Abstract
Children with autism exhibit a host of motor disorders including poor coordination, poor tool use and delayed learning of complex motor skills like riding a tricycle. Theory suggests that one of the crucial steps in motor learning is the ability to form internal models: to predict the sensory consequences of motor commands and learn from errors to improve performance on the next attempt. The cerebellum appears to be an important site for acquisition of internal models, and indeed the development of the cerebellum is abnormal in autism. Here, we examined autistic children on a range of tasks that required a change in the motor output in response to a change in the environment. We first considered a prism adaptation task in which the visual map of the environment was shifted. The children were asked to throw balls to visual targets with and without the prism goggles. We next considered a reaching task that required moving the handle of a novel tool (a robotic arm). The tool either imposed forces on the hand or displaced the cursor associated with the handle position. In all tasks, the children with autism adapted their motor output by forming a predictive internal model, as exhibited through after-effects. Surprisingly, the rates of acquisition and washout were indistinguishable from normally developing children. Therefore, the mechanisms of acquisition and adaptation of internal models in self-generated movements appeared normal in autism. Sparing of adaptation suggests that alternative mechanisms contribute to impaired motor skill development in autism. Furthermore, the findings may have therapeutic implications, highlighting a reliable mechanism by which children with autism can most effectively alter their behaviour.
Keywords: reach adaptation, prism adaptation, motor control, autism
Introduction
Impaired performance of skilled motor tasks and gestures is perhaps the most consistent motor finding associated with autism (Smith and Bryson, 1994; Rogers et al., 1996; Williams et al., 2004; Mostofsky et al., 2006). While impairments in imitation have been emphasized (Williams et al., 2001; Rogers et al., 2003), children with autism also exhibit deficits in skilled motor performance in response to command and with tool use (Rogers et al., 1996; Mostofsky et al., 2006). This is suggestive of a more generalized dyspraxia. In a developmental context, impaired performance of skilled gestures may be secondary to a fundamental problem with acquiring motor skills. Indeed, in our clinical practice we have noted that children with autism often show delayed learning of novel motor skills such as peddling a tricycle or pumping their legs on a swing (Gidley Larson and Mostofsky, 2006).
While tool use or riding a tricycle are complex behaviours, evidence from numerous motor adaptation tasks in the last decade suggests that successful motor control requires forming an internal model that accurately predicts the sensory consequences of motor commands (for a recent review, see Krakauer and Shadmehr, 2007). Evidence from imaging (Diedrichsen et al., 2007), neurophysiology (Pasalar et al., 2006) and patient studies (Martin et al., 1996b; Lang and Bastian, 1999; Maschke et al., 2004; Smith and Shadmehr, 2005; Chen et al., 2006) suggest that the cerebellum is one of the key structures required to form accurate internal models. The development of the cerebellum appears to be abnormal in autism, as evidenced by both post-mortem and imaging studies (Williams et al., 1980; Ritvo et al., 1986; Bauman and Kemper, 1994; Bailey et al., 1998; Fatemi et al., 2002). Careful investigation of motor adaptation may thereby provide insight into the neurological mechanisms contributing to impaired motor function and skill acquisition in these children.
There is currently one previously published study of motor adaptation in autism (Mostofsky et al., 2004). That study examined a ball catching task that had been previously shown to depend on cerebellar function (Lang and Bastian, 1999). During the task the mass of the ball but not its visual appearance was suddenly changed, requiring subjects to adapt to the new dynamics. The findings revealed normal adaptation in high-functioning children with autism (HFA), with rates of learning and post-adaptation after-effects that were similar to typically developing (TD) controls. The results suggested that the children with autism could learn from a mismatch between the predicted and observed proprioceptive and visual consequences of the ball impact on their hand. In a sense, these children appeared to have a normal ability to rapidly adapt the internal model that predicted the consequences of the ball's impact on their arm.
Ball catching is only one example of an adaptation task known to rely on the cerebellum. There are more complex tasks that involve learning to control novel tools (e.g. reach while holding a robotic arm), or adapting arm movements in response to a transformation of the visual information (e.g. via a prism). These differ from ball catching task in that some of the perturbations are purely visual instead of mechanical, and that subjects have never experienced any of these unusual perturbations. We therefore studied children with HFA and TD controls adapting arm movements across these tasks. The results of our study suggest that the ability to learn from sensory prediction errors and acquisition of internal models is intact in autistic children.
Methods
Participants
A total of 41 (21 HFA and 20 TD) children participated in this study. Participants were recruited from out-patient clinics at the Kennedy Krieger Institute, local Autism Society of America (ASA) chapters, postings at schools, social skills groups, paediatrician's offices and word of mouth. The participants were between the ages of 8 and 13 years, had a birth weight >2000 g, had no history of seizures, neurological disorders primarily affecting motor performance, traumatic brain injury, mental retardation or known prenatal illicit drug exposure. All participants had normal or corrected-to-normal vision. Lastly, MRI confirmed that none of the participants had structural abnormalities within the cerebellum or any other brain regions.
