Abstract
Microglial cells are activated during excitotoxin-induced neurodegeneration. However, the in vivo role of microglia activation in neurodegeneration has not yet been fully elucidated. To this end, we used Ikkβ conditional knockout mice (LysM-Cre/IkkβF/F) in which the Ikkβ gene is specifically deleted in cells of myeloid lineage, including microglia, in the CNS. This deletion reduced IκB kinase (IKK) activity in cultured primary microglia by up to 40% compared with wild-type (IkkβF/F), and lipopolysaccharide-induced proinflammatory gene expression was also compromised. Kainic acid (KA)-induced hippocampal neuronal cell death was reduced by 30% in LysM-Cre/IkkβF/F mice compared with wild-type mice. Reduced neuronal cell death was accompanied by decreased KA-induced glial cell activation and subsequent expression of proinflammatory genes such as tumour necrosis factor (TNF)-α and interleukin (IL)-1β. Similarly, neurons in organotypic hippocampal slice cultures (OHSCs) from LysM-Cre/IkkβF/F mouse brain were less susceptible to KA-induced excitotoxicity compared with wild-type OHSCs, due in part to decreased TNF-α and IL-1β expression. Based on these data, we concluded that IKK/nuclear factor-κB dependent microglia activation contributes to KA-induced hippocampal neuronal cell death in vivo through induction of inflammatory mediators.
Keywords: excitotoxicity, hippocampus, IKKβ; kainic acid, microglia
Introduction
Excitotoxicity is a mechanism that contributes to neuronal cell death following acute neuronal damage, including traumatic brain injury and stroke, and is implicated in chronic neurodegenerative diseases (Doble, 1999). It is well known that over-stimulation of the glutamate receptor is responsible for excitotoxic neuronal cell death (Doble, 1999). In this process, activated microglia are easily detected in and around the regions encompassing dying neurons (Andersson et al., 1991b). Several studies also suggest that activation of microglia may contribute to excitotoxin-induced neuronal cell death. Upon excitotoxic brain injury, proinflammatory cytokines are expressed by activated microglia (Barone and Feuerstein, 1999). In another study, inhibition of microglia activation and proliferation by a chemical inhibitor reduced excitotoxic spinal cord neuronal cell death (Tikka et al., 2001). The critical role of microglial production of tissue plasminogen activator (tPA) in excitotoxin-induced hippocampal neuronal cell death was also documented in a study using tPA-deficient mice (Tsirka et al., 1995). These previous reports support a model in which excitotoxic brain damage induces microglial cell activation, thereby augmenting neuronal cell death. However, it has also been argued that microglia protect hippocampal neurons from excitotoxic cell death (Bruce et al., 1996; Neumann et al., 2006; Simard and Rivest, 2007). In these studies, inhibition of microglial activation by glucocorticoid augmented kainic acid (KA)-mediated excitotoxicity, and tumour necrosis factor (TNF)-α, secreted by activated glial cells during excitotoxic brain damage, was found to protect hippocampal neurons from oxidative stress. Additionally, activated microglia have been shown to release neurotrophic factors, which promote neuronal survival against excitotoxic neuronal damage (Elkabes et al., 1996; Young et al., 1999). Therefore, it is conceivable that microglia are activated to protect neurons during excitotoxic brain damage. Thus, the in vivo role of microglial cell activation in excitotoxic neuronal cell death is still debatable. Nonetheless, in light of the in vitro neurotoxic effects of activated microglial cells, inflammatory microglial activation is regarded as an attractive therapeutic target for the treatment of various neurological disorders that accompany excitotoxic neuronal cell death (Block et al., 2007). In this regard, it is of critical importance to elucidate the in vivo role of microglia activation, as inhibitors of microglial activation could turn out to exacerbate brain damage if microglia activation in fact plays a neuroprotective role in vivo.
It is well known that nuclear factor-κB (NF-κB) activation plays a critical role in the microglial production of proinflammatory genes including TNF-α, interleukin-1β (IL-1β) and inducible nitric oxide synthase (iNOS) (Jana et al., 2001; Rasley et al., 2002; Moriyama et al., 2006). Upon stimulation, NF-κB is activated by IκB kinase (IKK) complex, in a manner dependent mainly on the IKKβ catalytic subunit (Karin, 1999). It was previously documented that NF-κB is activated in microglia in excitotoxic brain injury (Matsuoka et al., 1999; Acarin et al., 2000). This may account for inflammatory cytokine expression by microglia during excitotoxic neurodegeneration. Therefore, we reasoned that by deleting the Ikkβ gene in microglial cells, we might be able to inhibit inflammatory microglia activation. Further, by using these mice in an excitotoxic brain injury model, we can address the in vivo role of microglia activation in excitotoxin-induced neuronal cell death. We tested this hypothesis by using LysM-Cre/Ikkβ-floxed (LysM-Cre/IkkβF/F) mice, in which the Ikkβ gene was specifically deleted in cells of myeloid origin, including the microglia in the CNS (Greten et al., 2004).
Materials and Methods
Animals and genotyping
Myeloid cell type-specific Ikkβ-deficient (LysM-Cre/IkkβF/F) mice were generated by breeding Ikkβ-floxed (IkkβF/F) mice and LysM-Cre knock-in mice expressing Cre under the control of endogenous lysozyme M promoter as previously described (Clausen et al., 1999; Li et al., 2003). PCR genotyping was performed using primers, 5′-TGA CCC GGG AAT GAA TAG GA-3′ and 5′-GTC TTC AAC CTC CCA AGC CTT-3′, which amplify both the Ikkβ+ (220 bp) and IkkβF (310 bp) alleles. LysM-Cre mice were genotyped by PCR using the primer pair NLS-Cre (5′-CCC AAG AAG AAG AGG AAG GTG TCC-3′) and Cre8 (5′-CCC AGA AAT GCC AGA TTA CG-3′). Mice were housed at 23 ± 2°C with a 12 h light–dark cycle and food and water ad libitum. All surgical and experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University.
Primary glial culture from neonates and cortical neuron culture
Primary microglia cultures were prepared as previously described (Lee et al., 2000). Briefly, mixed glial cultures were prepared from postnatal day 1–3 wild-type and LysM-Cre/IkkβF/F mice. After removing meninges from the cerebral hemispheres, tissue was dissociated into a single-cell suspension by gentle trituration. Cells were cultured in glial culture media (DMEM supplemented with 10 mM HEPES, 10% FBS, 2 mM l-glutamine and 1× antibiotic/antimycotic) in 75 cm2 flasks at 37°C in a 5% CO2 incubator and the medium was changed every 5 days. Microglia were harvested from mixed glial cultures on day 14. After shaking at 200 r.p.m. for 4 h on an orbital shaker, the media from the cultures was collected and centrifuged at 800g for 10 min. Microglia were plated in glial culture media. After 30 min, dishes were washed with medium to remove unattached astrocytes. The purity of microglia was routinely monitored and was >98% as determined by histochemical staining with cluster of differentiation molecule 11b (CD11b) (1 : 200, Serotec Inc., Oxford, UK). After shaking on day 14, adherent cells were trypsinized and allowed to re-attach for 30 min. Unattached astrocytes were transferred to a new plate and cultured in glial culture medium. The purity of astrocytes in this culture was >95% by glial fibrillary acidic protein (GFAP) (1 : 10 000; DAKO, Denmark) immunostaining, and the remaining cells were identified as microglia or oligodendrocytes. Primary cortical neurons were prepared from E17 mouse embryos as previously described (Brewer et al., 1993). Microglia from adult or neonate mouse brains were prepared as described elsewhere (Slepko and Levi, 1996), and peritoneal macrophages (PMΦ) were prepared as described previously (Pfeiffer et al., 2001).
