP-2/CXCL6 expression in inflamed intestinal tissue. Eur J Immunol. 2004;34:1992–2000. doi: 10.1002/eji.200324807. [DOI] [PubMed] [Google Scholar]
- 35.Gijsbers K, Gouwy M, Struyf S, Wuyts A, Proost P, Opdenakker G, Penninckx F, Ectors N, Geboes K, Van DJ. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res. 2005;303:331–342. doi: 10.1016/j.yexcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 36.Zhu YM, Bagstaff SM, Woll PJ. Production and upregulation of granulocyte chemotactic protein-2/CXCL6 by IL-1beta and hypoxia in small cell lung cancer. Br J Cancer. 2006;94:1936–1941. doi: 10.1038/sj.bjc.6603177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzumori N, Zhao XX, Suzumori K. Increased granulocyte chemotactic protein-2 concentrations in peritoneal fluid of women with endometriosis. Acta Obstet Gynecol Scand. 2005;84:1141–1144. doi: 10.1111/j.0001-6349.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 38.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394–324. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 40.Rovai LE, Herschman HR, Smith JB. Cloning and characterization of the human granulocyte chemotactic protein-2 gene. J Immunol. 1997;158:5257–5266. [PubMed] [Google Scholar]
- 41.Wuyts A, Van ON, Haelens A, Samson I, Herdewijn P, Ben-Baruch A, Oppenheim JJ, Proost P, Van DJ. Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochemistry. 1997;36:2716–2723. doi: 10.1021/bi961999z. [DOI] [PubMed] [Google Scholar]
- 42.Wuyts A, Proost P, Lenaerts JP, Ben-Baruch A, Van DJ, Wang JM. Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur J Biochem. 1998;255:67–73. doi: 10.1046/j.1432-1327.1998.2550067.x. [DOI] [PubMed] [Google Scholar]
- 43.Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol. 1998;28:164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 44.Takata H, Tomiyama H, Fujiwara M, Kobayashi N, Takiguchi M. Cutting edge: expression of chemokine receptor CXCR1 on human effector CD8+ T cells. J Immunol. 2004;173:2231–2235. doi: 10.4049/jimmunol.173.4.2231. [DOI] [PubMed] [Google Scholar]
- 45.Chuntharapai A, Lee J, Hebert CA, Kim KJ. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J Immunol. 1994;153:5682–5688. [PubMed] [Google Scholar]
- 46.Morohashi H, Miyawaki T, Nomura H, Kuno K, Murakami S, Matsushima K, Mukaida N. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J Leukoc Biol. 1995;57:180–187. doi: 10.1002/jlb.57.1.180. [DOI] [PubMed] [Google Scholar]
- 47.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 48.Petering H, Gotze O, Kimmig D, Smolarski R, Kapp A, Elsner J. The biologic role of interleukin-8: functional analysis and expression of CXCR1 and CXCR2 on human eosinophils. Blood. 1999;93:694–702. [PubMed] [Google Scholar]
- 49.Modi WS, Chen ZQ. Localization of the human CXC chemokine subfamily on the long arm of chromosome 4 using radiation hybrids. Genomics. 1998;47:136–139. doi: 10.1006/geno.1997.5100. [DOI] [PubMed] [Google Scholar]
- 50.Wuyts A, Struyf S, Gijsbers K, Schutyser E, Put W, Conings R, Lenaerts JP, Geboes K, Opdenakker G, Menten P, Proost P, Van DJ. The CXC chemokine GCP-2/CXCL6 is predominantly induced in mesenchymal cells by interleukin-1beta and is down-regulated by interferon-gamma: comparison with interleukin-8/CXCL8. Lab Invest. 2003;83:23–34. doi: 10.1097/01.lab.0000048719.53282.00. [DOI] [PubMed] [Google Scholar]
- 51.Froyen G, Proost P, Ronsse I, Mitera T, Haelens A, Wuyts A, Opdenakker G, Van DJ, Billiau A. Cloning, bacterial expression and biological characterization of recombinant human granulocyte chemotactic protein-2 and differential expression of granulocyte chemotactic protein-2 and epithelial cell-derived neutrophil activating peptide-78 mRNAs. Eur J Biochem. 1997;243:762–769. doi: 10.1111/j.1432-1033.1997.00762.x. [DOI] [PubMed] [Google Scholar]
- 52.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 53.Rudack C, Sachse F, Alberty J. Primary role of growth-related oncogene-alpha and granulocyte chemotactic protein-2 as neutrophil chemoattractants in chronic rhinosinusitis. Clin Exp Allergy. 2006;36:748–759. doi: 10.1111/j.1365-2222.2006.02501.x. [DOI] [PubMed] [Google Scholar]
- 54.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Lu W, Gersting JA, Maheshwari A, Christensen RD, Calhoun DA. Developmental expression of chemokine receptor genes in the human fetus. Early Hum Dev. 2005;81:489–496. doi: 10.1016/j.earlhumdev.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 56.Mine S, Nasu K, Fukuda J, Sun B, Miyakawa I. Secretion of granulocyte chemotactic protein-2 by cultured human endometrial stromal cells. Fertil Steril. 2003;79:146–150. doi: 10.1016/s0015-0282(02)04552-1. [DOI] [PubMed] [Google Scholar]
- 57.Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994;9:253–258. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- 58.Kodama T, Hara T, Okamoto E, Kusunoki Y, Ohama K. Characteristic changes of large granular lymphocytes that strongly express CD56 in endometrium during the menstrual cycle and early pregnancy. Hum Reprod. 1998;13:1036–1043. doi: 10.1093/humrep/13.4.1036. [DOI] [PubMed] [Google Scholar]