All children in the HFA group met DSM-IV algorithm criteria for autism, confirmed using the Autism Diagnostic Interview—Revised (ADI-R; Lord et al., 1994) and Module 3 of the Autism Diagnostic Observation Schedule—Generic (ADOS-G; Lord et al., 2000) or an earlier edition of the ADOS (Lord et al., 1989).
Typically developing children were eligible for the study if they met the following criteria: no evidence of neurological disorder, no presence of autism spectrum disorders in any immediate family members (siblings, parents), free from diagnosis on a standardized psychiatric parent interview, the Diagnostic Interview for Children and Adolescents-IV (DICA-IV; Reich, 2000) (with the exception of simple phobia) and had no history or current use of any psychoactive medication.
Within the HFA group, five participants were taking stimulants, two were taking atypical neuroleptics and three were taking anti-depressant medications. Stimulant medications were discontinued the day prior to testing, but all other medications were taken as prescribed.
Intellectual functioning was assessed using the most current version of the Wechsler Intelligence Scale for Children (WISC) at the time of testing, WISC—3rd edition (Wechsler, 1991) (n = 6) or the WISC—4th edition (Wechsler, 2003) (n = 34), with the exception of one child whose intelligence was measured using the Differential Ability Scales (Elliott, 1990). All children had full scale IQ (FSIQ) ≥80 with the exception of two children with HFA. Both children had a FSIQ within 4 points of 80 and either their perceptual or verbal score was >90.
The study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and written assent was obtained from all participating children.
Procedures
Experiment 1: prism adaptation
Twenty children with HFA and 15 TD children participated in this task. Of the 20 HFA participants, 14 also completed at least one condition of the reach adaptation task, and of the 16 TD participants, 6 also completed at least one condition of the reach adaptation task. All participants were tested performing a prism adaptation task as has been previously described (Martin et al., 1996a, b). Subjects threw balls at a target during three periods: (i) ‘baseline’ with no prisms, (ii) ‘adaptation’ with prism goggles on [30 diopter (∼17°) Fresnel 3M Press-on plastic lenses, (3M Health Care, Specialties Division, St Paul, MN, USA)] and (iii) ‘post-adaptation’ with no prisms. The target was the centre 3 × 3 cm square on a wall grid, at shoulder height, 80 in. away. Subjects stood the entire time and no instructions were given about shoulder or trunk posture; however, participants were told to always keep their head looking forward at the target, and not to look down at their hands. They were also instructed to ‘throw with the same arm and to throw where you see the target’. Throughout the task, the examiner stood behind the subject and immediately recorded the grid position (3 cm increments) of impact of each throw. We recorded 10 baseline throws, 35 adaptation throws and 25 post-adaptation throws. We compared the performance of the two groups (HFA versus control) using repeated measures ANOVA across the following periods of the experiment: late baseline, early adaptation, late adaptation, early post-adaptation and late post-adaptation. The values for each period were calculated as the average of the first (or last) three trials within that period. Group data from the adaptation phase were fit to a single exponential decay function, with the time constant in trials representing the rate of adaptation (i.e. the number of trials to complete ∼63% of the adaptation).
Experiment 2: learning to control a novel tool
Participants were positioned in front of a horizontal screen (Fig. 1A). Using their right hand, they grasped the handle of a robot tool that was positioned below the screen (Shadmehr and Mussa-Ivaldi, 1994). The setup was identical to that used in Hwang et al. (2003): a smock was draped over the participant's shoulders to prevent view of the upper arm and the screen prevented view of the hand and the robotic tool. An overhead LCD projector painted the screen, displaying a cursor that represented handle position and a reach target (Fig. 1A). Participants were instructed to move the cursor to the target.
Fig. 1.
Experimental setup for the reach adaptation tasks. (A) The children were seated in front of a robotic arm and held its handle to manipulate position of a cursor on a projection screen. The goal of the task was to move the cursor from a home position to the target. Targets (1.5 × 1.5 cm2) were presented at one of six positions. In the force field condition, the robot produced a force proportional to the velocity of the handle. In the visual rotation condition, the position of the cursor with respect to the handle was rotated. (B) The experimental procedure. The children began the task with 300 trials in which no perturbations were applied. This was followed by 150 force field trials, then 100 trials of washout, then 150 visual rotation trials and finally 100 trials of washout. The order of field/visual blocks was randomly assigned for each child.
The behaviour of the robotic tool underwent two different kinds of changes and we considered the ability of the subjects to adapt to these changes. In one block of trials, the robot's dynamics changed as it produced a force field. In another block of trials, the robot's kinematics changed as it imposed a rotation on the position of the cursor with respect to the position of the handle. The experiments will be described in detail below.