Determination of loss of the IkkβF allele at the genomic level by real-time PCR
Genomic DNA (100 ng in 4 μl) was prepared from each sample, and mixed with SYBR Green PCR Master Mix (10 μl, Applied Biosystems, Foster City, CA, USA), primers (1 μl at 10 μM each) and H2O (5 μl). Real-time PCR was performed for 40 cycles of 95°C for 15 s and 60°C for 1 min using an ABI 7500 Real Time PCR System (Applied Biosystems, CA, USA). Primers, 5′-AAG ATG GGC AAA CTG TGA TGT G-3′ and 5′-CAT ACA GGC ATC CTG CAG AAC A-3′, were used to amplify the IkkβF allele, and primers, 5′-GGT GCA TGG TGT GTG AAG AC-3′ and 5′-CAT GCA TAC TAC CGC CAC AC-3′, were used to amplify the Tnfr1 gene as a control. The ratio of IkkβΔ and IkkβF signal was calculated after normalization to the Tnfr1 signal.
Real-time RT–PCR
Real-time RT–PCR was performed using SYBR Green PCR Master Mix as previously described (Lee et al., 2004). Reactions were performed in duplicate in a total volume of 10 μl, each containing 10 pM primer, 4 μl cDNA and 5 μl SYBR Green PCR Master Mix. The mRNA levels of each target gene were normalized to that of GAPDH mRNA. Fold-induction was calculated using the 2−ΔΔCT method as previously described (Livak and Schmittgen, 2001). All real-time RT–PCR experiments were performed at least three times, and the mean ± SEM values have been presented unless otherwise noted. The primer sequence information can be found in the Supplementary materials.
Stereotaxic injection and tissue processing
For intracerebroventricular (i.c.v.) injection of KA, 8- to 12-week-old male wild-type and LysM-Cre/IkkβF/F mice (22–25 g) were anesthetized by pentobarbital sodium (30 mg/kg, body weight, i.p.) and placed on a stereotaxic apparatus (Myneurolab, MO, USA). Animals were injected with PBS or KA (0.2 µg in 4.0 µl of PBS) at the speed of 0.5 µl/min into the right ventricle using a 26-G needle (stereotaxic coordinates in millimetre with reference to the bregma: AP, −2.0; ML, −2.9; DV, −3.8). After 5 min, the needle was removed with three intermediate steps over 3 min to minimize backflow, and the incision was cleaned with saline and sutured. Animals were kept on a warm pad until recovery. On either day 1 or day 3 after surgery, brains were removed from the mice after perfusion, immersed for 12 h in 4% PFA fixative at 4°C and serially cryoprotected in 10, 20 and 30% sucrose in PBS for 48 h at 4°C. Serial coronal sections (30 µm thickness) were cut on a cryostat and collected as free-floating sections in PBS. Sections were stored at −20°C until needed for histochemical studies.
Evaluating neuronal damage
For Nissl staining, hippocampal tissue sections were mounted on gelatin-coated slides, dried for 1 day at RT and stained with 0.5% cresyl violet. The numbers of cornu ammonis (CA) 1 and 3 neurons were counted at three levels of the dorsal hippocampus. Specifically, alternate sections were obtained at 1.6, 1.9 and 2.2 mm posterior to the bregma, and two regions from each level (six regions for each animal) were used to count cells in the CA1 region. The number of intact neurons within the CA1 layer was counted using a light microscope (BX51, Olympus, Japan) at 400× magnification and expressed as the number of CA1 neurons per millimeter of linear length as described previously (Choi et al., 2005). To maintain consistency across animals, a rectangular box (1.0 × 0.25 mm) was centred over the CA1 cell layer beginning 1.0 mm lateral to the midline and 0.5 mm medial to the CA2 subfield. Only neurons with normal visible nuclei were counted. The mean number of CA1 neurons per millimeter of linear length of the ipsilateral hemispheres was calculated for each treatment group. The number of CA3 pyramidal neurons was also counted under light microscope as described previously (Hernandez-Sanchez et al., 2001). Cell counts were made in a defined area (1.0 mm × 0.75 mm) centred over the CA3 region using three sections per brain. All assessments of histological sections were blindly performed.
Immunohistochemistry and quantitative analysis
Immunohistochemical analysis was performed as previously described (Park et al., 2006), and detailed methods can be found in the Supplementary materials. To perform quantitative analysis of CD11b and GFAP immunostaining, 3–4 sections per animal were selected and images were captured and analysed using MetaMorph software (Universal Imaging, PA, USA). One or two fields (200 µm × 200 µm or 100 µm × 100 µm) in each slide within the midpoint of hippocampal CA1 and CA3 regions encompassing all layers were selected for quantification, and the intensity of CD11b and GFAP immunoreactivity was evaluated by means of a relative optical density value. To quantify CD11b+/NG2+ cells, images were captured under a confocal laser scanning microscopy. The entire quantifying procedure was blindly performed.
Organotypic hippocampal slice cultures
Organotypic hippocampal slice cultures (OHSCs) were prepared and maintained as described previously (Jung et al., 2004). After 14 days in culture, slices were exposed to 50 µM KA following the previously reported protocol (Kristensen et al., 2001). Neuronal degeneration was quantified by the uptake of propidium iodide (PI) into the damaged cells (Macklis and Madison, 1990). To evaluate the effects of cytokines on KA-induced neuronal cell death, cultures were co-treated with KA and TNF-α/IL-1β or anti-TNF-α/IL-1β antibodies (R & D Systems, MN, USA) for 3 h, and then, incubated in recovery medium for 24 h with or without cytokines or blocking antibodies. Detailed methods can be found in the Supplementary materials.
Hippocampal EEG analysis
Methods for hippocampal electroencephalography (EEG) after KA injection are presented in Supplementary materials.
Transient middle cerebral artery occlusion
Transient Middle Cerebral Artery Occlusion (MCAO) was performed as previously described (Kim et al., 2004). Detailed methods can be found in the Supplementary materials.
Statistical analysis
The statistical significance of the differences between wild-type and LysM-Cre/IkkβF/F mice was determined by Student's t-test or ANOVA with a Fisher's post hoc test. All data are presented as mean ± SEM. Differences were considered significant when P < 0.05.
