Abstract
Peroxisome proliferator-activated receptor γ(PPARγ), a nuclear receptor and the target of anti-diabetic thiazolinedione drugs, is known as the master regulator of adipocyte biology. Although it regulates hundreds of adipocyte genes, PPARγ binding to endogenous genes has rarely been demonstrated. Here, utilizing chromatin immunoprecipitation (ChIP) coupled with whole genome tiling arrays, we identified 5299 genomic regions of PPARγ binding in mouse 3T3-L1 adipocytes. The consensus PPARγ/RXRα “DR-1”-binding motif was found at most of the sites, and ChIP for RXRα showed colocalization at nearly all locations tested. Bioinformatics analysis also revealed CCAAT/enhancer-binding protein (C/EBP)-binding motifs in the vicinity of most PPARγ-binding sites, and genome-wide analysis of C/EBPα binding demonstrated that it localized to 3350 of the locations bound by PPARγ. Importantly, most genes induced in adipogenesis were bound by both PPARγ and C/EBPα, while very few were PPARγ-specific. C/EBPβ also plays a role at many of these genes, such that both C/EBPα and β are required along with PPARγ for robust adipocyte-specific gene expression. Thus, PPARγ and C/EBP factors cooperatively orchestrate adipocyte biology by adjacent binding on an unanticipated scale.
Keywords: PPARγ, C/EBP, adipocyte, genome wide, ChIP–chip
Peroxisome proliferator-activated receptor γ (PPARγ), a member of the nuclear receptor superfamily of ligand-activated transcription factors, is the cellular target of anti-diabetic thiazolidinedione drugs (TZDs). PPARγ is both necessary (Rosen et al. 2002) and sufficient (Tontonoz et al. 1994c) for the differentiation of mouse fibroblasts into adipocytes, where PPARγ is expressed at its highest levels (Chawla et al. 1994; Tontonoz et al. 1994b). The adipogenic activity of PPARγ requires a functional DNA-binding domain (Tontonoz et al. 1994c), suggesting that this critical function involves binding directly to target genes. PPARγ is also important for major functions of mature adipocytes, including lipid metabolism, adipokine secretion, and insulin sensitivity (Rangwala and Lazar 2004). A number of animal models and naturally occurring human mutations have demonstrated that PPARγ plays critical roles in adipocyte development and function in vivo as well (Gray et al. 2005).
PPARγ regulates adipocyte biology together with members of the CCAAT/enhancer-binding protein (C/EBP) family. C/EBPβ and C/EBPδ are expressed early during adipogenesis (Cao et al. 1991; Yeh et al. 1995), and are involved in the induction of PPARγ (Wu et al. 1996; Hamm et al. 2001). C/EBPα is induced at later stages and is active in mature adipocytes (Darlington et al. 1998). Ectopic expression of C/EBPα (Freytag et al. 1994; Tontonoz et al. 1994c) or C/EBPβ (Wu et al. 1995; Yeh et al. 1995) can induce NIH-3T3 fibroblasts to differentiate, although this requires PPARγ (Wu et al. 1996). Coexpression of C/EBPα and PPARγ in NIH-3T3 cells has synergistic effects on adipogenic conversion and essentially obviates the need for hormonal stimulation (Tontonoz et al. 1994c). This suggests that the cooperation of PPARγ with C/EBP family members is necessary for optimal differentiation, although the precise mechanism of this cooperation remains unclear.
Based on in vitro studies and target genes identified in the literature, PPARγ binds consensus DNA elements as a heterodimer with RXRα in a head-to-tail orientation (Gearing et al. 1993; IJpenberg et al. 1997). Known PPARγ-binding sites contain the so-called “DR1” site; i.e., a direct repeat of the AGGTCA element conserved to various degrees and separated by a single nucleotide (Schoonjans et al. 1996). These conclusions are based on analysis of only ∼30 genes identified as PPARγ targets through reporter gene and gel shift assays and chromatin immunoprecipitation (ChIP) (Tontonoz et al. 1994a; IJpenberg et al. 1997; Robinson et al. 1998; Teboul et al. 2001; Chui et al. 2005; Guan et al. 2005; Yajima et al. 2007; Nakachi et al. 2008). In contrast, expression profiling studies during adipocyte differentiation and following PPARγ ligand treatment suggest that hundreds of genes may be regulated by PPARγ (Perera et al. 2006; Sears et al. 2007; Nakachi et al. 2008). Thus, the full range of PPARγ-binding sites in adipocytes, or cistrome (Lupien et al. 2008), remains largely unknown. It is also unclear where PPARγ-binding sites are located relative to transcription start sites (TSS), and whether other transcription factors colocalize with PPARγ to enhance or antagonize its activity.
Here, we used ChIP followed by DNA hybridization to whole-genome tiling arrays (ChIP–chip) to determine the PPARγ cistrome in mouse 3T3-L1 adipocytes. We identified 5299 binding regions, with a false discovery rate of 1%, most of which are novel and located in distal intergenic regions and introns rather than proximal promoters. The vast majority of regions bound by PPARγ are also bound by RXRα and contain the consensus DR1 element. Surprisingly, consensus C/EBP-binding motifs were identified within >90% of PPARγ recruitment sites, and direct ChIP–chip analysis confirmed colocalization of C/EBPα at the majority of PPARγ-binding regions. Examination of PPARγ and C/EBPα binding near genes up-regulated in differentiation revealed that 60% of the genes are bound by both factors, whereas 3% of the genes are bound by PPARγ only and 25% by C/EBPα alone. Furthermore, depletion of PPARγ and C/EBP factors in mature adipocytes led to synergistic decreases in expression of common target genes. This suggests that the mechanism by which PPARγ and C/EBP factors cooperatively orchestrate adipocyte differentiation involves binding to a largely overlapping set of gene targets.
Results
Identification and validation of novel PPARγ-binding sites
Genome wide ChIP–chip for PPARγ was employed on 3T3-L1 adipocytes harvested at day 10 post-hormonal induction of adipogenesis using standard techniques. The antibody against PPARγ was demonstrated to be specific (Supplemental Fig. S1A), and to enrich for known PPARγ target sites on the Fabp4/aP and Cd36 genes relative to negative control sites (Supplemental Fig. S1B). The ChIP DNA was amplified by 25 cycles of ligation-mediated PCR (Lee et al. 2006), and analysis of the amplified DNA at the Fabp4/aP and Cd36 sites indicated that no major bias was introduced by this procedure (Supplemental Fig. S1C). Three biological replicates for PPARγ and control IgG were hybridized to the whole-genome Mouse Tiling 2.0R Array Set (Affymetrix), and the data were analyzed using the model-based analysis of tiling arrays (MAT) (Carroll et al. 2006; Johnson et al. 2006), using the cutoffs of false discovery rate ≤1% and enrichment of PPARγ signal over IgG equal to or greather than twofold. This analysis identified 5299 unique regions of ∼1000-base-pair (bp) length, including known sites at Fabp4/aP2, Cd36, Lipe/Hsl, Olr1, and Me1 (Supplemental Table 1).
To validate the results of the PPARγ ChIP–chip, PPARγ enrichment was assayed by ChIP-quantitative PCR (QPCR) at 95 novel locations; 92 of these were true positives, suggesting an actual false discovery rate of ∼3%. Fifteen of the sites were also tested by ChIP-QPCR with two different PPARγ antibodies, which led to similar results as the original antibody used for the arrays (Supplemental Fig. S2). As additional validation, one of these antibodies was used for ChIP–chip on custom “PPARγ-binding site” arrays that densely tile 1431 randomly selected PPARγ-binding regions. Of those, 1370 (95.7%) were enriched using the alternative antibody on this novel chip platform. Taken together, these data indicate that the vast majority of the newly discovered sites are indeed bound by endogenous PPARγ in adipocytes.