Twelve children with HFA participated in the force perturbation trials while 13 children with HFA participated in the visual perturbation trials. A total of 10 TD children participated in both force and visual perturbation trials. Participants were first familiarized with the robot by performing movements in the absence of any perturbations (i.e. 300 null trials). Given the young age of our participants, a break of at least 1 h was taken after the first 250 null trials. Prior to beginning the adaptation trials, 50 null trials were given (for a total of 300 null trials). This was followed by 150 adaptation trials in which the robot applied a perturbation. In the force-field block, the perturbation was a clockwise curl field (Donchin et al., 2002), in which forces on the hand were a function of hand velocity:  with B = [0 13; –13 0] N/m/s. In the visual-rotation block, the perturbation was a clockwise 19° rotation of the motion of the cursor with respect to the motion of the hand. Catch-trial (trials in which the field was removed) were randomly placed at a rate of 1/6 of the trials. The adaptation trials were followed by 100 washout trials. The order of the perturbation blocks was counterbalanced among participants (Fig. 1B).
with B = [0 13; –13 0] N/m/s. In the visual-rotation block, the perturbation was a clockwise 19° rotation of the motion of the cursor with respect to the motion of the hand. Catch-trial (trials in which the field was removed) were randomly placed at a rate of 1/6 of the trials. The adaptation trials were followed by 100 washout trials. The order of the perturbation blocks was counterbalanced among participants (Fig. 1B).
Participants were instructed to keep the cursor at ‘home’ (centre square) until a 6 mm target square appeared in one of six positions 10 cm distance from the centre square, then to move the cursor to the target square as quickly and smoothly as possible. The targets appeared in a pseudo-random order; the order was the same for all participants and was the same for both adaptation and washout blocks. The target square would appear only after the participant placed the cursor in the home position. When the target square appeared, the home target would disappear and the participant would make the movement. If the cursor was placed in target within 500 ± 50 ms the target box would ‘explode’ and give a pleasant sound. However, a buzzing sound would be presented if the velocity during the movement exceeded 0.55 m/s or if the max velocity never got past 0.20 m/s. The participant earned 1 point for every explosion and lost 1 point for every buzz. The points earned were later cashed in for a prize. Short breaks were given throughout the tasks as needed. Our dependent measure was Perpendicular Displacement (PD), defined as the distance from a straight line (from the position of the hand before movement to the centre of the target) at maximum velocity (Fig. 2). Learning indices (LI) (Donchin et al., 2002) were determined using the following equation:
Early in training, PDs are small in the catch trials and large in the field trials, the LI is close to zero. Late in training, PDs in catch trials should be large and PDs in field trials should be small, so LI rises toward 1. The maximum value is around 0.8 when the probability of catch trial is 1/6. LI was calculated on PDs averaged over 15 consecutive movements.
Fig. 2.
Prism adaptation. (A) Throwing errors on the prism task for the typically developing children and the children with HFA across the baseline (no prisms), adaptation (with prism goggles) and post-adaptation (no prisms) phases. Deviations to the left of the target are negative values and deviations to the right of the target are positive values, 0 represents the target. Error bars are SD. (B) The average errors across selected trials (first or last three trials in a period). There were no significant differences in group performance and there was no significant [(group) × (trial)] interaction (P > 0.05), indicating that the HFA and TD groups performed similarly across all phases of the experiment. Error bars are SD.
For the purpose of analyses, the PD of every 15 movements was averaged across each block for every subject, yielding 20 null data points, 10 data points for each adaptation block and 7 data points for each washout block. Using this average PD, 9 LI were calculated for each subject across each of the adaptation blocks. We compared the LI of the two groups (HFA versus control) using repeated measures ANOVA across each adaptation block.
Analysis
Specific details regarding statistical analyses are explained in each section respectively. In all experiments repeated measures ANOVA was used to examine change (i.e. learning) across blocks of trials. For each experiment we report the main effects of group (diagnosis) and trial/block, as well as the effect of the [(group) × (trial/block)] interaction; the latter was used to examine for group differences in learning.
Results
Given that each experiment had a different number of participants, IQ data for each sample are given in the respective results sections. Recent findings suggest that measures of perceptually based reasoning are more valid for assessment of intellectual ability in children with autism spectrum disorders (Mottron, 2004) than is full-scale IQ. Perceptual reasoning index (PRI) from the WISC-4 and the performance IQ (PIQ) from the WISC-3 were therefore used to compare intellect across groups.
Demographically, the entire sample was predominantly Caucasian (81% Caucasian, 14% African-American and 2% Hispanic), right-handed (81%) and male (74%). The socio-economic status of the group fell in the ‘medium–high’ range, according to Hollingshead guidelines (Hollingshead, 1975), with a mean score of 55 points (range 27–66). There were no significant differences between the groups on gender, ethnicity, handedness, socio-economic status or ADOS score.