Results
The Ikkβ gene is deleted in microglial cells of LysM-Cre/IkkβF/F mice
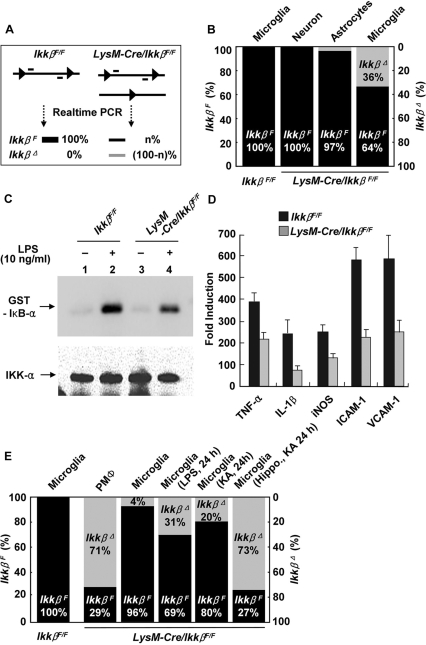
Previously, we reported that the Ikkβ gene is tissue-specifically deleted in myeloid cells of LysM-Cre/IkkβF/F mice, in which the Cre transgene is constitutively expressed in cells of myeloid origin (Park et al., 2002; Greten et al., 2004). Since microglia belong to the myeloid lineage, have characteristics of macrophages/monocytes and constitutively express the lysozyme gene (Perry et al., 1985; Zucker-Franklin et al., 1987; Hao et al., 1991), we tested whether Ikkβ is deleted in microglial cells of LysM-Cre/IkkβF/F mice. First, we cultured primary microglial cells from neonatal LysM-Cre/IkkβF/F mice and measured the Ikkβ deletion rate at the genomic level with real-time PCR using a specific primer set designed to detect undeleted-Ikkβ alleles, as previously described (Li et al., 2003). In cultured primary microglia of LysM-Cre/IkkβF/F mice, 36 ± 3% of the Ikkβ alleles in the population were deleted, while deletion of Ikkβ was not detected in primary cortical neurons (Fig. 1B). In primary cultured astrocytes, the deletion frequency was only 3 ± 1%, probably due to contamination of the astrocyte culture with microglia (Fig. 1B). We then tested whether deletion of Ikkβ in microglia correlates with reduced IKK activity using an in vitro kinase assay. Lipopolysaccharide (LPS)-stimulated IKK activity in primary microglial cells from LysM-Cre/IkkβF/F mice was decreased by 40% compared with IKK activity in wild-type (IkkβF/F) microglia (Fig. 1C). These results suggest that ∼40% of the microglial cells lost Ikkβ alleles. Similarly, LPS-induced expression of proinflammatory genes such as TNF-α, IL-1β, iNOS, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) was decreased by 30–60% (Fig. 1D). These data demonstrate that cultured microglial cells from LysM-Cre/IkkβF/F mice are less responsive to LPS stimulation. Since microglial cell cultures are derived from neonates, it is possible that the deletion rate may not represent the Ikkβ deletion rate in adult mice in vivo. To test this, we harvested microglial cells directly from adult mice using a previously reported procedure (Slepko and Levi, 1996) and assessed the Ikkβ deletion rate. In microglia directly isolated from 8-week-old adult LysM-Cre/IkkβF/F cerebra, only 4% of the Ikkβ alleles were deleted, although we observed 71 ± 2% Ikkβ deletion in PMΦ (Fig. 1E), which is consistent with a previous study (Greten et al., 2004). The Ikkβ deletion rate in the microglia population directly isolated from neonate mice cerebra was ∼20% (data not shown), which is higher than the deletion rate in adult mouse microglia in vivo, but still lower than the rate in primary cultured microglia. These results show that Ikkβ is more easily deleted in primary cultured microglia, which are in a more activated state than microglia in vivo (Eder et al., 1999; Hurley et al., 1999), and implies that the in vivo Ikkβ deletion rate may be increased upon microglia activation. To test this, we stimulated LysM-Cre/IkkβF/F microglia in vivo by i.c.v. LPS injection. One day after LPS injection, in microglia isolated from the whole cerebrum, the Ikkβ deletion rate increased to 31 ± 2% and injection of KA increased the Ikkβ deletion rate to 20 ± 3% (Fig. 1E). In microglia isolated from the hippocampus, where KA-induced microglial activation is most prominent, the deletion rate increased to 73 ± 2% (Fig. 1E). Taken together, these data show that KA-injection enhances microglia-specific Ikkβ deletion in LysM-Cre/IkkβF/F mice, and suggest that microglia in LysM-Cre/IkkβF/F mice are less responsive to inflammatory or stress stimulation.
Fig. 1.
Microglia-specific Ikkβ gene deletion in the LysM-Cre/IkkβF/F mice. (A and B) Primary microglia and astrocytes were cultured from neonatal wild-type (IkkβF/F) or LysM-Cre/IkkβF/F mice, and cortical neurons were isolated and cultured from E17 embryos of LysM-Cre/IkkβF/F mice. Genomic DNA from each primary cell culture was analysed by real-time PCR to determine the rate of Ikkβ deletion (A). Percentages of undeleted and deleted IkkβF alleles are shown in black and grey bars, respectively (B). (C) Primary microglia from wild-type and LysM-Cre/IkkβF/F mice were stimulated with LPS (10 ng/ml) for 30 min, and cell lysates were used to measure IKK activity. Samples were resolved on SDS–PAGE, and radiolabelled substrates were exposed using a PhosphorImager (upper panel). The same membrane was probed with anti-IKKα antibody to normalize IKK loading (lower panel). (D) Primary microglia from either wild-type (black bars) or LysM-Cre/IkkβF/F mice (grey bars) were stimulated with LPS (10 ng/ml) for 3 h. Total RNA was prepared and used to determine the mRNA expression levels of TNF-α, IL-1β, iNOS, ICAM-1 and VCAM-1, which are represented as fold-induction. (E) Microglia and PMΦ from adult wild-type or LysM-Cre/IkkβF/F mice were used to prepare genomic DNA to determine the Ikkβ gene deletion rate. Percentages of undeleted and deleted IkkβF alleles are shown in black and grey bars, respectively.
KA-induced death of hippocampal neurons is reduced in LysM-Cre/IkkβF/F mice
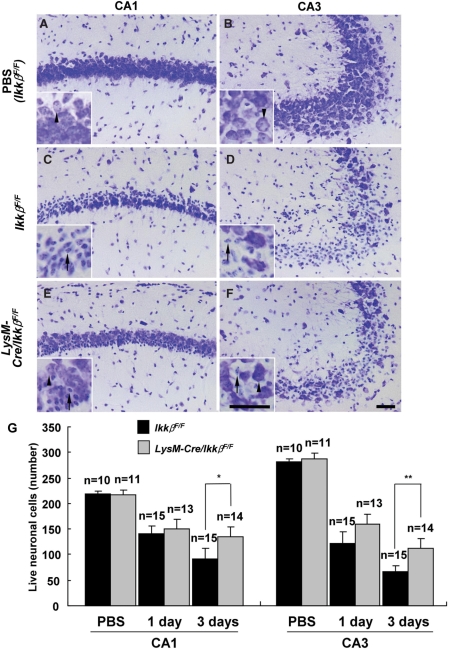
To determine the in vivo role of IKKβ-mediated microglial cell activation in excitotoxin-induced hippocampal neuronal cell death, we introduced KA directly into the brains of wild-type and LysM-Cre/IkkβF/F mice. The i.c.v. KA introduction is a well-established excitotoxicity model that induces behavioural manifestations of seizures in mice and selective hippocampal cell death (Cho et al., 2003). We did not observe any discernible differences between wild-type and LysM-Cre/IkkβF/F mice in terms of seizure-like behaviour or hippocampal EEG analysis [power spectrum main frequency range, 1 day after KA injection: 0.5–3 Hz in wild-type mice (n = 5) versus 0.7–1.7 Hz in LysM-Cre/IkkβF/F mice (n = 4); 3 days after KA injection: 0.5–2 Hz in wild-type mice (n = 5) versus 0.5–2 Hz in LysM-Cre/IkkβF/F mice (n = 4)]. Hippocampal neuronal cell death was measured by cresyl violet staining. After KA administration, a characteristic loss of pyramidal neurons in the CA1 and CA3 subfields of the ipsilateral hippocampus was observed in wild-type mice (Fig. 2C and D), whereas no obvious neuronal loss was observed in the ipsilateral hippocampus of PBS-injected wild-type mice (Fig. 2A and B). The extent of hippocampal neuronal loss was significantly reduced in LysM-Cre/IkkβF/F mice (Fig. 2E and F). Three days after KA injection, the number of live neuronal cells in the CA1 and CA3 subfields of wild-type mice decreased by 58% and 76%, respectively, whereas in LysM-Cre/IkkβF/F mice, live neuronal cells in the CA1 and CA3 areas were reduced by only 38% and 60%, respectively (Fig. 2G). Comparable levels of neuronal loss were obtained by counting neuronal cells by immunohistochemistry using anti-NeuN antibody (data not shown). Interestingly, however, the reduction in neuronal loss in LysM-Cre/IkkβF/F mice compared with wild-type mice was not prominent 1 day after KA injection (Fig. 2G).