To test the effect of ligand, PPARγ ChIP–chip was performed on adipocytes treated with 1 μM rosiglitazone for 24 h, using an array that interrogates mouse chromosomes 6, 8, and 16. Using normalization to IgG and a statistical cutoff of FDR ≤5%, 185 binding regions were found, of which only seven had not been previously identified in the genome-wide PPARγ ChIP–chip performed in the absence of exogenous ligand (data not shown). These seven sites were located either in gene-poor regions or near nonadipocyte genes and had low enrichment values, suggesting that they may be false positives. Indeed, ChIP-QPCR analysis of rosiglitazone-treated adipocytes at the seven sites did not reveal substantial PPARγ enrichment over IgG (data not shown). These findings indicate that there is little or no additional binding of PPARγ upon exogenous ligand stimulation, which is consistent with reported in vitro data indicating that DNA binding by PPARγ is ligand-independent (Li and Glass 2004; Lehrke and Lazar 2005).
Location of novel PPARγ-binding regions relative to known genes
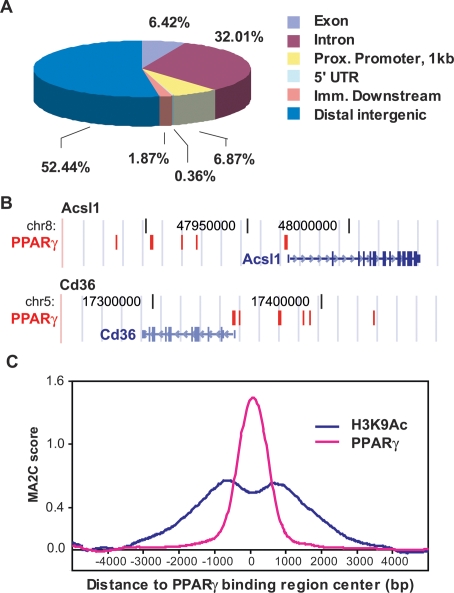
The cis-regulatory element annotation system, CEAS (Ji et al. 2006), was next employed to map the novel PPARγ-binding regions relative to annotated genes in the mouse genome. This analysis revealed that <7% of the genomic locations of PPARγ binding in adipocytes are at proximal promoters, defined here as <1 kb from the TSS (Fig. 1A). Expanding the definition of proximal promoter to 10 kb from the TSS increased the percentage of binding regions falling in this category only to 12.6% (670 out of 5299 sites). By contrast, >50% of the sites fall within distal intergenic regions (defined as >1 kb 5′ from the TSS, and >1 kb 3′ from the end of the gene), and many other sites (32%) are located in introns (Fig. 1A). Importantly, many regions of PPARγ binding are clustered such that a single gene may have multiple sites in its proximity, as can be seen for the Acsl1 (acyl-CoA synthetase 1) and Cd36 genes in Figure 1B.
Figure 1.
Location analysis of PPARγ-binding sites. (A) PPARγ-binding regions were mapped relative to their nearest RefSeq genes using CEAS (Ji et al. 2006). Proximal (prox.) promoter was defined as ≤1 kb upstream from the TSS. Immediate (imm.) downstream was defined as ≤1 kb downstream from the 3′ end of the gene. Distal intergenic refers to all locations outside the boundaries of a gene and the 1 kb flanking the gene on either end. (UTR) Untranslated region. (B) PPARγ-binding regions are frequently clustered around target genes. Two PPARγ target genes, Cd36 and Acsl1, are shown in their native chromosomal locations according to the February 2006 Mouse Genome Assembly (mm8) in the UCSC Genome Browser (http://genome.ucsc.edu). Red blocks represent regions of enriched PPARγ-binding signal. Vertical lines within the genes represent exons, horizontal lines represent introns, and arrowheads represent the direction of transcription. (C) Enrichment of acetylation at Lys 9 of histone 3 (H3K9Ac) in the regions of PPARγ binding. Shown are the average ChIP–chip profiles for PPARγ and H3K9Ac across 740 PPARγ-binding regions located >10 kb from a TSS. MA2C score refers to the enrichment at each location along the 10 kb distance that was tiled on the custom array for each region.
Functionality of novel PPARγ-binding sites
To assess the functionality of novel PPARγ-binding sites uncovered by ChIP–chip, 12 PPARγ enrichment regions were subcloned into a luciferase reporter plasmid, upstream of an SV40 minimal promoter. Upon transfection together with PPARγ and RXRα expression vectors into 293T cells, nine of the 12 reporters displayed PPARγ-dependent activity (Supplemental Fig. S3A). The sites were selected to represent a variety of distances to the closest TSS, ranging from −134 to +44 kb. No correlation was found between distance and activity. Three of the reporter constructs were analyzed further by electroporation into mature 3T3-L1 adipocytes, and all three were able to drive luciferase activity (Supplemental Fig. S3B). These findings suggest that many of the novel PPARγ-binding sites are potentially functional, including sites that are >100 kb away from the TSS.
To investigate the relationship between distal PPARγ binding and histone modification, ChIP–chip for acetylated Lys 9 on histone 3 (H3K9ac) was performed using a custom array containing 740 PPARγ-binding sites located >10 kb from a TSS. H3K9Ac, which is a well-documented signature of enhancers (Roh et al. 2007), was markedly enriched in the vicinity of PPARγ binding (Fig. 1C). Furthermore, H3K9Ac occupancy was increased in 67% (498 out of 740) of the PPARγ-binding regions in adipocytes compared to preadipocytes (P = 1.30e–31 by paired t-test). Thus, the adipocyte specificity and physical proximity of H3K9Ac enrichment to PPARγ binding suggest a functional relationship.
The vast majority of PPARγ binding occurs along with RXRα
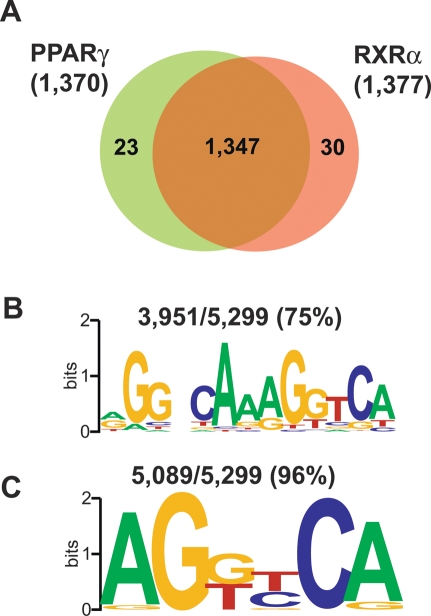
Although it is believed that PPARγ binds DNA as a heterodimer with RXRα, it is unclear whether this pattern of binding applies to all PPARγ sites in living cells. ChIP–chip for PPARγ and RXRα using the custom “PPARγ-binding site” arrays described above revealed nearly identical binding patterns such that of the 1370 locations bound by PPARγ on these arrays, 1347 (98.3%) also had binding for RXRα (Fig. 2A). The specificity of the RXRα ChIP–chip was confirmed with an additional antibody raised against a different region of the protein, which demonstrated that 96% of the RXRα-binding sites identified initially were also enriched with the second antibody (Supplemental Fig. S4A). The RXRα ChIP–chip results were further validated by ChIP-QPCR (Supplemental Fig. S4B). As an important control, only RXRα was recruited to the LXR response element in the fatty acid synthase promoter (Joseph et al. 2002; Matsukuma et al. 2007), which PPARγ does not bind (Supplemental Fig. S4B), indicating that the colocalization observed on PPARγ sites is not due to antibody cross-reactivity.
Figure 2.
RXRα heterodimerization and DR1 enrichment at novel PPARγ-binding sites. (A) Overlap in binding between PPARγ and RXRα across 1431 PPARγ-binding regions identified previously in the genome-wide search and interrogated in the custom “PPARγ-binding site” arrays. Shown in parentheses is the number of enriched regions for each antibody. (B,C) Enriched motif analysis of the PPARγ sites using TRANSFAC and JASPAR PWMs. (B) A DR1-like element was found in 75% of the sites. (C) Ninety-six percent of the novel PPARγ-binding regions contain at least the half site of the consensus PPARγ response element.