Experiment 1: prism adaptation
Twenty children with HFA and 16 TD children participated in this task. There were no significant differences in the mean age or in the mean PRI/PIQ from the WISC-4/3 between the two groups (Table 1).
Table 1.
Demographic variables
| Gender |
Age |
PRI/PIQ |
ADOS |
|||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | SD | M | SD | M | SD | |
| Experiment 1: prism adaptation | ||||||||
| HFA n = 20 | 17 | 3 | 10.9 | 1.8 | 110.0 | 14.9 | 14.3 | 4.0 |
| Control n = 16 | 11 | 6 | 10.8 | 1.3 | 112.8 | 11.6 | – | – |
| Experiment 2: reach adaptation | ||||||||
| HFA n = 15 | 13 | 2 | 11.1 | 1.6 | 108.9 | 15.6 | 14.4 | 3.7 |
| Control n = 10 | 8 | 2 | 11.7 | 1.5 | 117.0 | 12.0 | – | – |
Figure 2A shows the trial-by-trial behaviour on the prism task for the TD and HFA groups. Note the similar extent and time course of adaptation, and the similar post-adaptation after-effects. Adaptation rates were similar between groups: 4.1 trials for the control group and 4.32 trials for the HFA group. Figure 2B shows the average data across selected trials (i.e. first or last three trials in a period), which were used for statistical tests. Both groups showed the initial errors in early adaptation trials, reduced errors in late adaptation trials and the after-effects in early post-adaptation trials. Repeated measures ANOVA showed a significant effect of period, which demonstrates that behaviour changed in both groups during the task [F(4,136) = 122; P < 0.001]. There was also a group effect caused by a slight offset in control versus HFA group behaviour [F(1,34) = 5.0; P = 0.03]. Most importantly, there was no group by period interaction [F(4,136) = 1.8; P = 0.13] suggesting that the HFA group did not adapt differently across period than controls.
Experiment 2: learning control of a novel tool
Fifteen children with HFA and 10 TD children participated in both tasks. There were no significant differences in the mean age or in the mean PRI/PIQ from the WISC-4/3 between the two groups (Table 1).
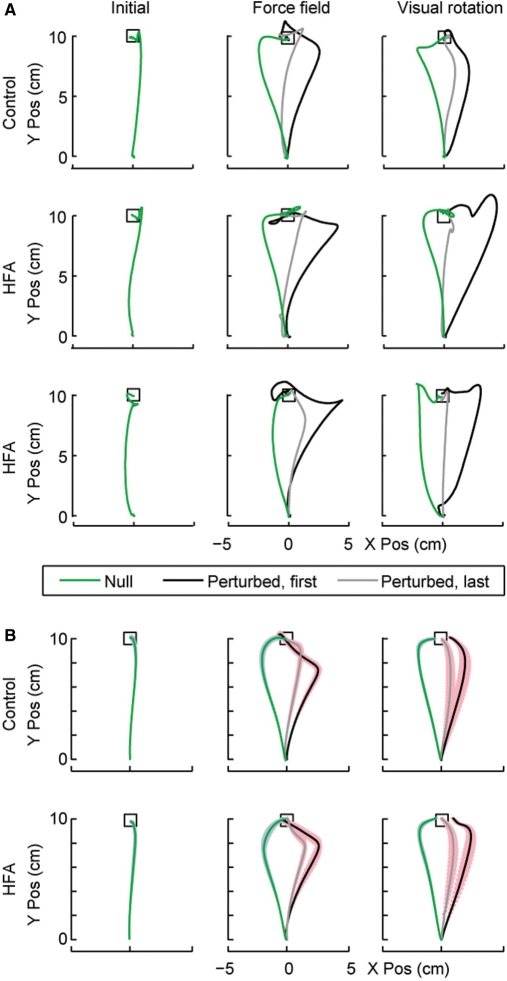
Reach trajectories of two typical HFA children and a TD child are shown in Fig. 3A for the force and rotation tasks. The mean trajectories across children are shown in Fig. 3B. All children exhibited clear indications of adaptation: reduced errors in field trials and increased errors in catch trials. The errors (angular error at 200 ms, or perpendicular displacement from a straight line at peak velocity, PD) of every 15 movement were averaged across each block for every subject, yielding 20 null data points (the last four are shown in Fig. 4A, null block), 10 data points for each adaptation block and 7 data points for each washout block (Fig. 4A). The two measures of error were very similar. Using PD as our measure of movement error, repeated measures ANOVA revealed that while reach errors declined with increased trials in both the TD and HFA groups in both the force field and the visual rotation tasks [force field F(9,180) = 32.4; P < 0.0001; visual rotation F(9,189) = 48.6; P <.0001], there was no significant effect of group [force field F(1,20) = 0.7; P = 0.43, visual rotation F(1,21) = 0.2; P = 0.60] and there was no significant interaction [force field F(9,180) = 1.2; P = 0.27; visual rotation F(9,189) = 1.1; P = 0.34], suggesting that there were no differences in rates of adaptation.