Fig. 2.
KA-induced hippocampal neuronal cell death is decreased in LysM-Cre/IkkβF/F mice. Wild-type (IkkβF/F) (A–D) and LysM-Cre/IkkβF/F (E and F) mice were i.c.v. injected with either PBS (A and B) or KA (0.2 µg in 4 µl PBS) (C–F). Cryosections (30 µm thick) were stained with cresyl violet. Arrows indicate dying neurons and arrow heads show live cells. Scale bars: 50 µm. (G) The rate of neuronal loss in the CA1 or CA3 area of ipsilateral side hippocampus was evaluated by counting live cells. Data are presented as mean ± SEM. (Student's t-test, *P < 0.05, **P < 0.01; versus wild-type mice.)
KA-induced glial cell activation is reduced in LysM-Cre/IkkβF/F mice
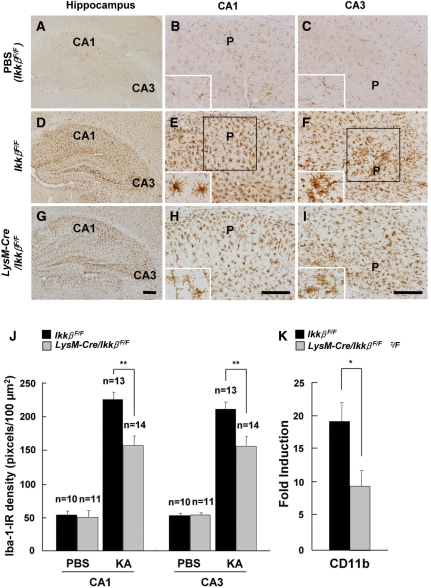
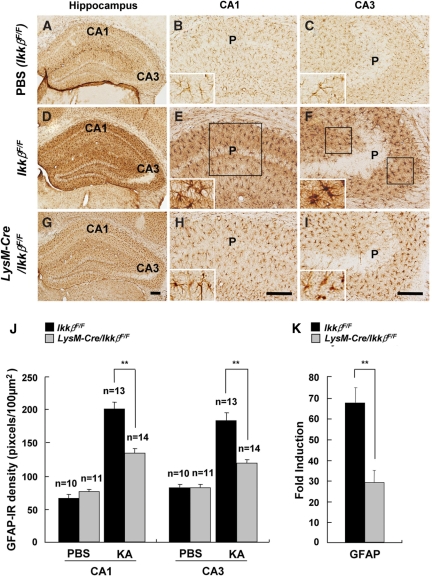
To assess the effects of Ikkβ deletion on KA-treated hippocampal glial cells, activation of microglia and astrocytes was analysed by immunohistochemistry using anti-Iba-1 and anti-GFAP antibodies, respectively (Figs 3 and 4). In PBS-treated wild-type mice, ionized calcium binding adaptor molecule-1 (Iba-1)-immunoreactive (IR) microglia were in their resting form (Fig. 3A–C). In KA-injected wild-type mice, along with hippocampal neuronal loss, the number of Iba-1-IR cells was remarkably increased in both the CA1 and CA3 regions of the ipsilateral hippocampus (Fig. 3D–F). Iba-1-IR microglia showed activated cell morphology, with enlarged cell bodies and thicker processes. In KA-injected LysM-Cre/IkkβF/F mice, however, microglia activation was notably suppressed. There were fewer Iba-1-IR cells, and their morphology was more ramified (Fig. 3G–I). Quantitatively, Iba-1 expression was decreased by 30% (Fig. 3J). The suppression of microglial activation in LysM-Cre/IkkβF/F mice was also confirmed by measuring the CD11b mRNA levels (Fig. 3K). The extent of microglial activation in the hippocampus was well correlated with the rate of neuronal loss in the adjacent region (data not shown). Similarly, astrocyte activation in the ipsilateral hippocampus after KA-treatment was also attenuated in LysM-Cre/IkkβF/F mice compared with wild-type mice (Fig. 4A–K). These results show that microglia-specific Ikkβ deletion suppresses KA-induced microglia activation in the hippocampus in vivo and that microglia activation may influence astrocyte activation.
Fig. 3.
KA-induced microglia activation is reduced in LysM-Cre/IkkβF/F mice. Wild-type (IkkβF/F) (A–F) and LysM-Cre/IkkβF/F (G–I) mice were i.c.v. injected with either PBS (A–C) or KA (D–I). After 3 days, mice were sacrificed and cryosections were immunostained with anti-Iba-1 (A–I) antibodies. P: pyramidal cell layer. Scale bars: 100 µm. (J) Expression levels of Iba-1 in the CA1 and CA3 subfields of wild-type and LysM-Cre/IkkβF/F mice were quantified. (K) The mRNA levels of CD11b in the ipsilateral hippocampus were examined 36 h after KA injection. Data are presented as mean ± SEM. (Student's t-test, *P < 0.05, **P < 0.01; versus wild-type mice.)
Fig. 4.
KA-induced astrocyte activation is reduced in LysM-Cre/IkkβF/F mice. Wild-type (IkkβF/F) (A–F) and LysM-Cre/IkkβF/F (G–I) mice were i.c.v. injected with either PBS (A–C) or KA (D–I). After 3 days, mice were sacrificed and cryosections were immunostained with anti-GFAP (A–I) antibodies. P: pyramidal cell layer. Scale bars: 100 µm. (J) Expression levels of GFAP in the CA1 and CA3 subfields of wild-type and LysM-Cre/IkkβF/F mice were quantified. (K) The mRNA levels of GFAP in the ipsilateral hippocampus were examined 36 h after KA injection. Data are presented as mean ± SEM. (Student's t-test, *P < 0.05, **P < 0.01; versus wild-type mice.)
Microglial IKKβ deletion is responsible for the attenuation of KA-induced hippocampal neuronal cell death
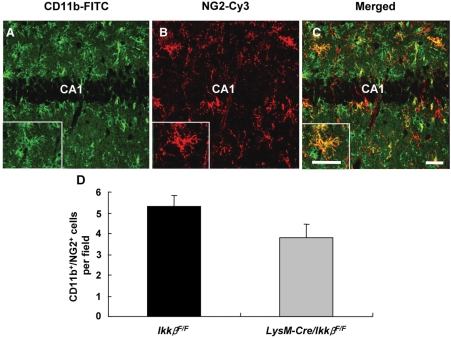
Our results from LysM-Cre/IkkβF/F mice suggest that microglia-specific Ikkβ deletion decreases KA-induced hippocampal neuronal cell death. However, they do not rule out the possibility that other myeloid cells such as macrophages or neutrophils from the periphery are involved, since the Ikkβ gene is also deleted in these cell types (Greten et al., 2004). To test this possibility, we examined macrophage and neutrophil infiltration of the brain parenchyma following KA treatment. For macrophage detection, we used antibodies for neuron-glial antigen 2 (NG2) and CD11b. It has been previously reported that blood-derived macrophages are double-positive for NG2 and CD11b, while oligodendrocyte precursor cells are single-positive for NG2 (Bu et al., 2001; Jones et al., 2002). A subpopulation of CD11b-IR cells co-expressing NG2 (CD11b+/NG2+) was detected in the ipsilateral hippocampus of KA-injected wild-type mice (Fig. 5A–C), whereas no obvious CD11b+/NG2+ cells were observed in the PBS-injected ipsilateral hippocampus or KA-injected contralateral hippocampus (data not shown). Interestingly, the number of CD11b+/NG2+ cells was slightly reduced in the LysM-Cre/IkkβF/F mice (3.8 ± 0.7/field) compared with the wild-type mice (5.3 ± 0.5/field), though this was not statistically significant (Fig. 5D). In addition, we did not observe significant neutrophil infiltration in hippocampal parenchyma in either control or LysM-Cre/IkkβF/F mice after KA injection (data not shown), which is consistent with findings from a previous report (Andersson et al., 1991a). These data suggest that it is not likely that reduced KA-induced neuronal loss in LysM-Cre/IkkβF/F mice is due to a reduction in myeloid cell infiltration in these mice.
