Abstract
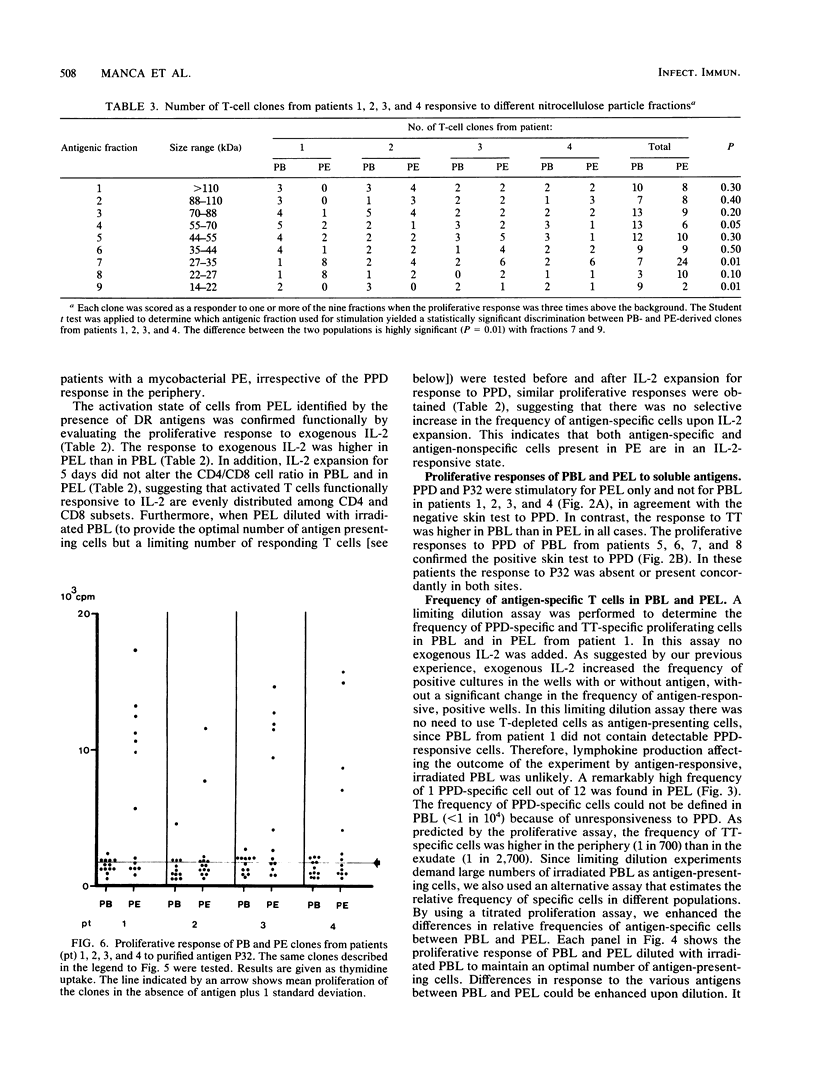
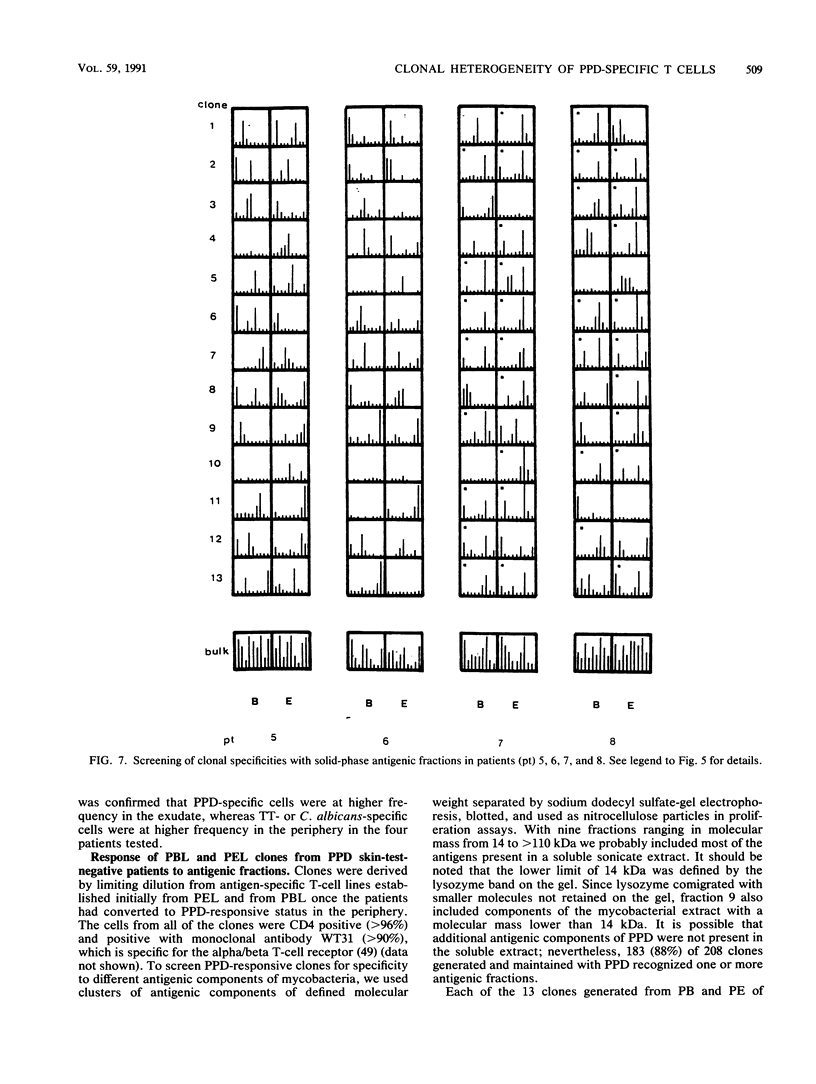
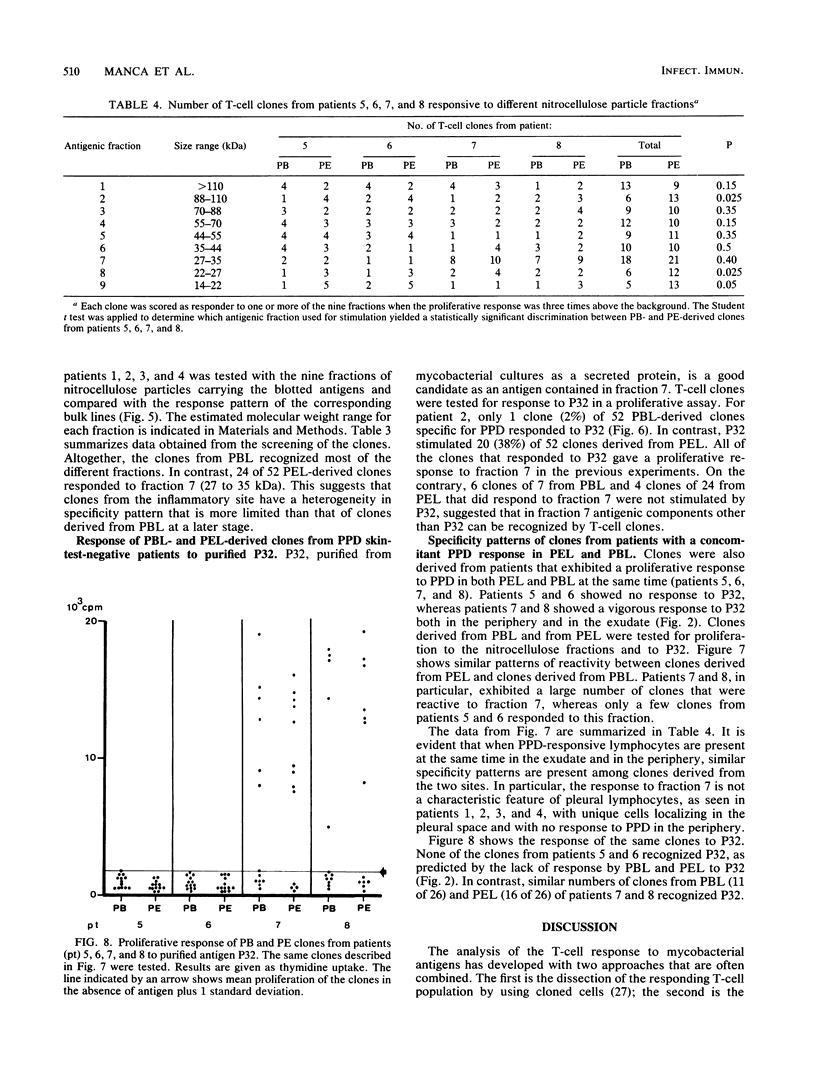
To detect possible differences in phenotype and fine specificity for mycobacterial antigens between CD4-positive T cells from peripheral blood (PB) and from inflammatory sites, we identified four patients presenting with a mycobacterial pleural exudate (PE) rich in PPD-specific lymphocytes and with a negative skin test to tuberculin purified protein derivative (PPD) and a negative proliferative response of PB lymphocytes to PPD at the same time. Several weeks after chemotherapy, these patients converted to PPD responsiveness in the periphery, and PPD-specific clones could be generated from PB at this stage. The phenotypic comparison of PE lymphocytes and concomitant PB lymphocytes obtained before treatment showed an increase of CD8 cells and a high frequency of HLA-DR-positive activated T cells in PE. The frequency of tetanus toxoid-specific and Candida albicans-specific proliferating T cells was lower than that of PPD-specific cells in PE but not in PB. PPD-specific clones were derived initially from PE and from PB once the patients had converted to PPD responsiveness. The two sets of clones from each patient were compared for proliferative response to mycobacterial antigen clusters of defined molecular weight ranges. A large number of PE-derived clones (36%) responded to a fraction of 27 to 35 kDa, whereas only one clone from PB responded to the same fraction. The purified antigen P32 (32 kDa), a soluble mycobacterial protein, stimulated PE-derived clones that were responsive to the 37- to 27-kDa fraction but did not stimulate PB-derived clones. The data demonstrate that PE- and PB-derived lymphocytes differ both in phenotype and in fine specificity, suggesting a limited clonal heterogeneity of T cells localizing at the inflammatory site in tuberculous patients without a PPD response in the periphery. Therefore T cells compartmentalized at inflammatory sites provide information that is different from that provided by T cells in the periphery.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Augustin A., Kubo R. T., Sim G. K. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989 Jul 20;340(6230):239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Mistry S. D., Cooper C. L., Pirmez C., Rea T. H., Modlin R. L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989 Feb 15;142(4):1114–1119. [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Converse P. J., Ottenhoff T. H., Gebre N., Ehrenberg J. P., Kiessling R. Cellular, humoral, and gamma interferon responses to Mycobacterium leprae and BCG antigens in healthy individuals exposed to leprosy. Scand J Immunol. 1988 May;27(5):515–525. doi: 10.1111/j.1365-3083.1988.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Cooper C. L., Mueller C., Sinchaisri T. A., Pirmez C., Chan J., Kaplan G., Young S. M., Weissman I. L., Bloom B. R., Rea T. H. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989 May 1;169(5):1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J. Immunization against tuberculosis: what kind of vaccine? Infect Immun. 1988 Nov;56(11):2769–2773. doi: 10.1128/iai.56.11.2769-2773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A., Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989 Jan;10(1):23–28. doi: 10.1016/0167-5699(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Tsuyuguchi I. Frequency of tuberculin-reactive T-lymphocytes in pleural fluid and blood from patients with tuberculous pleurisy. Chest. 1986 Apr;89(4):530–532. doi: 10.1378/chest.89.4.530. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Duby A. D., Lee S. J., Benjamin D., Seidman J. G., Weiner H. L. Oligoclonal T lymphocytes in the cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1988 Apr 1;167(4):1313–1322. doi: 10.1084/jem.167.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Happ M. P., Kubo R. T., Palmer E., Born W. K., O'Brien R. L. Limited receptor repertoire in a mycobacteria-reactive subset of gamma delta T lymphocytes. Nature. 1989 Dec 7;342(6250):696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Hilgers J., ten Berg R., Van Vooren J. P., De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988 Dec;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., O'Hehir R. E., Young D. B. The use of nitrocellulose immunoblots for the analysis of antigen recognition by T lymphocytes. J Immunol Methods. 1988 May 25;110(1):1–10. doi: 10.1016/0022-1759(88)90076-2. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987 Jan;60(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Lee S. P., Stoker N. G., Grant K. A., Handzel Z. T., Hussain R., McAdam K. P., Dockrell H. M. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect Immun. 1989 Aug;57(8):2475–2480. doi: 10.1128/iai.57.8.2475-2480.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveton C., Barnass S., Champion B., Lucas S., De Souza B., Nicol M., Banerjee D., Rook G. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. 1989 Feb;57(2):390–395. doi: 10.1128/iai.57.2.390-395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby P. C., Sacks D. L. Identification of antigens recognized by T cells in human leishmaniasis: analysis of T-cell clones by immunoblotting. Infect Immun. 1989 Oct;57(10):2971–2976. doi: 10.1128/iai.57.10.2971-2976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli M. C., Finn O. J. T cell receptor beta-chain selection in human allograft rejection. J Immunol. 1989 Jan 1;142(1):81–86. [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. Dominant T-cell receptor beta-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol. 1990 Jan;31(1):121–126. doi: 10.1111/j.1365-3083.1990.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Modrow S., Karr R. W., Young R. A., Kaufmann S. H. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989 Nov 1;143(9):2844–2849. [PubMed] [Google Scholar]
- Narayanan R. B., Bhutani L. K., Sharma A. K., Nath I. T cell subsets in leprosy lesions: in situ characterization using monoclonal antibodies. Clin Exp Immunol. 1983 Mar;51(3):421–429. [PMC free article] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Klatser P. R., Ivanyi J., Elferink D. G., de Wit M. Y., de Vries R. R. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature. 1986 Jan 2;319(6048):66–68. doi: 10.1038/319066a0. [DOI] [PubMed] [Google Scholar]
- Padula S. J., Sgroi D. C., Lingenheld E. G., Love J. T., Jr, Chou C. H., Clark R. B. T cell receptor beta chain gene rearrangement shared by murine T cell lines derived from a site of autoimmune inflammation. J Clin Invest. 1988 Jun;81(6):1810–1818. doi: 10.1172/JCI113524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A., Scoging A., Mehlert A., Young D. B., Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988 Dec;18(12):1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- Reynolds S. R., Kunkel S. L., Thomas D. W., Higashi G. I. T cell clones for antigen selection and lymphokine production in murine Schistosomiasis mansoni. J Immunol. 1990 Apr 1;144(7):2757–2762. [PubMed] [Google Scholar]