Fig. 3.
(A) Reach trajectories of the cursor positions for two HFA children and one TD child. The left column shows a single reaching movement for each child in the null condition (last trial in the null field). The middle column shows reach trajectories in the force field block. The black line is the first field trial and the grey line is the last field trial. The green line is the last catch trial (null field) in this block. The right column shows reach trajectories in the visual rotation block. The black line is the first rotation trial and the grey line is the last rotation trial. The green line is the last catch trial (no rotation) in this block. The trajectories begin at the centre “home” square and the upper box is the target. (B) Mean reach trajectories for cursor positions for the HFA children and the controls. Starting positions and overall movement direction have been aligned across subjects before averaging, and only the first 700 ms of the movement are shown. The coloured patch behind each line represents the SEM of the movements across children at each time point. In other respects, the format is as in A.
Fig. 4.
Reach adaptation. (A) Errors during reach adaptation. The top sub-plot shows the angular error at 200 ms in the movement (with respect to a straight line connecting home position to the target). The bottom sub-plot shows the PD at maximum hand speed with respect to a straight line. Bin size is 15 trials. The data is average error across subjects. Error bars are SEM. The solid lines show the error in field or visual rotation trials, while the dashed lines show the errors in catch trials. (B) LI were calculated on PDs averaged over 15 consecutive movements. Zero indicates no adaptation, and 1 indicates complete adaptation. Given the fact that catch trials produce unlearning (Thoroughman and Shadmehr, 2000), the maximum possible learning index is about 0.85.
Similarly, repeated measure ANOVAs of the washout trials revealed that across both groups of subjects, there was a significant effect of trial in the post-adaptation washout trials [force field F(6,114) = 20.5; P < 0.0001; visual rotation F(6,120) = 18.7; P < 0.0001], and there were no significant effects of group [force field F(1,19) = 1.4; P = 0.25; visual rotation F(1,20) = 1.2; P = 0.28] or interaction [force field F(6,114) = 1.2; P = 0.32; visual rotation F(6,120) = 0.6; P = 0.76]. Therefore, both groups demonstrated similar patterns of post-adaptation after effects.
Analysis of the learning index (LI) produced very similar results (Fig. 4B). While there was a significant effect of trial [force field F(8,152) = 23.9; P < 0.0001; visual rotation F(8,152) = 36; P < 0.0001], there was no significant effect of group or interaction (all P-values > 0.24).
Discussion
We considered three well-studied motor adaptation protocols to test the ability of children with autism to acquire internal models of action. Two experiments involved learning to control a novel tool (reach adaptation with a robotic arm), while the third involved learning to compensate for a transformation on the visual input. As evidenced by the after-effects of adaptation, the children with autism improved their performances through formation of predictive internal models, with rates of acquisition and forgetting that were not different from normally developing children.
The ability to adapt voluntary movements to novel conditions introduced by prisms, or to novel tools introduced by robots, is thought to depend on the integrity of the cerebellum. For example, adaptation to distorting prisms (Weiner et al., 1983), visual transformations (Sanes et al., 1990) and force fields (Maschke et al., 2004; Smith and Shadmehr, 2005), as well as many non-reaching adaptation tasks (for review, see Ito, 2002), are impaired with cerebellar damage. Other conditions that have been consistently implicated in prism adaptation are posterior parietal damage (Welch and Goldstein, 1972; Newport and Jackson, 2006) and schizophrenia (Bigelow et al., 2006). In contrast, studies on amnesia (Milner et al., 1998; Shadmehr et al., 1998), Alzheimer's and Korsakoff's (Weiner et al., 1983) have not found any effect on adaptation of reaching movements.
However, the role of basal ganglia in learning of such skills has been more controversial. While impairments in motor adaptation have been found in patients in Parkinson's disease (Boller et al., 1984; Canavan et al., 1990; Contreras-Vidal and Buch, 2003) and Huntington's disease (Paulsen et al., 1993), a number of well-designed studies have failed to see any evidence for an impairment in either of these conditions, especially during initial learning (Weiner et al., 1983; Stern et al., 1988; Fernandez-Ruiz et al., 2003; Marinelli et al., 2008). Several authors have suggested that this could be explained because some paradigms allow for explicit learning or strategizing, and that in these cases basal ganglia disorders can affect learning (Contreras-Vidal and Buch, 2003; Fernandez-Ruiz et al., 2003; Marinelli et al., 2008). A recent report found normal learning in PD but abnormal consolidation of the motor memories (Marinelli et al., 2008). Therefore, while there is little doubt that cerebellar disorders generally produce impaired learning in the motor tasks that were studied here, the role of the basal ganglia in the learning process remains poorly understood (Shadmehr and Krakauer, 2008).