Fig. 5.
Microglial IKKβ deletion does not block immune cell recruitment from the periphery. (A–C) Infiltration of peripheral monocytes in the ipsilateral hippocampus was examined by immunostaining with anti-CD11b and anti-NG2 antibodies 3 days after KA injection in wild-type and LysM-Cre/IkkβF/F mice. Scale bars: 50 µm. (D) CD11b+/NG2+ cells were counted in 27 optical fields (350 µm × 350 µm) per animal group (two fields per section, two sections per animal in the seven animals per group). Data are expressed as mean ± SEM. (Student's t-test, P = 0.09; versus wild-type mice.)
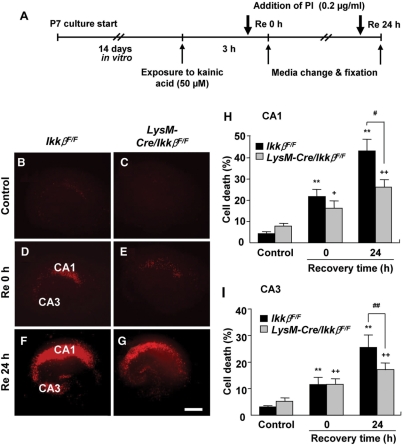
To further exclude the contribution of blood-derived macrophages or neutrophils, we adopted an OHSC system, and tested the effects of Ikkβ deletion in microglia on excitotoxicity ex vivo. Hippocampal slices from wild-type and LysM-Cre/IkkβF/F mice were maintained in culture medium for 2 weeks prior to KA stimulation to eliminate any blood-derived macrophages or neutrophils (Fig. 6A). Stimulated cultures were evaluated using cellular uptake of PI as a measure of excitotoxic neuronal damage. Immediately after a 3 h exposure to KA (0 h of recovery), the fluorescence values of PI uptake in the CA1 and CA3 areas were slightly increased to 21.1 ± 3.2% and 11.5 ± 2.7%, respectively, in wild-type OHSCs, and similar levels of PI uptake were detected in the LysM-Cre/IkkβF/F OHSCs (Fig. 6D, E, H and I). Upon 24 h recovery after KA exposure, PI uptake in the CA1 and CA3 regions of wild-type OHSCs was further increased to 42.5 ± 5.5% and 25.5 ± 4.7%, respectively. However, in OHSCs from LysM-Cre/IkkβF/F mice, the increase in PI uptake during the recovery period was significantly attenuated compared with wild-type: it increased to only 25.6 ± 3.6% and 17.1 ± 2.6%, respectively (Fig. 6F–I). In control OHSCs, however, a slightly higher level of basal PI uptake was detected in slices from LysM-Cre/IkkβF/F mice. Taken together, these data argue that Ikkβ deletion in microglia is responsible for the attenuation of KA-induced hippocampal neuronal cell death and that the IKKβ/NF-κB signalling pathway in microglia may play an important role in KA-induced excitotoxicity in the hippocampus.
Fig. 6.
Neurons in OHSCs from LysM-Cre/IkkβF/F mice are less susceptible to KA-induced excitotoxicity. (A) Schematic representation of the protocols used to study KA excitotoxicity in OHSCs. OHSCs were maintained in slice culture medium for 2 weeks, then exposed to KA (50 µM) for 3 h. After a media change, OHSCs were either directly fixed (D and E), or allowed to recover in fresh media for 24 h before fixation (F and G). One hour prior to fixation, PI (0.2 μg/ml) was added to the media. Neuronal cell death in OHSCs was determined by PI uptake in the CA1 and CA3 pyramidal cell layers. The extent of neuronal degeneration was quantified by PI fluorescence intensity (H and I), and representative images are shown (B–G). Scale bars: 200 µm. Data are presented as mean ± SEM. (ANOVA test with a Fisher's post hoc test, **P < 0.01, versus control wild-type OHSCs;+P < 0.05, ++P < 0.01, versus control LysM-Cre/IkkβF/F OHSCs; #P < 0.05, ##P < 0.01, versus KA-treated wild-type OHSCs.)
KA-induced proinflammatory gene expression is reduced in LysM-Cre/IkkβF/F mice
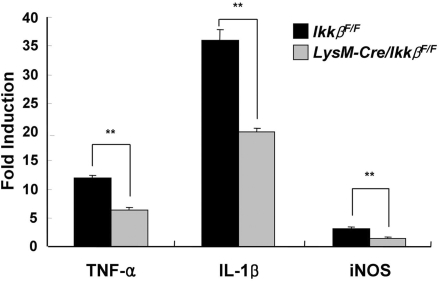
In an attempt to elucidate the mechanisms underlying the difference in excitotoxic susceptibility of wild-type and LysM-Cre/IkkβF/F mice, we measured the mRNA expression levels of proinflammatory NF-κB-target genes such as TNF-α, IL-1β and iNOS in the hippocampus. These genes have been implicated in excitotoxic hippocampal neuronal cell death (De Simoni et al., 2000; Rizzi et al., 2003). The mRNA levels of TNF-α, IL-1β and iNOS in the hippocampi of KA-stimulated wild-type mice increased 12-, 35- and 3-fold, respectively (Fig. 7). KA-induced expression of these proinflammatory genes in hippocampi of LysM-Cre/IkkβF/F mice, however, was attenuated by 30–50%. These data demonstrate that KA-induced inflammatory gene expression is reduced in LysM-Cre/IkkβF/F mice.
Fig. 7.
KA-induced proinflammatory gene expression is reduced in LysM-Cre/IkkβF/F mice. Wild-type and LysM-Cre/IkkβF/F mice were i.c.v. injected with KA or PBS. After 36 h, total RNA was prepared from ipsilateral hippocampus and used for quantitative real-time PCR to measure TNF-α, IL-1β and iNOS mRNA levels. The relative mRNA expression levels in KA-injected mice compared with those in PBS-injected mice are presented as fold induction (**P < 0.01 by Student's t-test; versus wild-type mice; n > 5).