PPARγ binding occurs primarily at DR1 consensus sites
Since PPARγ and RXRα colocalize at the PPARγ-binding regions, we examined whether the sites contain DR1 elements. For this purpose we scanned the sequences of the PPARγ-binding regions using position weight matrices (PWMs) for known transcription factors from the TRANSAC and JASPAR databases (see Materials and Methods), with the entire mouse genome as a background model. The highest scoring motifs in this analysis represented different DR1 matrices enriched 3.7–9.5-fold over what is expected from the frequency of the motifs in the genome. Of the 5299 PPARγ-binding regions that we identified, 3951 (75%) contain a DR1 element (Fig. 2B), and >95% of all regions contain a consensus half-site (Fig. 2C). Together with the high degree of colocalization with RXRα, this suggests that in living cells the heterodimer binds to DR1 elements, although one of the half-sites may be highly degenerate. Importantly, the DR1 element is predicted to occur in excess of 600,000 times in the mouse genome, whereas the PPARγ cistrome described here contains <1% of that number as sites of actual recruitment. Thus, there is selectivity for the DR1 sites that are actually occupied by PPARγ.
Enrichment of the C/EBP-binding motif at the majority of novel PPARγ-binding regions
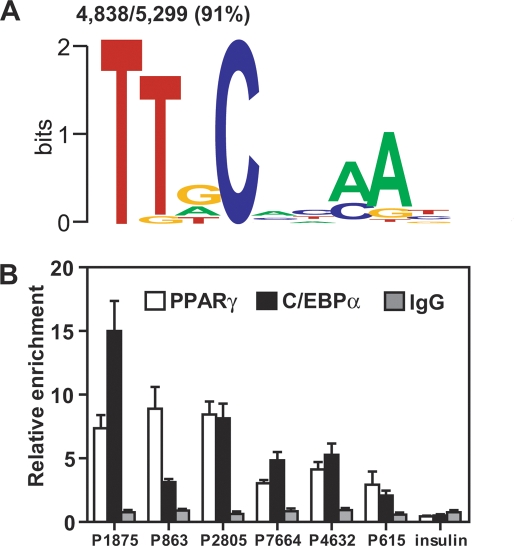
Having identified the DR1 motif at PPARγ-binding sites, we next asked whether binding elements for other transcription factors were present nearby. This analysis determined that the consensus motif for C/EBP factors was highly enriched within the PPARγ-binding regions such that 91% of the genomic regions bound by PPARγ contain at least one C/EBP motif (Fig. 3A). ChIP-QPCR for the most abundant C/EBP isoform in adipocytes, C/EBPα, demonstrated that C/EBPα was indeed present near a number of PPARγ-binding regions with C/EBP motifs identified computationally (Fig. 3B).
Figure 3.
C/EBP response elements are found at the vast majority of PPARγ-binding regions. (A) Enrichment of C/EBP motifs. The PPARγ-binding locations were mined as in Figure 2, B and C. Shown is the logo of one C/EBP PWM among several that were enriched. (B) ChIP-QPCR analysis for C/EBPα and PPARγ at several novel PPARγ-binding regions that were computationally predicted to contain C/EBP response elements (see Supplemental Tables 1 and 5 for identification and location of the PPARγ-binding sites). An area of the insulin gene served as negative control for PPARγ and C/EBPα binding. Data are normalized to a site in the Arbp/36b4 gene and presented as mean ± SE, n = 3.
Genome-wide analysis of C/EBPα binding in adipocytes reveals widespread overlap with PPARγ
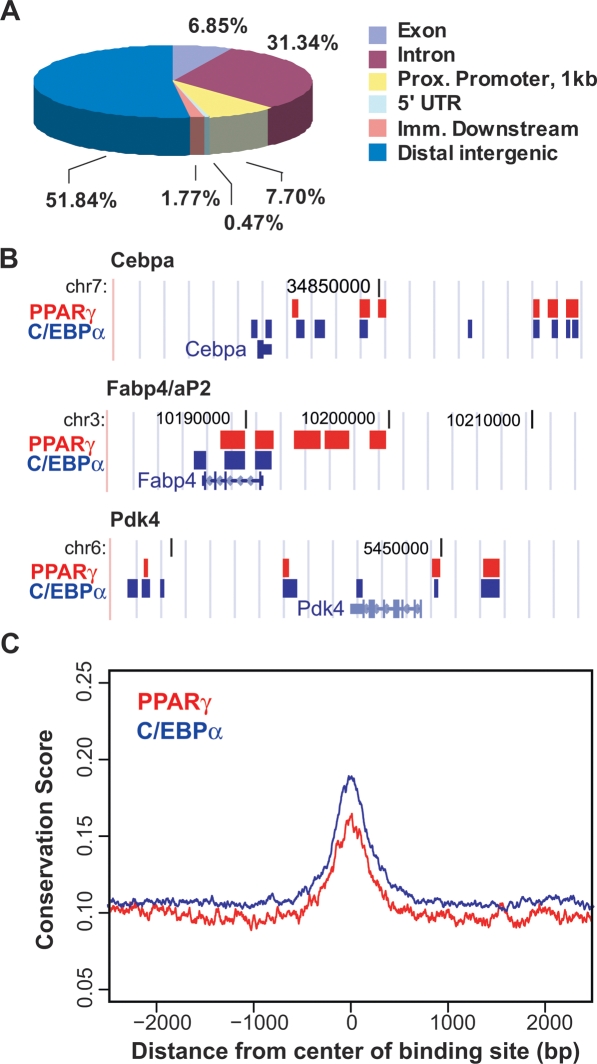
The preceding analyses suggested that C/EBPα binding in adipocytes overlaps that of PPARγ to an extraordinary degree. This was directly tested by genome-wide ChIP–chip to determine the C/EBPα cistrome in adipocytes. Two biological replicates for C/EBPα ChIP–chip were analyzed using MAT, normalizing the data to IgG controls. Using the stringent cutoffs of false discovery rate ≤1% and enrichment of C/EBPα signal over IgG twofold or more, C/EBPα binding was detected at 16,760 unique locations (Supplemental Table 2). Analysis of the C/EBPα data set relative to known genes revealed that, like PPARγ, C/EBPα was predominantly localized to distal intergenic regions and introns, with relatively few sites present in proximal promoters (Fig. 4A). The distribution of binding regions relative to TSSs was also very similar for the two factors (Supplemental Fig. S5). Examination of binding at the Cebpa, Fabp4/aP2, and Pdk4 (pyruvate dehydrogenase kinase, isoenzyme 4) genes illustrates that, as for PPARγ, C/EBPα binding occurs in clusters (Fig. 4B). Finally, CEAS was used to show that there is a high degree of conservation of C/EBPα and PPARγ sites among higher eukaryotes (Fig. 4C), suggesting that the findings are likely to be relevant across species.
Figure 4.
Location analysis of C/EBPα binding. (A) Mapping of C/EBPα-binding regions on genome-wide scale relative to RefSeq mouse genes. The analysis was performed as in Figure 1A. (B) C/EBPα and PPARγ binding in relation to three target genes, Cebpa, Fabp4/aP2, and Pdk4. The genes are shown as in Figure 1B, in the native chromosomal locations according to the mm8 Assembly in the UCSC Genome Browser (http://genome.ucsc.edu). Blue blocks represent regions of C/EBPα enriched ChIP signal, while red blocks represent PPARγ enrichment. (C) Average plot for conservation of all PPARγ- and C/EBPα-binding regions among higher eukaryotes.
With the elucidation of the PPARγ and C/EBPα adipocyte cistromes, the extent to which their binding overlapped could be assessed in an unbiased manner. PPARγ and C/EBPα binding overlap was generated in the University of California at Santa Cruz (UCSC) Genome Browser (http://genome.ucsc.edu) (Kent et al. 2002; Karolchik et al. 2004), such that sites were considered overlapping if there was at least 1 bp in common between the binding regions, which average ∼1000 bp in length. Remarkably, and in agreement with the bioinformatics predictions, C/EBPα binds nearby at >60% of the locations bound by PPARγ (Fig. 5A). Several regions expected to have binding for both PPARγ and C/EBPα or each of the factors alone were validated using ChIP-QPCR with two different C/EBPα antibodies, ruling out the possibility of cross-reactivity of the C/EBPα antibody with PPARγ (Supplemental Fig. S6).