Given the highly consistent finding from post-mortem studies revealing cerebellar pathology in autism (Williams et al., 1980; Ritvo et al., 1986; Bauman and Kemper, 1994; Bailey et al., 1998; Fatemi et al., 2002); it should follow that children with autism would demonstrate impairment in motor adaptation. However, our findings here suggest otherwise. Our data suggest that motor adaptation is normal in children with autism. One possible interpretation is that cerebellar function is still largely intact in autism, despite neuroanatomical changes observed in individuals with the disorder. If this is true, then the deficits in motor function and skill acquisition (i.e. gait, coordination, balance, rhythmicity, motor planning/sequencing, imitation and dyspraxia; for review see Gidley Larson and Mostofsky, 2006) seen in autism may instead be due to dysfunction within other regions critical for motor/procedural learning (i.e. frontal, parietal, basal ganglia) or abnormalities in connections between these regions.
In particular, parietal regions are critical for the storage and implementation of spatial and temporal representations of movement formulas (Heilman and Gonzalez Rothi, 2003). These representations are necessary for acquiring and executing novel motor sequences. Children with autism also show impairments in motor control and planning (i.e. impairments in producing correct grip position for picking up an item) (Hughes, 1996), dysrythmic movements (Jansiewicz et al., 2006), and functions important to motor/procedural learning for which the frontal lobe and basal ganglia are integral (Rinehart et al., 2001; Lehericy et al., 2005; Monchi et al., 2006; Rinehart et al., 2006a, b). Findings from neuroimaging studies in individuals with autism provide evidence for abnormalities in these regions (Piven et al., 1996; Abell et al., 1999; Carper and Courchesne, 2000; Carper et al., 2002; McAlonan et al., 2002; Hardan et al., 2003; Carper and Courchesne, 2005). Further, it has been suggested that autism is associated with an overgrowth of localized cortical connections with undergrowth of more distant connections between cerebral cortical regions and subcortical structures (Herbert et al., 2004, 2005; Happe and Frith, 2006), with resulting impaired complex information processing (Minshew et al., 1997) and ‘weak central coherence’ (Shah and Frith, 1993). Thus, the deficits in motor function and in motor skill acquisition seen in autism may be due to abnormalities in neural connections across a distributed network.
Alternatively, it is possible that the cerebellar lesions found in individuals with autism are reflective of abnormal development and may be of different clinical significance than acquired cerebellar lesions. Studies examining the effect of cerebellar lesions on motor adaptation have thus far focused on humans and macaque monkeys with acquired lesions (Martin et al., 1996b; Baizer et al., 1999). The findings reveal that motor adaptation relies on cerebellar mechanisms to learn motor patterns through trial and error (Lang and Bastian, 1999). However, there is little known about the effect of cerebellar lesions occurring early in brain development. Given the developmental context of autism, compensatory mechanisms may exist leading to normal adaptation.
Adaptation of movement is a basic function central to successful performance of simple tasks necessary for survival. More specifically, humans are constantly adjusting their internal model to account for the effects of the external environment (i.e. moving while holding and object, weight of object, etc.), as well as internal changes (i.e. fatigue, growth, etc.). For instance, in order to reach out, grab a hold of food, and bring it to one's mouth to eat, the internal model must constantly be adjusting for the distance of the food from the body, the type of grip required to grasp the food, the weight of the food, the movement trajectory of the arm from the table to the mouth, the width of the mouth, etc. Given that motor adaptation may be critical for human development and survival, in the face of cerebellar lesions occurring early in development, adaptation may be preserved at the expense of other cerebellar functions.
In an fMRI study of attention and simple motor function in individuals with autism, Allen and Courchesne (2003) reported that cerebellar activation in the autism group, compared to a typically developing control group, spread from the areas normally associated with simple motor tasks (paleocerebellum ipsilateral to movement) to include regions of the cerebellum not associated with simple motor tasks (contralateral and posterior cerebellum). The authors posited that an ‘early loss of Purkinje neurons might cause more primitive functions normally subserved by paleocerebellar regions to be displaced into the neocerebellum at the cost of tissue that subserves cognitive function’ (p. 271). As such, a loss of Purkinje cells in early developing brains of children with autism may result in preferential sparing of motor adaptation, which is central to survival, resulting in less availability of cerebellar resources necessary for other motor and non-motor functions.