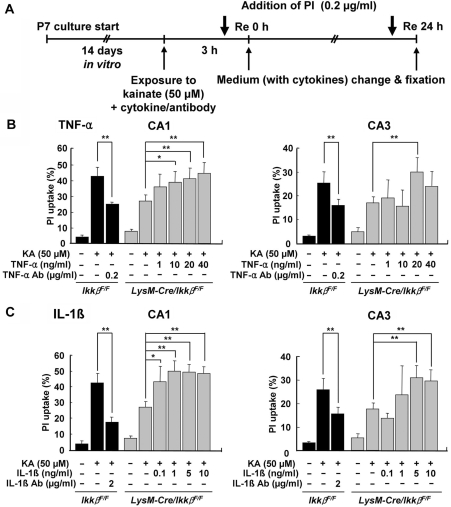
TNF-α and IL-1β contribute to KA-induced hippocampal cell death in OHSCs
We then tested the effects of proinflammatory cytokines on excitotoxicity in OHSCs (Fig. 8). Treatment of KA-stimulated LysM-Cre/IkkβF/F OHSCs with TNF-α (10–40 ng/ml in CA1; 20 ng/ml in CA3) completely elevated the cell death rate to the level seen in wild-type OHSCs (Fig. 8B). Likewise, treatment with IL-1β (0.1–10 ng/ml in CA1; 5–10 ng/ml in CA3) enhanced the KA-mediated excitotoxicity in LysM-Cre/IkkβF/F OHSCs (Fig. 8C). The specificity of the cytokines was confirmed using blocking antibodies against TNF-α and IL-1β in this experiment (data not shown). Furthermore, the addition of anti-TNF-α or anti-IL-1β blocking antibodies in the wild-type OHSCs reduced KA-mediated excitotoxicity by 30–60% (Fig. 8B and C). Taken together, these data suggest that TNF-α and IL-1β expression in wild-type OHSCs potentiates KA excitotoxicity, and that decreased expression of these cytokines in LysM-Cre/IkkβF/F OHSCs partly accounts for the decreased KA excitotoxicity.
Fig. 8.
Treatment with TNF-α and IL-1β potentiates KA-induced excitotoxicity in OHSCs from LysM-Cre/IkkβF/F mice. (A) Schematic representation of the protocols used to study the effects of TNF-α or IL-1β on KA-induced neuronal cell death in OHSCs. LysM-Cre/IkkβF/F OHSCs were co-treated with KA (50 µM) and cytokines for 3 h, then incubated in recovery medium with or without various concentrations of TNF-α (B) or IL-1β (C). In addition, wild-type OHSCs were treated with KA, in the presence or absence of either anti-TNF-α (B) or anti-IL-1β (C) blocking antibodies. After 24 h of recovery, OHSCs were fixed and cellular PI uptake was measured in the CA1 and CA3 pyramidal cell layers. The extent of neuronal degeneration was quantified by fluorescence intensity of PI (B and C). Data are expressed as mean ± SEM. (ANOVA test with a Fisher's post hoc test; *P < 0.05, **P < 0.01; versus KA-treated LysM-Cre/IkkβF/F OHSCs.)
Ischaemic brain damage and microglia activation after transient MCAO is reduced in LysM-Cre/IkkβF/F mice
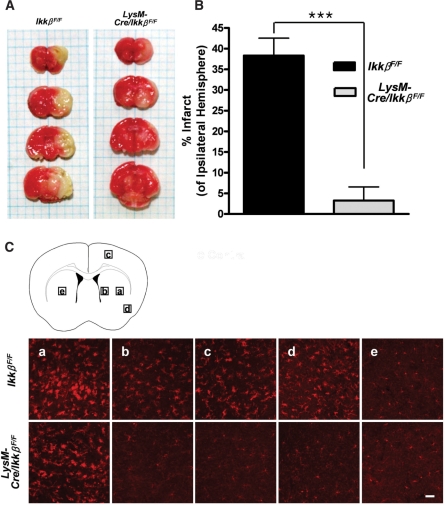
To verify the neuroprotective effects of microglial Ikkβ deletion in a more physiologically relevant disease model, we induced ischaemic brain damage by MCAO in LysM-Cre/IkkβF/F mice. It is well known that excitotoxicity is one of the underlying mechanisms of ischaemic neurodegeneration (Doble, 1999). A 1-h MCAO followed by a 3-day reperfusion period induced ∼40% degeneration of the ipsilateral brain, as calculated by infarct volume (Fig. 9A and B). In LysM-Cre/IkkβF/F mice, however, the infarct size decreased to <10%. We then tested microglia activation after MCAO by Iba-1 immunostaining. As previously reported (Schilling et al., 2003), ischaemic injury induced microglia activation around the infarct region in wild-type mice (Fig. 9C). The levels of microglia activation were variable depending on the distance to the infarct region: the closer to the infarct region, the stronger the microglia activation (Fig. 9C, compare A and B). Microglia activation was also detected in LysM-Cre/IkkβF/F mice. However, the activation levels were much weaker than those of wild-type mice (Fig. 9C, compare upper panels versus lower panels). These data demonstrate that LysM-Cre/IkkβF/F mice are less susceptible to MCAO-induced ischaemic brain damage and microglia activation.
Fig. 9.
Infarct size and microglial activation following MCAO are reduced in LysM-Cre/IkkβF/F mice. Wild-type and LysM-Cre/IkkβF/F mice were subjected to transient MCAO for 1 h and reperfused. (A) After 71 h, the brains were removed, cut into 2-mm thick blocks and stained with triphenyl tetrazolium chloride. (B) The infarct area was measured and expressed as the percentage of the ipsilateral hemisphere. Data are presented as mean ± SEM. (***P < 0.001 by Student's t-test; versus wild-type mice; n = 4). (C) Cryosections of the second blocks were stained with anti-Iba-1 antibody. Representative images of five different regions were captured and are presented (ipsilateral: a–d, contralateral: e). Scale bars: 50 μm.
Discussion
To address the in vivo role of inflammatory microglia activation in excitotoxicity, we employed myeloid cell type-specific Ikkβconditional knockout (LysM-Cre/IkkβF/F) mice. In primary cultured microglia from neonate LysM-Cre/IkkβF/F mice, we found that the Ikkβ deletion frequency was ∼36%, but the deletion frequency in microglia isolated directly from adult or neonate LysM-Cre/IkkβF/F mice was much lower. This difference may be due to the fact that microglia are in their resting state in vivo and then become spontaneously activated during in vitro culture, resulting in upregulation of the lysozyme M gene (Ohmi et al., 2003). This explanation accounts for the increase in the Ikkβ deletion rate to ∼73% in microglia from KA-treated LysM-Cre/IkkβF/F ipsilateral hippocampus (Fig. 1E). Interestingly, we detected a higher microglial Ikkβ deletion frequency in neonate mice compared with adult mice. This implies that lysozyme M gene expression is developmentally regulated in microglia, which may also contribute to the enhanced Ikkβ deletion frequency observed in the primary cultured microglia. In addition, our in vitro data demonstrate that the Ikkβ gene is deleted in microglia, but not in astrocytes or in neurons of the LysM-Cre/IkkβF/F brain and, in the absence of IKKβ in microglia, IKKβ/NF-κB-dependent inflammatory gene expression is attenuated. These data argue that LysM-Cre/IkkβF/F mice can be used to investigate the in vivo role of microglia activation in excitotoxic neurodegeneration.
It should be noted that, in these mice, only partial deletion (73% maximum) of the IkkβF allele in the KA-activated hippocampal microglia population was achieved. The incomplete deletion of the IkkβF allele in the entire population of microglial cells resembles the situation in macrophages, where the IkkβF deletion rate rarely exceeded 75% in bone marrow-derived macrophages from LysM-Cre/IkkβF/F mice (Greten et al., 2004). The incomplete deletion of a target gene is often a drawback of using tissue-specific conditional knockout mice. Although a study using conventional knockout mice does not face such problems, it does not provide researchers with cell type-specific information either. Indeed, it was reported that p50 knockout mice are more vulnerable to KA-induced excitotoxicity, indicating a beneficial role of NF-κB activation in these mice (Yu et al., 1999). However, such effects were attributed mainly to NF-κB activation in neurons, but not in microglia. Similarly, the neuroprotective function of microglial activation was suggested in a recent study using MyD88 knockout mice (Simard and Rivest, 2007), in which the effects of MyD88 deletion in microglia versus non-microglial cells could not be differentiated. Our in vivo data, however, indicate that IKKβ deletion in microglia exerts protective effects against KA-induced excitotoxic hippocampal neuronal cell death. Thus far, several reports have suggested a neurotoxic role of microglia in excitotoxicity. However, most of these studies are based on circumstantial evidence supported by in vitro experiments using cultured microglia. In this regard, our study using Ikkβ conditional knockout mice, conclusively demonstrates the in vivo role of the IKK/NF-κB-mediated microglia activation in excitotoxicity.