Figure 5.
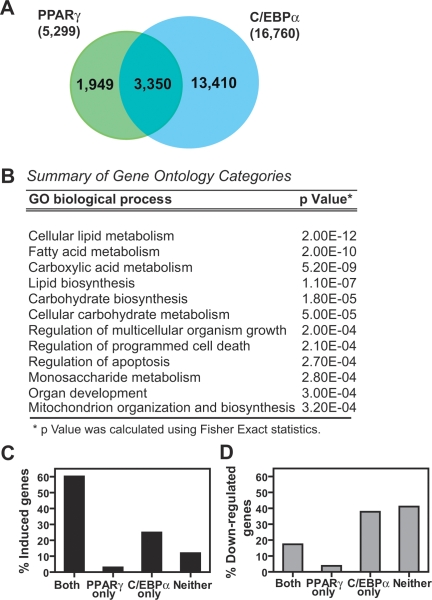
Extent of PPARγ and C/EBPα binding overlap and its association with gene expression during adipocyte differentiation. (A) Overlap between the binding of PPARγ and C/EBPα on genome-wide scale. Shown are the numbers of regions found to be shared by the two factors—i.e., having at least 1 bp in common—or unique to each factor. (B) Summary of gene ontology (GO) categories of the nearest genes to regions with overlapping PPARγ and C/EBPα binding. In this analysis, only binding regions whose nearest gene was within 50 kb were considered. (C) Association between factor binding and genes induced in adipogenesis. Shown are the percent genes up-regulated more than threefold and containing binding sites within 50 kb of the gene start site for both factors (Both), PPARγ alone, C/EBPα alone, or neither factor (Neither). (D) The association between genes down-regulated in adipocyte differentiation and PPARγ and C/EBPα binding was analyzed as in C.
The functional significance of nearby binding of PPARγ and C/EBPα was addressed by examination of the genes located near overlapping regions. For each PPARγ region that overlaps with C/EBPα binding, the nearest gene was determined as well as the distance from the TSS to the center of the PPARγ-binding region. Gene Ontology analysis of the closest genes identified by this approach revealed strong enrichment of metabolic processes, such as fatty acid and carboxylic acid metabolism, lipid biosynthesis, carbohydrate biosynthesis, and others (Fig. 5B). The analysis shown was performed for the 1996 sites whose nearest gene was within 50 kb, and was minimally affected by changing the cutoff to 20 or 100 kb. These findings suggest that both PPARγ and C/EBPα may be necessary for expression of adipocyte-specific genes.
To explore this possibility further, PPARγ and C/EBPα binding was examined relative to expression of genes regulated during adipogenesis. Microarray profiling of preadipocytes and mature adipocytes identified 834 genes up-regulated threefold or more and 877 genes down-regulated threefold or more (P ≤ 0.001) during the differentiation process (Supplemental Tables 3, 4). Remarkably, >60% of the up-regulated genes had binding for both PPARγ and C/EBPα within 50 kb of their TSSs, while only 3% of the genes were bound by PPARγ alone (Fig. 5C). By contrast, the down-regulated genes were not enriched for binding of both factors (Fig. 5D), indicating that the colocalization of PPARγ and C/EBPα is unique to genes that are highly induced in adipogenesis. Furthermore, there was little change in the fraction of genes bound by C/EBPα alone (Fig. 5C,D). Importantly, increasing the threshold distance from 50 kb up to 100 kb in 10 kb intervals did not significantly alter the percentage of genes with binding sites (Supplemental Fig. S7), suggesting that no bias had been introduced by setting the distance at 50 kb.
C/EBPs are required for the expression of genes bound by PPARγ and C/EBPα
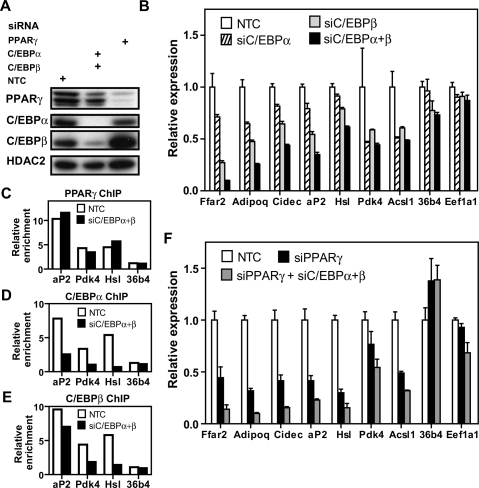
To assess whether binding of C/EBPα is required for the expression of genes bound by both PPARγ and C/EBPα, each transcription factor was depleted from mature adipocytes using siRNA (Fig. 6A). Surprisingly, knockdown of C/EBPα produced small changes in the mRNA levels of target genes such as adiponectin and aP2, which are well-characterized C/EBPα targets (Fig. 6B; Christy et al. 1989; Park et al. 2004). We hypothesized that this may be due to compensation by C/EBPβ, which has been reported to be present and active in differentiated adipocytes (MacDougald et al. 1995). Indeed, ChIP-QPCR in mature adipocytes revealed C/EBPβ binding to several newly discovered and previously known C/EBP targets (Supplemental Fig. S8A). This was confirmed on a larger scale using the custom “PPARγ-binding site” arrays described earlier. C/EBPα binding was found near many of the PPARγ-binding sites, such that 1117 out of 1370 PPARγ-occupied regions also had C/EBPα binding, as can be seen on the Cd36 gene (Supplemental Fig. S8B). ChIP–chip for C/EBPβ and C/EBPα showed nearly identical binding profiles, with 1140 out of 1150 (99.1%) C/EBPα locations also bound by C/EBPβ (Supplemental Fig. S8C,D). The C/EBPα and C/EBPβ antibodies were shown to be specific by immunoblotting, ruling out an artifact due to antibody cross-reactivity (Supplemental Fig. S9A). The specificity of C/EBPα and C/EBPβ binding was confirmed further by ChIP–chip using additional antibodies and the custom array containing 740 PPARγ-binding regions. Importantly, the vast majority of sites initially identified on this array for C/EBPα and C/EBPβ were validated with the new antibodies (Supplemental Fig. S9B,C), indicating that the binding results are robust regardless of the antibody used for ChIP.
Figure 6.
Effects of C/EBP depletion on expression of genes on which PPARγ and the C/EBPs colocalize. (A) Immunoblot analysis demonstrating the efficiency of siRNA-mediated knockdown of C/EBPα, C/EBPβ, PPARγ, or nontarget contol (NTC). HDAC2 represents a loading control. (B) QPCR analysis of gene expression following 24 h of siRNA-mediated knockdown. All of the genes shown were found to have binding sites for PPARγ and C/EBPα, except eukaryotic translation elongation factor 1 α 1 (Eef1α1) and 36b4, which were used as controls. Data were normalized to the housekeeping gene Pabpc1, and are presented as mean ± SE, n = 3. (C–E) ChIP-QPCR analysis of factor binding at several target sites following 24 h of C/EBPα and β or NTC knockdown. Data are normalized to a nontarget genomic site and IgG enrichment. Shown is a representative ChIP-QPCR experiment. (C) PPARγ enrichment. (D) C/EBPα enrichment. (E) C/EBPβ enrichment. (F) QPCR analysis of gene expression following 24 h of siRNA-mediated knockdown of PPARγ alone and together with C/EBPα and C/EBPβ. Analysis was performed as in B above.