Along these lines it may also be possible that children with autism rely on explicit, declarative mechanisms to guide more procedurally based motor adaptation and learning. Parents of children with HFA and Asperger's syndrome commonly report above-average ability to memorize scripted information (Gidley Larson and Mostofsky, 2006) and published studies suggest that individuals with autism show both impaired procedural learning (Mostofsky et al., 2000) and excessive reliance on explicit/declarative learning when acquiring predictive knowledge (Klinger and Dawson, 2001; Walenski et al., 2008). In the reach adaptation task that we considered here, performance improvements rely not only on the implicit memory systems, but are also aided by the declarative system (Hwang et al., 2006). Although the influence of the declarative system is thought to be small, it is possible that in children with autism it plays a more prominent role. Because generalization patterns exhibited by the declarative contributions are distinct from the implicit system (Malfait and Ostry, 2004), future experiments may be able to test whether the robust performance exhibited by the autistic children is due to their declarative system.
Given the central role of adaptation to survival, it is possible that for individuals with autism, abnormalities in the cerebellum and other areas critical to motor learning necessitate recruitment of circuits involved in declarative learning. Manipulations to adaptation paradigms, such as gradual perturbation that minimizes explicit awareness, would help to examining this hypothesis. Techniques that examine neural activity associated with these functions (e.g. fMRI) would also be useful. Specifically fMRI would help to determine whether individuals with HFA demonstrate compensated use of cerebellar regions or employ different brain regions, such as the basal ganglia, in motor adaptation.
A limitation of the current and previously published studies on motor adaptation (Mostofsky et al., 2004) in autism is that examination focused on uni-manual upper-limb adaptation. Gait abnormalities are often reported in autism (DeMyer et al., 1981; Vilensky et al., 1981; Rinehart et al., 2006a, b), in contrast to upper limb movements, gait adaptation relies on more medial cerebellar regions. Examination of gait adaptation may provide further insight into the neurological basis of autism. A second limitation of this study is that while the acquisition of internal models of sensorimotor adaptation appears to be normal in children with autism, it is unclear whether there would be any form of generalization of these newly acquired internal models on future performance. Thus, further research examining performance over days and/or weeks is warranted in order to determine consolidation as well as generalization towards the performance of future movements.
Lastly, it is possible that in the various tests of motor learning, performance of the HFA children was comparable to healthy controls because our sample size was too small to detect significant differences. To quantify the likelihood of this, we performed a power analysis. When a two-sided t-test for independent samples was used to compare the control and autism groups’ final LI at a significance level of 5%, our sample size had 80% power to detect a difference in learning index of 0.15 (1.3 SD) for the force field task, and of 0.20 (1.3 SD) for the visual rotation task. The same analysis was done for the prism after-effects, and it showed that our sample size had 80% power to detect a 16 cm (1.0 SD) difference. The ability to detect differences in the mean of two groups that are within 1.5 SD of each other indicates that our experiment had power to detect reasonably small differences in performance. Therefore, it is unlikely that our findings represent a type II error.
In summary, children with autism demonstrated normal motor adaptation in a number of tasks that required acquisition of an internal model. These findings are consistent with previous findings of normal adaptation (Mostofsky et al., 2004) and are despite the overwhelming evidence of cerebellar pathology in individuals with autism.
Funding
National Alliance for Autism Research/Autism Speaks; National Institutes of Health (K02 NS 044850 to S.H.M., RO1NS048527 to S.H.M.); Johns Hopkins General Clinical Research Center (M01 RR00052); The Binational Science Foundation (2005513 to R.S. and O.D.).
Acknowledgement
We would also like to thank Megan Roeder for help with subject recruitment and testing.
Glossary
Abbreviations:
- HFA
high-functioning children with autism
- LI
learning index
- PD
perpendicular displacement
- PRI
perceptual reasoning index
- TD
typically developing
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–73. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Baizer J, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol. 1999;81:1960–5. doi: 10.1152/jn.1999.81.4.1960. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism. Baltimore, MD: The Johns Hopkins University Press; 1994. [Google Scholar]
- Bigelow NO, Turner BM, Andreasen NC, Paulsen JS, O’Leary DS, Ho BC. Prism adaptation in schizophrenia. Brain Cogn. 2006;61:235–42. doi: 10.1016/j.bandc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Boller F, Passafiume D, Keefe N, Rogers K, Morrow L, Kim Y. Visuospatial impairment in Parkinson's disease. Role of perceptual and motor factors. Arch Neurol. 1984;41:485–90. doi: 10.1001/archneur.1984.04050170031011. [DOI] [PubMed] [Google Scholar]
- Canavan AG, Passingham RE, Marsden CD, Quinn N, Wyke M, Polkey CE. Prism adaptation and other tasks involving spatial abilities in patients with Parkinson's disease, patients with frontal lobe lesions and patients with unilateral temporal lobectomies. Neuropsychologia. 1990;28:969–84. doi: 10.1016/0028-3932(90)90112-2. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123(Pt 4):836–44. doi: 10.1093/brain/123.4.836. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–33. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–51. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R. Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex. 2006;16:1462–73. doi: 10.1093/cercor/bhj087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Buch ER. Effects of Parkinson's disease on visuomotor adaptation. Exp Brain Res. 2003;150:25–32. doi: 10.1007/s00221-003-1403-y. [DOI] [PubMed] [Google Scholar]