Interestingly, the attenuation of excitotoxicity in LysM-Cre/IkkβF/F mice was not statistically significant 1 day after injection, but was substantial 3 days after i.c.v. administration of KA (Fig. 2). It has been reported that introduction of KA into the brain induces excitotoxicity through two different mechanisms. Primary hippocampal damage is induced within 24 h by KA-mediated seizure activity, while further delayed neuronal cell death follows this initial damage after 2–3 days (Doble, 1999). The absence of any significant difference in terms of hippocampal EEG activity argues against that reduced neuronal death in the knockout mice is due to reduced seizures in these mice. Rather, our data suggest that IKKβ-mediated microglia activation contributes to delayed excitotoxic neurodegeneration in the later stage. These results are consistent with those of previous reports showing that inflammatory mediators contribute to excitotoxic neurodegeneration at later stages (Giulian and Vaca, 1993). Notably, we observed attenuation of glial cell activation not only in microglia, but also in astrocytes of LysM-Cre/IkkβF/F mice (Fig. 4A–K). Considering that Ikkβ is deleted only in microglia, it is likely that KA-induced astrocyte activation is secondary to microglia activation.
Thus far, it is not clear how microglia become activated upon KA stimulation. Although direct microglial activation by KA has been reported (Noda et al., 2000), we were not able to detect any proinflammatory gene expression after KA treatment of cultured hippocampal glial cells (data not shown). Therefore, it is more likely that microglia are indirectly activated by KA-damaged neurons. In this regard, it is of interest that high-mobility group box-1 (HMGB-1), a non-histone DNA-binding protein, was recently reported to be released by damaged neurons in the ischaemic brain, thus activating microglia (Kim et al., 2006). It has also been documented that HMGB-1 exerts its cytokine-like function by activating toll-like receptor (TLR) 2 and 4 on innate immune cells (Park et al., 2004). In the CNS, TLR2 and 4 are constitutively expressed on microglia (Olson and Miller, 2004). Considering that IKK/NF-κB activation is a major downstream signal of TLR, it is tempting to speculate that, in our excitotoxicity model, microglia are activated by TLR binding to HMGB-1 released from KA-damaged hippocampal neurons. This can be addressed in future studies using TLR2- or 4-deficient mice.
It should be noted that, in this study, we did not find direct in vivo evidence that the reduction in neuronal loss in the knockout mice was due to IKKβ deletion in microglia, since IKKβ in these mice was also deleted in other myeloid lineage cells. However, indirect evidence suggests microglia-specific effects on excitotoxicity. First, in immunohistochemistry tests, we did not observe a statistically significant reduction in macrophage infiltration in LysM-Cre/IkkβF/F mice after KA administration. More importantly, neurons in OHSCs from LysM-Cre/IkkβF/F mice were relatively resistant to the KA-induced excitotoxicity. In such an OHSC model, the effects of blood-derived myeloid cells were minimized, since hippocampal slices were cultured in vitro without blood supply for 2 weeks before the experiment. Considering these data, we concluded that microglia-specific IKKβ deletion plays a major role in the attenuation of excitotoxicity.
In ex vivo experiments, excitotoxic hippocampal cell death was reduced by 30–40% in the OHSCs of LysM-Cre/IkkβF/F mice, which is reminiscent of the reduction rate in vivo. However, the kinetics of the cell death were dissimilar. In the in vivo system, we did not observe a statistically significant difference in the cell death rate between wild-type and LysM-Cre/IkkβF/F mice 24 h after KA injection, whereas in OHSCs, the decrease in cell death was prominent after 24 h of recovery (Figs 2 and 6). This can be simply attributed to the temporal difference in KA accessibility to hippocampal neurons in vivo versus ex vivo. Alternatively, this can be explained by the difference in the deletion rate of Ikkβ at the time of stimulation. Since hippocampal slices were prepared from neonate mice, it is likely that the microglial Ikkβ deletion rate of OHSCs is higher than the in vivo rate of adult mice, which may account for the difference.
To elucidate the basis of neurotoxic effects of IKKβ activation, we monitored the expression of several putative neurotoxic mediators that can be induced upon IKKβ activation in microglia. KA-induced TNF-α, IL-1β and iNOS gene expression was reduced in the ipsilateral hippocampus of LysM-Cre/IkkβF/F mice (Fig. 7). In addition, exogenous addition of TNF-α and IL-1β to LysM-Cre/IkkβF/F OHSCs enhanced their excitotoxic susceptibility (Fig. 8). These data imply that IKKβ-dependent expression of these inflammatory cytokines may be, at least in part, responsible for the delayed excitotoxicity. The neurotoxic effects of IL-1β and iNOS in excitotoxicity are well documented (Hara et al., 1997). Likewise, TNF-α has been implicated as a critical mediator of neuronal cell death in cerebral ischaemia (Meistrell et al., 1997). However, it has also been reported that TNF-α expression during excitotoxic damage plays a neuroprotective role (Cheng et al., 1994). Furthermore, TNF receptor-deficient mice are more susceptible to excitotoxic brain injury, which also suggests a neuroprotective role of TNF-α in vivo (Bruce et al., 1996). Thus far, there is no clear explanation for these discrepancies. It should be noted, however, that the TNF receptor gene is deleted in all brain cells of the knockout mice, including neurons and glia, from early development. In LysM-Cre/IkkβF/F mice, however, TNF-α production is altered only in microglia and only after excitotoxic brain injury, which may account for the different results. Moreover, deletion of microglial Ikkβ reduced the expression of other inflammatory mediators. Therefore, the neurotoxic effects of microglial activation might be due to the concerted effects of various IKKβ target genes. In our study, we measured expression of proinflammatory cytokines in vivo, but confirmed their neurotoxic effects ex vivo using OHSCs. Therefore, it is formally possible that another IKKβ-dependent gene not tested in this study contributes to delayed neuronal cell death in vivo. For instance, microglial production of tPA has been proposed as a major player in delayed type excitotoxicity. However, in our system, there were no significant differences in mRNA expression of this gene after KA stimulation between wild-type and LysM-Cre/IkkβF/F mice (data not shown).
Finally, our data from MCAO model suggest that the neuroprotective role of microglial Ikkβ deletion is involved in a more physiological neurodegeneration. Interestingly, the attenuation rate of brain damage in LysM-Cre/IkkβF/F mice in a MCAO model was much higher than in a KA-excitotoxicity model. It has been documented that peripheral immune cells such as macrophages and neutrophils infiltrate the brain parenchyma after ischaemic brain injury, which contributes to neurodegeneration (Schroeter et al., 1994; Villa et al., 2007). In LysM-Cre/IkkβF/F mice, Ikkβ is also deleted in monocytes and neutrophils (Greten et al., 2004). Therefore, it is possible that the decrease in infarct size in LysM-Cre/IkkβF/F mice is not only due to microglial Ikkβ deletion, but also to Ikkβ deletion in peripheral immune cells, which may account for the difference in the inhibition rate between ischaemic injury versus KA-excitotoxicity. In this study, we did not delve further into the relative contribution of the Ikkβ deletion in peripheral immune cells versus microglia in the MCAO model.