Thus, C/EBPβ and C/EBPα are bound to C/EBP sites in mature adipocytes, suggesting that these factors act redundantly. Because knockdown of C/EBPβ gave similar results in target gene expression as C/EBPα (Fig. 6B), the effect of simultaneous depletion of C/EBPα and C/EBPβ was examined. Importantly, the expression levels of several genes shown to be co-occupied by PPARγ and C/EBPα were substantially reduced in the absence of both C/EBPα and C/EBPβ (Fig. 6B). To rule out the possibility that the small decrease in PPARγ levels produced by C/EBP knockdown (see Fig. 6A) is responsible for the effects on gene expression, we examined the recruitment of PPARγ to a number of binding sites in conditions of C/EBPα and β depletion. There was little or no decrease in occupancy compared to control cells (Fig. 6C), suggesting that the transcriptional activity of PPARγ is unlikely to be altered under these conditions. On the other hand, C/EBPα and β knockdown produced the expected decreases in occupancy by these factors at the same target sites (Fig. 6D,E). Similarly, PPARγ knockdown led to a substantial reduction of PPARγ recruitment (Supplemental Fig. S10A) and very little change in C/EBPβ occupancy (Supplemental Fig. S10B), although C/EBPα binding was somewhat decreased (Supplemental Fig. S10C). As expected, PPARγ knockdown led to down-regulation of target genes within 24 h of siRNA electroporation (Fig. 6F). However, the effects on gene expression were substantially larger when PPARγ and both C/EBPs were simultaneously depleted (Fig. 6F), suggesting that the factors have synergistic roles in activating transcription. Taken together, these data demonstrate that the C/EBPs not only bind near PPARγ at genes induced during adipogenesis, but also cooperate with PPARγ to regulate the expression of these genes in adipocytes.
Discussion
In this study, an unbiased approach was taken to characterize the PPARγ cistrome in mouse 3T3-L1 adipocytes. Five thousand two hundred ninety-nine genomic binding sites were identified with a high degree of confidence. Investigation of the mechanism by which PPARγ associates with these sites led to a number of discoveries about global PPARγ function. PPARγ binds primarily far from TSS, and typically associates with DR1 elements as a heterodimer with RXRα. Importantly, a new level of collaboration between PPARγ and C/EBP factors was uncovered, which involves colocalization at a surprisingly large number of target genes. Thus, the current study advances understanding of PPARγ-dependent gene regulation in adipocytes by identifying a large number of novel binding sites and potential gene targets. It also highlights the fact that rather than functioning separately, PPARγ and other factors such as the C/EBPs are likely part of a complex transcriptional network that regulates gene expression in spatially and temporally coordinated manner.
PPARγ binding occurs primarily in distal intergenic regions and introns, with few sites localizing to proximal promoters. This distribution is consistent with what has been shown for other transcription factors, including estrogen receptor (Carroll et al. 2006), androgen receptor (Bolton et al. 2007), and FoxA1 (Lupien et al. 2008). Such findings underscore the advantages of whole-genome approaches and the limitations of current methods searching for factor binding within gene promoters, including promoter bashing and promoter tiling arrays. In fact, a recent study involving adipocyte PPARγ ChIP and hybridization to a proximal promoter tiling array discovered only 167 binding sites while interrogating 16,592 promoters (Nakachi et al. 2008).
A potential concern about sites located tens and hundreds of kilobases away from TSS is their functionality. However, a large number of PPARγ-binding regions located >10 kb from a TSS were enriched for H3K9Ac in adipocytes but not preadipocytes, suggesting that PPARγ bound at such sites may be recruiting histone acetyltransferases. Furthermore, the ability of 12 PPARγ-binding regions tested to function as enhancer elements was independent of distance to the nearest gene in the context of a minimal promoter. Similar results have been reported for other nuclear receptors such as vitamin D (Kim et al. 2007), estrogen (Carroll et al. 2005), and glucocorticoid receptors (Anderson et al. 2007). To explain the abundance of distal binding sites, it has been proposed that transcription factors bound to distal sites direct DNA looping such that coactivators and chromatin remodelers at the distal enhancers are brought in proximity to TSS of target genes, facilitating the recruitment of polymerase (West and Fraser 2005). Such communication between enhancers and promoters has been demonstrated for many factors including androgen receptor (Wang et al. 2005), and estrogen receptor (Carroll et al. 2005). Similarly, PPARγ may also be able to orchestrate DNA looping as a mechanism of long-range gene regulation, which would account for the presence of large numbers of distal PPARγ-binding sites. Further understanding of PPARγ action will involve characterizing its role in long-range chromatin interactions and elucidating the mechanism by which such high-order chromatin structures lead to active transcription.
Using a custom array of PPAR-binding sites, RXRα recruitment was observed at ∼98% of adipocyte PPARγ-binding locations. This strongly suggests that PPARγ heterodimerizes with RXRα at most if not all of its binding sites in adipocytes, although binding of RXR as a homodimer at some locations cannot be excluded (IJpenberg et al. 2004). The PPARγ/RXRα binding occurred predominantly at DR1 elements, although some of these sites vary substantially from the consensus. The composite motif derived from in vivo DR1 elements illustrates that the spacer position favors adenosine, and the 3′ half-site is more highly conserved. This is consistent with observations in vitro that RXRα, which occupies the 3′ half-site (DiRenzo et al. 1997), has higher sequence stringency compared to PPARγ occupying the 5′ site (Temple et al. 2005). Although previous studies have suggested the existence of a conserved extended 5′ sequence (IJpenberg et al. 1997; Temple et al. 2005), we did not find a strong preference. This does not rule out the possibility that within relatively degenerate DR1 elements there is a subset for which the flanking positions are better conserved, for example, to enhance low-affinity PPARγ binding (IJpenberg et al. 1997).
A surprising discovery in this study was that C/EBP motifs are present within 91% of the PPARγ-binding regions. Genome-wide ChIP–chip was used to demonstrate that C/EBPα binding overlaps with >60% of the PPARγ target locations. Gene ontology analysis of the nearest genes for such overlapping sites revealed a substantial enrichment for lipid metabolism processes. Combining the binding data with an expression microarray from adipocyte differentiation revealed that 60% of the up-regulated genes have binding sites for both PPARγ and C/EBPα within 50 kb of the TSS. This suggests that the colocalization of PPARγ and C/EBPα occurs specifically at genes that are activated in differentiation and participate in major adipocyte-specific functions such as triglyceride synthesis and lipid storage. Consistent with previous reports (MacDougald et al. 1995), we also found that C/EBPβ continues to occupy C/EBP sites even in mature adipocytes. Knockdown of PPARγ or the combination of C/EBPα and β clearly showed that both sets of factors are essential for optimal expression of adipocyte genes. Furthermore, simultaneous knockdown of the three factors even more dramatically decreases the expression of a number of genes, suggesting that there may be a large class of genes for which PPARγ and the C/EBPs play synergistic roles for activation.
These findings suggest a much more prominent role for C/EBP factors in mature adipocytes than has been appreciated. Because the C/EBP cistrome in adipocytes was previously unknown, it was believed that C/EBPβ function is limited to early adipogenesis, while the role of C/EBPα is to maintain PPARγ expression and insulin sensitivity in mature cells (Wu et al. 1999; Elberg et al. 2000; Rosen et al. 2002). In contrast, the present data suggest that the majority of adipocyte genes are not regulated by PPARγ alone but rather require C/EBPα and C/EBPβ binding as well. The extent of this collaboration could not have been predicted from previous findings since until now only a small number of genes were known to have binding sites for both factors. Thus, by characterizing the widespread colocalization of the factors and its functional significance in metabolic gene expression, the current findings contribute to understanding the mechanism by which PPARγ and C/EBPs cooperatively regulate adipocyte biology.
Although the molecular details of this cooperation remain to be elucidated, it is possible that the ability of each factor to recruit coactivators and chromatin remodelers at adjacent sites leads to synergistic effects on transcriptional activation. Alternatively, the presence of one factor may facilitate binding of the other; for example, by opening chromatin and making binding sites accessible. In this sense, C/EBPβ, which is activated earlier in adipogenesis than either PPARγ or C/EBPα, may serve as a pioneer factor that directs changes in chromatin marks and nucleosome positioning allowing the other factors to bind when they are expressed. In fact, it has been proposed that C/EBPβ is bound to promoters of genes such as Cebpa prior to transcriptional activation, and it is associated with corepressors and histone deacetylases, which are displaced upon activation of PPARγ, allowing for gene expression to occur (Zuo et al. 2006). Finally, it is likely that PPARγ and the C/EBPs bind in proximity to other transcription factors that remain to be characterized and may enhance or antagonize transcriptional activity. Future studies are necessary to characterize the chromatin context in which PPARγ and C/EBP binding occurs, including histone modification profiles, binding site accessibility, as well as recruitment of cofactors, chromatin- and histone-modifying machinery, and other transcription factors.