- Rossi G. A., Balbi B., Manca F. Tuberculous pleural effusions. Evidence for selective presence of PPD-specific T-lymphocytes at site of inflammation in the early phase of the infection. Am Rev Respir Dis. 1987 Sep;136(3):575–579. doi: 10.1164/ajrccm/136.3.575. [DOI] [PubMed] [Google Scholar]
- Rouse R. V., Reichert R. A., Gallatin W. M., Weissman I. L., Butcher E. C. Localization of lymphocyte subpopulations in peripheral lymphoid organs: directed lymphocyte migration and segregation into specific microenvironments. Am J Anat. 1984 Jul;170(3):391–405. doi: 10.1002/aja.1001700313. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H., Tsuyuguchi I. Analysis of T cell subsets by monoclonal antibodies in patients with tuberculosis after in vitro stimulation with purified protein derivative of tuberculin. Clin Exp Immunol. 1984 Aug;57(2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Ford W. L. The recirculating lymphocyte pool of the rat: a systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 1983 May;49(1):83–94. [PMC free article] [PubMed] [Google Scholar]
- Spits H., Borst J., Tax W., Capel P. J., Terhorst C., de Vries J. E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985 Sep;135(3):1922–1928. [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Takashima T., Collins F. M. T-cell-mediated immunity in persistent Mycobacterium intracellulare infections in mice. Infect Immun. 1988 Nov;56(11):2782–2787. doi: 10.1128/iai.56.11.2782-2787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Van der Zee R., Van Eden W., Meloen R. H., Noordzij A., Van Embden J. D. Efficient mapping and characterization of a T cell epitope by the simultaneous synthesis of multiple peptides. Eur J Immunol. 1989 Jan;19(1):43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Leprosy: understanding protective immunity. Immunol Today. 1989 Jul;10(7):218–221. doi: 10.1016/0167-5699(89)90253-3. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]