In summary, we demonstrated, in this study, that microglia-specific IKKβ activation potentiates KA-induced excitotoxic hippocampal neuronal cell death. These results suggest that IKKβ-dependent inflammatory cytokine expression in microglia contributes to the potentiation of excitotoxic injury.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
Neurobiology Research Program at the Korea Ministry of Science and Technology, Republic of Korea (M10412000014-07N1200-01410); Korea Research Foundation Grant (KRF-2005-070-C00096). National Institutes of Health grants for Knockout mouse generation in San Diego (ES04151, ES006376 and AI043477); Korea Research Foundation Grant funded by the Korean Government (MOEHRD, KRF-2006-351-E00016 to I.-H.Cho).
Glossary
Abbreviations:
- CA1
cornu ammonis 1
- CA3
cornu ammonis 3
- CD11b
cluster of differentiation molecule 11b
- GFAP
glial fibrillary acidic protein
- HMGB-1
high-mobility group box-1
- Iba-1
ionized calcium binding adaptor molecule-1
- IL-1β
interleukin-1β
- IKK
IκB kinase
- IR
immunoreactive
- KA
kainic acid
- LPS
lipopolysaccharide
- MCAO
middle cerebral artery occlusion
- NF-κB
nuclear factor-kappa B
- NG2
neuron-glial antigen 2
- OHSCs
organotypic hippocampal slice cultures
- PI
propidium iodide
- PMΦ
peritoneal macrophages
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor-α
- tPA
tissue plasminogen activator
References
- Acarin L, Gonzalez B, Castellano B. STAT3 and NFkappaB activation precedes glial reactivity in the excitotoxically injured young cortex but not in the corresponding distal thalamic nuclei. J Neuropathol Exp Neurol. 2000;59:151–63. doi: 10.1093/jnen/59.2.151. [DOI] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. The CNS acute inflammatory response to excitotoxic neuronal cell death. Immunol Lett. 1991a;30:177–81. doi: 10.1016/0165-2478(91)90022-3. [DOI] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration. Neuroscience. 1991b;42:201–14. doi: 10.1016/0306-4522(91)90159-l. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–34. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–94. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–53. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Cho IH, Im JY, Kim D, Kim KS, Lee JK, Han PL. Protective effects of extracellular glutathione against Zn2+-induced cell death in vitro and in vivo. J Neurosci Res. 2003;74:736–43. doi: 10.1002/jnr.10794. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–90. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–33. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Eder C, Schilling T, Heinemann U, Haas D, Hailer N, Nitsch R. Morphological, immunophenotypical and electrophysiological properties of resting microglia in vitro. Eur J Neurosci. 1999;11:4251–61. doi: 10.1046/j.1460-9568.1999.00852.x. [DOI] [PubMed] [Google Scholar]
- Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–21. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Vaca K. Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke. 1993;24:I84–90. [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hao C, Richardson A, Fedoroff S. Macrophage-like cells originate from neuroepithelium in culture: characterization and properties of the macrophage-like cells. Int J Dev Neurosci. 1991;9:1–14. doi: 10.1016/0736-5748(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–12. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Sanchez C, Basile AS, Fedorova I, Arima H, Stannard B, Fernandez AM, et al. Mice transgenically overexpressing sulfonylurea receptor 1 in forebrain resist seizure induction and excitotoxic neuron death. Proc Natl Acad Sci USA. 2001;98:3549–54. doi: 10.1073/pnas.051012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SD, Walter SA, Semple-Rowland SL, Streit WJ. Cytokine transcripts expressed by microglia in vitro are not expressed by ameboid microglia of the developing rat central nervous system. Glia. 1999;25:304–9. doi: 10.1002/(sici)1098-1136(19990201)25:3<304::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276:44527–33. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Park SJ, Park JS, Lee KE. Glucose/oxygen deprivation induces the alteration of synapsin I and phosphosynapsin. Brain Res. 2004;996:47–54. doi: 10.1016/j.brainres.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–74. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–21. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004;189:122–30. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Kristensen BW, Noraberg J, Zimmer J. Comparison of excitotoxic profiles of ATPA, AMPA, KA and NMDA in organotypic hippocampal slice cultures. Brain Res. 2001;917:21–44. doi: 10.1016/s0006-8993(01)02900-6. [DOI] [PubMed] [Google Scholar]
- Lee S, Hong J, Choi SY, Oh SB, Park K, Kim JS, et al. CpG oligodeoxynucleotides induce expression of proinflammatory cytokines and chemokines in astrocytes: the role of c-Jun N-terminal kinase in CpG ODN-mediated NF-kappaB activation. J Neuroimmunol. 2004;153:50–63. doi: 10.1016/j.jneuroim.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Zhou T, Choi C, Wang Z, Benveniste EN. Differential regulation and function of Fas expression on glial cells. J Immunol. 2000;164:1277–85. doi: 10.4049/jimmunol.164.3.1277. [DOI] [PubMed] [Google Scholar]
- Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170:4630–7. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Macklis JD, Madison RD. Progressive incorporation of propidium iodide in cultured mouse neurons correlates with declining electrophysiological status: a fluorescence scale of membrane integrity. J Neurosci Methods. 1990;31:43–6. doi: 10.1016/0165-0270(90)90007-3. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Kitamura Y, Okazaki M, Terai K, Taniguchi T. Kainic acid-induced activation of nuclear factor-kappaB in rat hippocampus. Exp Brain Res. 1999;124:215–22. doi: 10.1007/s002210050616. [DOI] [PubMed] [Google Scholar]
- Meistrell ME 3rd, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, et al. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–8. [PubMed] [Google Scholar]
- Moriyama N, Taniguchi M, Miyano K, Miyoshi M, Watanabe T. ANP inhibits LPS-induced stimulation of rat microglial cells by suppressing NF-kappaB and AP-1 activations. Biochem Biophys Res Commun. 2006;350:322–8. doi: 10.1016/j.bbrc.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–6. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–8. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–7. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–51. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, et al. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–56. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–26. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in mouse peritoneal macrophages activated in vitro and in vivo: evidence against an essential role of peroxynitrite. FASEB J. 2001;15:2355–64. doi: 10.1096/fj.01-0295com. [DOI] [PubMed] [Google Scholar]
- Rasley A, Anguita J, Marriott I. Borrelia burgdorferi induces inflammatory mediator production by murine microglia. J Neuroimmunol. 2002;130:22–31. doi: 10.1016/s0165-5728(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003;14:494–503. doi: 10.1016/j.nbd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Neuroprotective effects of resident microglia following acute brain injury. J Comp Neurol. 2007;504:716–29. doi: 10.1002/cne.21469. [DOI] [PubMed] [Google Scholar]
- Slepko N, Levi G. Progressive activation of adult microglial cells in vitro. Glia. 1996;16:241–46. doi: 10.1002/(SICI)1098-1136(199603)16:3<241::AID-GLIA6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–4. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Villa P, Triulzi S, Cavalieri B, Di Bitondo R, Bertini R, Barbera S, et al. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med. 2007;13:125–33. doi: 10.2119/2007-00008.Villa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Hirst WD, Solito E, Wilkin GP. De novo expression of lipocortin-1 in reactive microglia and astrocytes in kainic acid lesioned rat cerebellum. Glia. 1999;26:333–43. doi: 10.1002/(sici)1098-1136(199906)26:4<333::aid-glia7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhou D, Bruce-Keller AJ, Kindy MS, Mattson MP. Lack of the p50 subunit of nuclear factor-kappaB increases the vulnerability of hippocampal neurons to excitotoxic injury. J Neurosci. 1999;19:8856–65. doi: 10.1523/JNEUROSCI.19-20-08856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D, Warfel A, Grusky G, Frangione B, Teitel D. Novel monocyte-like properties of microglial/astroglial cells. Constitutive secretion of lysozyme and cystatin-C. Lab Invest. 1987;57:176–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.