Materials and methods
Cell culture
3T3-L1 preadipocytes were obtained from American Type Culture Collection and grown to confluence in growth medium consisting of high-glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum (U.S. Biotechnologies) and 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen). Two days post-confluence, differentiation medium was added, consisting of growth medium supplemented with 1 μM dexamethasone, 10 μg/mL bovine insulin, and 0.5 mM isobutyl-1-methylxanthine (Sigma). Cells were grown in differentiation medium for 3 d, followed by 2 d in growth medium with insulin, followed by growth medium only. 293T cells were grown in high-glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum (U.S. Biotechnologies), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen).
ChIP
Cells were cross-linked in 1% Formaldehyde (Fisher) for 10 min, followed by quenching with 1/20 volume of 2.5 M glycine solution, and two washes with 1× PBS. Nuclear extracts were prepared by dounce homogenizing in nuclear lysis buffer (20 mM HEPES, 0.25 M sucrose, 3 mM MgCl2, 0.2% NP-40, 3 mM β-mercaptoethanol, 0.4 mM PMSF, Complete protease inhibitor tablets from Roche). Chromatin fragmentation was performed by sonication in ChIP SDS lysis buffer (50 mM HEPES, 1% SDS, 10 mM EDTA), using Bioruptor (Diagenode). Proteins were immunoprecipitated in ChIP dilution buffer (50 mM Hepes/NaOH at ph 7.5, 155 mM NaCl, 1.1% Triton X-100, 0.11% Na-deoxycholate, 1 mM PMSF, Complete protease inhibitor tablet), using anti-C/EBPα antibodies (sc-61 and sc-9314, Santa Cruz Biotechnologies), anti-PPARγ antibodies (sc-7196 and sc-1984, Santa Cruz Biotechnologies; 81b8, Cell Signaling Technologies), anti-C/EBPβ antibodies (sc-746x and sc-150x, Santa Cruz Biotechnologies), anti-RXRα antibodies (sc-553 and sc-774, Santa Cruz Biotechnologies) or nonspecific rabbit IgG control (Sigma). Cross-linking was reversed overnight at 65°C, and DNA isolated using phenol/chloroform/isoamyl alcohol. For ChIP-QPCR, enrichment was measured using Power SYBR Green PCR Mastermix and the PRISM 7500 instrument (Applied Biosystems). Analysis was performed by the standard curve method and normalization to a nontarget control region of the 36b4 or insulin genes. Primer sequences used for QPCR analysis can be found in Supplemental Table 5. For acetylation ChIP, an anti-H3K9Ac antibody (06-942, Upstate Biotechnologies) and anti-histone 3 (H3) antibody (ab1791, Abcam) were used and the ChIP was performed as described previously (Steger et al. 2008).
ChIP–chip
Whole-genome ChIP–chip was performed using Mouse Tiling 2.0R Array Set (Affymetrix), following ligation-mediated PCR (Lee et al. 2006), limited DNase I (Ambion) digestion to fragment DNA to average size of ∼200–300 bp, and labeling with biotin (Perkin Elmer). Arrays were hybridized, washed, and scanned as per manufacturer’s instructions. The custom “PPARγ-binding site” arrays contain 1431 PPARγ-binding regions from the whole-genome data set. One of the arrays contains 740 distal regions, ≥10 kb away from the TSS of the nearest gene, while the second array contains 691 PPARγ-binding regions that are located within 10 kb of the nearest gene or within the exons of a gene. Regions of enriched PPARγ signal over IgG based on the genome-wide study were centered within 10 kb of genomic sequence. Their nonrepetitive chromosomal sequence based on the February 2006 Mouse Genome Assembly (mm8) was retrieved from the UCSC Genome Browser (http://genome.ucsc.edu), and tiled with overlapping 60 mer oligonucleotides. The arrays were printed by Agilent (http://www.agilent.com). For transcription factors, amplification of the ChIP, and input DNA was carried out as above, followed by labeling with Cy5 (ChIP DNA) and Cy3 (input DNA). Hybridization was performed according to manufacturer’s protocol and as previously described (Steger et al. 2008). ChIP–chip on the custom arrays was performed in replicate for PPARγ ChIP with 81b8 antibody, RXRα ChIP with sc-553 antibody, and for IgG. One hybridization was performed for each of the following ChIP experiments: RXRα ChIP with sc-774 antibody, C/EBPα with sc-61 antibody, C/EBPα with sc-9314 antibody, C/EBPβ with sc-150 antibody, and C/EBPβ with sc-746 antibody.
ChIP–chip analysis
Whole-genome arrays were analyzed with MAT (Johnson et al. 2006), with probes remapped to the February 2006 Mouse Genome Assembly (mm8) using xMAN (Li et al. 2008). The threshold cutoffs for binding regions were FDR ≤1%, and enrichment of PPARγ or C/EBPα over IgG twofold or more. PPARγ and C/EBPα binding overlap was generated in the UCSC Genome Browser (http://genome.ucsc.edu) such that sites were considered overlapping if there was at least 1 bp in common between the binding regions. Screen shots of PPARγ- and C/EBPα-binding regions relative to individual RefSeq genes in their native chromosomal locations were obtained from the UCSC Genome Browser. Custom tiling-arrays were analyzed with MA2C (Song et al. 2007), after reformatting of Agilent data files. For transcription factors, the threshold cutoffs for binding regions were a P-value ≤10−3. If the center 1 kb of any given 10-kb tiled region overlapped with an MA2C peak by at least 100 bp, the region was regarded as bound. To generate the average H3K9Ac profile, the H3K9Ac signal at each probe was first normalized to H3 signal. All 10-kb regions were aligned at the center where PPARγ binding occurs, followed by calculation of the average signal for each location. A 500-bp sliding window was used to smooth the profiles and the data were normalized to the average signal in the 1 kb at each end. For comparison of adipocyte and preadipocyte acetylation, the average H3K9Ac signal for the center 2 kb of each region was obtained and normalized to the average H3 signal in the same 2 kb area and to the signal in the 1 kb at each end. The average signals generated for adipocytes and preadipocytes for each region were compared, and paired t-test was used to determine whether overall the increase in acetylation signal for adipocytes was significant.
Mapping of binding regions to known genes and conservation analysis
The distribution of PPARγ- and C/EBPα-binding sites relative to known genes was generated using CEAS (Ji et al. 2006) according to gene coordinates in the February 2006 Mouse Genome Assembly (mm8). For each data set, the ChIP regions were aligned at the center and uniformly expanded to 2500 bp at each end, and at each position the average phastCons score (Siepel et al. 2005) retrieved from UCSC Genome Browser (http://genome.ucsc.edu) was generated for the average conservation plot.
Enriched motif analysis
An updated version of CEAS (Ji et al. 2006) was used to obtain the enriched transcription factor motifs located in ChIP regions. The 526 well-defined PWMs used in the analysis were from TRANSFAC (Matys et al. 2003) and JASPAR (Sandelin et al. 2004). The enrichment of motifs within the PPARγ ChIP–chip data set was calculated relative to the frequency of motif occurrence in the entire mouse genome.
Nearest gene analysis
For each PPARγ- or C/EBPα-binding region, the nearest gene was determined, and the distance from the center of the binding region to the TSS of the gene was calculated based on the February 2006 Mouse Genome Assembly (mm8). Gene Ontology (GO) analysis was performed using DAVID (http://david.abcc.ncifcrf.gov) (Dennis et al. 2003), by examining the biological processes in which nearest genes are involved, using RefSeq mRNA IDs and the entire mouse genome as a background model. P-values were calculated using Fisher Exact statistics. The GO analysis was performed for the 1996 PPARγ regions with overlapping C/EBPα binding and distance to the nearest gene ≤50 kb. Changing the cutoff to 20 or 100 kb did not substantially alter the GO analysis outcome.