- 59.Chard T. Cytokines in implantation. Hum Reprod Update. 1995;1:385–396. doi: 10.1093/humupd/1.4.385. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Velasco JA, Arici A. Chemokines and human reproduction. Fertil Steril. 1999;71:983–993. doi: 10.1016/s0015-0282(99)00120-x. [DOI] [PubMed] [Google Scholar]
- 61.Rice A, Chard T. Cytokines in implantation. Cytokine Growth Factor Rev. 1998;9:287–296. doi: 10.1016/s1359-6101(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 62.Starkey PM, Sargent IL, Redman CW. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988;65:129–134. [PMC free article] [PubMed] [Google Scholar]
- 63.Liggins G. Cervical ripening as an inflammatory reaction. In: Ellwood D, Anderson A, editors. The cervix in pregnancy and labour. Edinburgh: Churchill Livingstone; 1981. pp. 1–9. [Google Scholar]
- 64.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 65.Sakamoto Y, Moran P, Searle RF, Bulmer JN, Robson SC. Interleukin-8 is involved in cervical dilatation but not in prelabour cervical ripening. Clin Exp Immunol. 2004;138:151–157. doi: 10.1111/j.1365-2249.2004.02584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sennstrom MB, Ekman G, Westergren-Thorsson G, Malmstrom A, Bystrom B, Endresen U, Mlambo N, Norman M, Stabi B, Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375–381. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- 67.Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol. 1997;74:89–92. doi: 10.1016/s0301-2115(97)02757-7. [DOI] [PubMed] [Google Scholar]
- 68.Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005;3:39. doi: 10.1186/1477-7827-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J., Jr. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Hebisch G, Grauaug AA, Neumaier-Wagner PM, Stallmach T, Huch A, Huch R. The relationship between cervical dilatation, interleukin-6 and interleukin-8 during term labor. Acta Obstet Gynecol Scand. 2001;80:840–848. doi: 10.1034/j.1600-0412.2001.080009840.x. [DOI] [PubMed] [Google Scholar]
- 71.Kemp B, Winkler M, Maas A, Maul H, Ruck P, Reineke T, Rath W. Cytokine concentrations in the amniotic fluid during parturition at term: correlation to lower uterine segment values and to labor. Acta Obstet Gynecol Scand. 2002;81:938–942. doi: 10.1034/j.1600-0412.2002.811007.x. [DOI] [PubMed] [Google Scholar]
- 72.Laham N, Rice GE, Bishop GJ, Ransome C, Brennecke SP. Interleukin 8 concentrations in amniotic fluid and peripheral venous plasma during human pregnancy and parturition. Acta Endocrinol (Copenh) 1993;129:220–224. doi: 10.1530/acta.0.1290220. [DOI] [PubMed] [Google Scholar]
- 73.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–88. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchley C, Bennet P, Thomton S, editors. Preterm Birth. London: RCOG Press; 2004. pp. 28–60. [Google Scholar]
- 75.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 76.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 79.Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Cliver SP, Hauth JC. The Alabama Preterm Birth Study: intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. Am J Obstet Gynecol. 2006;195:1533–1537. doi: 10.1016/j.ajog.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 80.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 81.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112 Supp l:16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 82.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 83.Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Dev Med Child Neurol. 1993;35:553–558. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 89.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, Bracken MB. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 90.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N Y Acad Sci. 2001;943:225–234. doi: 10.1111/j.1749-6632.2001.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 91.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 92.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev. 2002;60:S19–S25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 93.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 94.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24 Suppl A:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 96.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 97.Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med. 2004;32:391–399. doi: 10.1515/JPM.2004.134. [DOI] [PubMed] [Google Scholar]
- 98.Mohan AR, Loudon JA, Bennett PR. Molecular and biochemical mechanisms of preterm labour. Semin Fetal Neonatal Med. 2004;9:437–444. doi: 10.1016/j.siny.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 99.Romero R, Durum SK, Dinarello CA, et al. Interleukin-1: A signal for the initiation of labor in chorioamnionitis; 33rd Annual Meeting for the Society for Gynecologic Investigation; Toronto, Ontario, Canada. 1986. Ref Type: Conference Proceeding. [Google Scholar]
- 100.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 101.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005;84:516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 102.Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol. 1996;175:830–833. doi: 10.1016/s0002-9378(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 103.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]