Association between gene expression changes during adipocyte differentiation and PPARγ and C/EBPα binding
Genes having PPARγ or C/EBPα binding within certain distance were defined as those having at least one binding region within the distance relative to the TSS. For each category of differentially expressed genes, the percentage of genes having PPARγ- or C/EBPα-binding regions within 10–100 kb was calculated. The location distributions of PPARγ or C/EBPα sites relative to the TSS of all RefSeq genes were also calculated.
Plasmids, transfections, and luciferase reporter assays
Twelve novel PPARγ-binding sites were selected based on distance from nearest TSS. Each ∼1000-bp enriched region was amplified from genomic NIH 3T3 DNA (primer sequences in Supplemental Table 6) using PfuTurbo Hotstart Polymerase (Stratagene) and cloned into the pCR-Blunt II-TOPO vector (Invitrogen). Following restriction enzyme digest, the regions were inserted into the multiple cloning site of the pGL3-Promoter vector (Promega). 293T cells were transfected overnight with 100 ng of pGL3-Promoter construct, 3 ng of pRL-CMV renilla vector, as well as 50 ng each of pCMX-PPARγ and pCMX-RXRα, or 100 ng of pCMX empty in 24-well plates using Lipofectamine 2000 (Invitrogen) according to manufacturer’s protocol. Luciferase activity was measured using Dual Luciferase Reporter Assay (Promega), normalizing firefly luciferase to renilla activity. For C/EBP factor overexpression, 293T cells were transfected overnight in 12-well plates with 50 ng of pSG5-C/EBPα or pSG5-C/EBPβ, or an empty pSG5 vector. For 3T3-L1 transfections, mature adipocytes were detached using Trypsin (Invitrogen) and Collagenase (Roche), washed, resuspended in Buffer V (AMAXA), and mixed with siRNA oligo (Dharmacon) or plasmid DNA. For luciferase assays, 2 μg of pGL3-Promoter construct and 6 ng of renilla vector were used per electroporation. For knockdown experiments, 2 or 3 nmol of siRNA oligo per transcription factor was used. Where necessary, the electroporation was supplemented with nontarget control (NTC) oligo in order to maintain equivalent quantity of siRNA across treatment groups. All electroporations were performed using Nucleofector II and Cell Line Nucleofector Kit V (AMAXA). The sequences of the siRNA oligos used for transcription factor knockdown are as follows: PPARγ, CAACAGGCCUC AUGAAGAAUU; C/EBPα, CCUGAGAGCUCCUUGGUCAUU; C/EBPβ, GAAAAGAGGCGUAUGUAUAUU.
RNA isolation, QPCR, and gene expression profiling
RNA was isolated using RNeasy Mini Kit (Qiagen), followed by reverse transcription of 0.2–0.8 μg of RNA with the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) following manufacturer’s instructions. QPCR was performed using primers as described (Steger et al. 2008), Power SYBR Green PCR Mastermix (Applied Biosystems), and the PRISM 7500 instrument (Applied Biosystems). Analysis was performed using the standard curve method and normalization of all genes of interest to the housekeeping control Pabpc1. Gene expression profiling was carried out using the MOE430 version 2.0 Mouse Array (Affymetrix) by hybridizing RNA from preadipocytes and mature adipocytes in triplicate.
Gene expression analyses
The gene expression data were normalized and summarized with RMA algorithm (Irizarry et al. 2003) and an updated RefSeq probe set definition (Dai et al. 2005). Differentially expressed genes were determined by using a t-test with a P-value ≤10−3 and a fold change >3.
Immunoblotting
Cell protein was extracted on ice in cold whole-cell extract buffer (0.15 M NaCl, 0.05 M Tris at pH 7.4, 0.005 M EDTA, 0.5% NP-40) supplemented with Complete protease inhibitors (Roche). SDS-PAGE was performed using 4%–20% Tris-glycine gels (Invitrogen), followed by transfer to PVDF membranes (Invitrogen). The primary antibodies used for immunoblotting were as follows: anti-C/EBPα (sc-61, Santa Cruz Biotechnologies), anti-C/EBPβ (sc-150x, Santa Cruz Biotechnologies), anti-PPARγ (sc-7273, Santa Cruz Biotechnologies), anti-HDAC2 (sc-7899, Santa Cruz Biotechnologies), and anti-Ran (610,341, BD Biosciences). After incubation with horseradish peroxidase-conjugated secondary antibodies, blots were developed using the enhanced chemiluminescent substrate kit from Amersham.
Acknowledgments
We thank members of the Lazar laboratory for insightful discussions and reagents, Elisabetta Manduchi (University of Pennsylvania) for help with computational analysis, and Mathieu Lupien and Myles Brown (Dana-Farber Cancer Institute) for helpful discussion. This work was supported by NIH R01 DK4970 (to M.A.L.) and NIH R01 HG004069-02 (to X.S.L.).
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1709008.
References
- Anderson P.D., Mehta N.N., Wolfe M.L., Hinkle C.C., Pruscino L., Comiskey L.L., Tabita-Martinez J., Sellers K.F., Rickels M.R., Ahima R.S., et al. Innate immunity modulates adipokines in humans. J. Clin. Endocrinol. Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- Bolton E.C., So A.Y., Chaivorapol C., Haqq C.M., Li H., Yamamoto K.R. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes & Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Umek R.M., McKnight S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes & Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Carroll J.S., Liu X.S., Brodsky A.S., Li W., Meyer C.A., Szary A.J., Eeckhoute J., Shao W., Hestermann E.V., Geistlinger T.R., et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Carroll J.S., Meyer C.A., Song J., Li W., Geistlinger T.R., Eeckhoute J., Brodsky A.S., Keeton E.K., Fertuck K.C., Hall G.F., et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Chawla A., Schwarz E.J., Dimaculangan D.D., Lazar M.A. Peroxisome proliferator-activated receptor (PPAR) γ: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- Christy R.J., Yang V.W., Ntambi J.M., Geiman D.E., Landschulz W.H., Friedman A.D., Nakabeppu Y., Kelly T.J., Lane M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes & Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Chui P.C., Guan H.P., Lehrke M., Lazar M.A. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Invest. 2005;115:2244–2256. doi: 10.1172/JCI24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Wang P., Boyd A.D., Kostov G., Athey B., Jones E.G., Bunney W.E., Myers R.M., Speed T.P., Akil H., et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G.J., Ross S.E., MacDougald O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- Dennis G., Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C., Lempicki R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:3. doi: 10.1186/gb-2003-4-9-r60. [DOI] [PubMed] [Google Scholar]
- DiRenzo J., Soderstrom M., Kurokawa R., Ogliastro M.H., Ricote M., Ingrey S., Horlein A., Rosenfeld M.G., Glass C.K. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol. Cell. Biol. 1997;17:2166– 2176. doi: 10.1128/mcb.17.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberg G., Gimble J.M., Tsai S.Y. Modulation of the murine peroxisome proliferator-activated receptor γ 2 promoter activity by CCAAT/enhancer-binding proteins. J. Biol. Chem. 2000;275:27815–27822. doi: 10.1074/jbc.M003593200. [DOI] [PubMed] [Google Scholar]
- Freytag S.O., Paielli D.L., Gilbert J.D. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes & Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- Gearing K.L., Gottlicher M., Teboul M., Widmark E., Gustafsson J.A. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc. Natl. Acad. Sci. 1993;90:1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.L., Dalla Nora E., Vidal-Puig A.J. Mouse models of PPAR-γ deficiency: Dissecting PPAR-γ’s role in metabolic homoeostasis. Biochem. Soc. Trans. 2005;33:1053– 1058. doi: 10.1042/BST0331053. [DOI] [PubMed] [Google Scholar]
- Guan H.P., Ishizuka T., Chui P.C., Lehrke M., Lazar M.A. Corepressors selectively control the transcriptional activity of PPARγ in adipocytes. Genes & Dev. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J.K., Park B.H., Farmer S.R. A role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 2001;276:18464–18471. doi: 10.1074/jbc.M100797200. [DOI] [PubMed] [Google Scholar]
- IJpenberg A., Jeannin E., Wahli W., Desvergne B. Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J. Biol. Chem. 1997;272:20108–20117. doi: 10.1074/jbc.272.32.20108. [DOI] [PubMed] [Google Scholar]
- IJpenberg A., Tan N.S., Gelman L., Kersten S., Seydoux J., Xu J., Metzger D., Canaple L., Chambon P., Wahli W., et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004;23:2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Ji X., Li W., Song J., Wei L., Liu X.S. CEAS: Cis-regulatory element annotation system. Nucleic Acids Res. 2006;34 (Web Server issue):W551–W554. doi: 10.1093/nar/gkl322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.E., Li W., Meyer C.A., Gottardo R., Carroll J.S., Brown M., Liu X.S. Model-based analysis of tiling-arrays for ChIP–chip. Proc. Natl. Acad. Sci. 2006;103:12457–12462. doi: 10.1073/pnas.0601180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.B., Laffitte B.A., Patel P.H., Watson M.A., Matsukuma K.E., Walczak R., Collins J.L., Osborne T.F., Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- Karolchik D., Hinrichs A.S., Furey T.S., Roskin K.M., Sugnet C.W., Haussler D., Kent W.J. The UCSC table browser data retrieval tool. Nucleic Acids Res. 2004;32:D493–D496. doi: 10.1093/nar/gkh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Yamazaki M., Zella L.A., Meyer M.B., Fretz J.A., Shevde N.K., Pike J.W. Multiple enhancer regions located at significant distances upstream of the transcriptional start site mediate RANKL gene expression in response to 1,25-dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2007;103:430–434. doi: 10.1016/j.jsbmb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.I., Johnstone S.E., Young R.A. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protocols. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M., Lazar M.A. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Li A.C., Glass C.K. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid Res. 2004;45:2161–2173. doi: 10.1194/jlr.R400010-JLR200. [DOI] [PubMed] [Google Scholar]
- Li W., Carroll J.S., Brown M., Liu S. xMAN: Extreme mapping of oligonucleotides. BMC Genomics. 2008;9 (Suppl. 1):S20. doi: 10.1186/1471-2164-9-S1-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M., Eeckhoute J., Meyer C.A., Wang Q., Zhang Y., Li W., Carroll J.S., Liu X.S., Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougald O.A., Cornelius P., Liu R., Lane M.D. Insulin regulates transcription of the CCAAT/enhancer binding protein (C/EBP) α, β, and δ genes in fully-differentiated 3T3-L1 adipocytes. J. Biol. Chem. 1995;270:647–654. doi: 10.1074/jbc.270.2.647. [DOI] [PubMed] [Google Scholar]
- Matsukuma K.E., Wang L., Bennett M.K., Osborne T.F. A key role for orphan nuclear receptor liver receptor homologue-1 in activation of fatty acid synthase promoter by liver X receptor. J. Biol. Chem. 2007;282:20164–20171. doi: 10.1074/jbc.M702895200. [DOI] [PubMed] [Google Scholar]
- Matys V., Fricke E., Geffers R., Gossling E., Haubrock M., Hehl R., Hornischer K., Karas D., Kel A.E., Kel-Margoulis O.V., et al. TRANSFAC: Transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakachi Y., Yagi K., Nikaido I., Bono H., Tonouchi M., Schonbach C., Okazaki Y.2008Identification of novel PPARγ target genes by integrated analysis of ChIP-on-chip and microarray expression data during adipocyte differentiation Biochem. Biophys. Res. Commun. 372 :362–366. [DOI] [PubMed] [Google Scholar]
- Park S.K., Oh S.Y., Lee M.Y., Yoon S., Kim K.S., Kim J.W. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes. 2004;53:2757–2766. doi: 10.2337/diabetes.53.11.2757. [DOI] [PubMed] [Google Scholar]
- Perera R.J., Marcusson E.G., Koo S., Kang X., Kim Y., White N., Dean N.M. Identification of novel PPARγ target genes in primary human adipocytes. Gene. 2006;369:90–99. doi: 10.1016/j.gene.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Rangwala S.M., Lazar M.A. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Robinson C.E., Wu X., Morris D.C., Gimble J.M. DNA bending is induced by binding of the peroxisome proliferator-activated receptor γ 2 heterodimer to its response element in the murine lipoprotein lipase promoter. Biochem. Biophys. Res. Commun. 1998;244:671–677. doi: 10.1006/bbrc.1998.8305. [DOI] [PubMed] [Google Scholar]
- Roh T.Y., Wei G., Farrell C.M., Zhao K. Genome-wide prediction of conserved and nonconserved enhancers by histone acetylation patterns. Genome Res. 2007;17:74–81. doi: 10.1101/gr.5767907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E.D., Hsu C.H., Wang X., Sakai S., Freeman M.W., Gonzalez F.J., Spiegelman B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes & Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A., Alkema W., Engstrom P., Wasserman W.W., Lenhard B. JASPAR: An open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004;32:D91–D94. doi: 10.1093/nar/gkh012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonjans K., Staels B., Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- Sears D.D., Hsiao A., Ofrecio J.M., Chapman J., He W., Olefsky J.M. Selective modulation of promoter recruitment and transcriptional activity of PPARγ. Biochem. Biophys. Res. Commun. 2007;364:515–521. doi: 10.1016/j.bbrc.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A., Bejerano G., Pedersen J.S., Hinrichs A.S., Hou M., Rosenbloom K., Clawson H., Spieth J., Hillier L.W., Richards S., et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.S., Johnson W.E., Zhu X., Zhang X., Li W., Manrai A.K., Liu J.S., Chen R., Liu X.S. Model-based analysis of two-color arrays (MA2C) Genome Biol. 2007;8:R178. doi: 10.1186/gb-2007-8-8-r178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger D.J., Lefterova M.I., Ying L., Stonestrom A.J., Schupp M., Zhuo D., Vakoc A.L., Kim J.E., Chen J., Lazar M.A., et al. Mol. Cell. Biol. Vol. 28. 2008. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. pp. 2825–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teboul L., Febbraio M., Gaillard D., Amri E.Z., Silverstein R., Grimaldi P.A. Structural and functional characterization of the mouse fatty acid translocase promoter: Activation during adipose differentiation. Biochem. J. 2001;360:305–312. doi: 10.1042/0264-6021:3600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple K.A., Cohen R.N., Wondisford S.R., Yu C., Deplewski D., Wondisford F.E. An intact DNA-binding domain is not required for peroxisome proliferator-activated receptor γ (PPARγ) binding and activation on some PPAR response elements. J. Biol. Chem. 2005;280:3529–3540. doi: 10.1074/jbc.M411422200. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Graves R.A., Budavari A.I., Erdjument-Bromage H., Lui M., Hu E., Tempst P., Spiegelman B.M. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPARγ and RXRα. Nucleic Acids Res. 1994a;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPARγ2: Tissue-specific regulator of an adipocyte enhancer. Genes & Dev. 1994b;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994c;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Carroll J.S., Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell. 2005;19:631–642. doi: 10.1016/j.molcel.2005.07.018. [DOI] [PubMed] [Google Scholar]
- West A.G., Fraser P. Remote control of gene transcription. Hum. Mol. Genet. 2005;14:R101–R111. doi: 10.1093/hmg/ddi104. [DOI] [PubMed] [Google Scholar]
- Wu Z., Xie Y., Bucher N.L., Farmer S.R. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes & Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- Wu Z., Bucher N.L., Farmer S.R. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Rosen E.D., Brun R., Hauser S., Adelmant G., Troy A.E., McKeon C., Darlington G.J., Spiegelman B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Yajima H., Kobayashi Y., Kanaya T., Horino Y. Identification of peroxisome-proliferator responsive element in the mouse HSL gene. Biochem. Biophys. Res. Commun. 2007;352:526–531. doi: 10.1016/j.bbrc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Yeh W.C., Cao Z., Classon M., McKnight S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes & Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Zuo Y., Qiang L., Farmer S.R. Activation of CCAAT/enhancer-binding protein (C/EBP) α expression by C/EBPβbeta during adipogenesis requires a peroxisome proliferator-activated receptor-γ-associated repression of HDAC1 at the C/ebpα gene promoter. J. Biol. Chem. 2006;281:7960–7967. doi: 10.1074/jbc.M510682200. [DOI] [PubMed] [Google Scholar]