- DeMyer M, Hingtgen J, Jackson R. Infantile autism reviewed: a decade of research. Schizophr Bull. 1981;7:388–451. doi: 10.1093/schbul/7.3.388. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Criscimagna-Hemminger SE, Shadmehr R. Dissociating timing and coordination as functions of the cerebellum. J Neurosci. 2007;27:6291–301. doi: 10.1523/JNEUROSCI.0061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Sawaki L, Madpu G, Cohen L, Shadmehr R. Mechanisms influencing acquisition and recall of motor memories. J Neurophysiol. 2002;88:2114–23. doi: 10.1152/jn.2002.88.4.2114. [DOI] [PubMed] [Google Scholar]
- Elliott CD. San Antonio, TX: The Psychological Corporation; 1990. Differential ability scales. [Google Scholar]
- Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–5. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Diaz R, Hall-Haro C, Vergara P, Mischner J, Nunez L, et al. Normal prism adaptation but reduced after-effect in basal ganglia disorders using a throwing task. Eur J Neurosci. 2003;18:689–94. doi: 10.1046/j.1460-9568.2003.02785.x. [DOI] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: a neurological disorder of early brain development. London: MacKeith Press; 2006. [Google Scholar]
- Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Kilpatrick M, Keshavan MS, Minshew NJ. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. J Child Neurol. 2003;18:317–24. doi: 10.1177/08830738030180050801. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Gonzalez Rothi LJ. Apraxia. New York: Oxford University Press; 2003. [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–26. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Hughes C. Brief report: planning problems in autism at the level of motor control. J Autism Dev Disord. 1996;26:99–107. doi: 10.1007/BF02276237. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Donchin O, Smith MA, Shadmehr R. A gain-field encoding of limb position and velocity in the internal model of arm dynamics. PLoS Biol. 2003;1:E25. doi: 10.1371/journal.pbio.0000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EJ, Smith MA, Shadmehr R. Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Exp Brain Res. 2006;173:425–37. doi: 10.1007/s00221-006-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Ann NY Acad Sci. 2002. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning; p. 978. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa RJ, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. J Autism Dev Disord. 2006;36:613–21. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Klinger L, Dawson G. Prototype formation in autism. Dev Psycholopathol. 2001;13:111–24. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Krakauer J, Shadmehr R. Towards a computational neuropsychology of action. Prog Brain Res. 2007;165:383–94. doi: 10.1016/S0079-6123(06)65024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J Neurophysiol. 1999;82:2108–19. doi: 10.1152/jn.1999.82.5.2108. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 2005;102:12566–71. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Malfait N, Ostry D. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci. 2004;24:8084–9. doi: 10.1523/JNEUROSCI.1742-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, et al. Parkinsonism and related disorders. 2008. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian A, Thach WT. Throwing while looking through prisms II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996a;119:1199–211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996b;119(Pt 4):1183–98. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez C, Ebner T, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–8. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain. 2002;125:1594–606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire L, Kandel E. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–68. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–16. [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–64. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Bunoski R, Morton S, Goldberg MC, Bastian A. Children with autism adapt normally during a catching task implicating the cerebellum. Neurocase. 2004;10:60–4. doi: 10.1080/13554790490960503. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J Int Neuropsychol Soc. 2006;12:314–26. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. J Int Neuropsychol Soc. 2000;6:752–9. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Mottron L. Matching strategies in cognitive research with individuals with high-functioning autism: current practices, instrument biases, and recommendations. J Autism Dev Disord. 2004;34:19–27. doi: 10.1023/b:jadd.0000018070.88380.83. [DOI] [PubMed] [Google Scholar]
- Newport R, Jackson S. Posterior parietal cortex and the dissociable components of prism adaptation. Neuropsychlogia. 2006;44:2757–65. doi: 10.1016/j.neuropsychologia.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Pasalar S, Roitman A, Durfee W, Ebner T. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;11:1404–11. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Butters N, Salmon D, Heindel W, Swenson M. Prism adaptation in Alzheimer's and Huntington's disease. Neuropsychology. 1993;7:73–81. [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 1996;35:530–6. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Movement preparation in high-functioning autism and Asperger disorder: a serial choice reaction time task involving motor reprogramming. J Autism Dev Disord. 2001;31:79–88. doi: 10.1023/a:1005617831035. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Bradshaw JL, Iansek R, Enticott PG, McGinley J. Gait function in high-functioning autism and Asperger's disorder: evidence for basal-ganglia and cerebellar involvement? Eur Child Adolesc Psychiatry. 2006a;15:256–64. doi: 10.1007/s00787-006-0530-y. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Iansek R, McGinley J, Brereton AV, Enticott P, et al. Gait function in newly diagnosed children with autism: Cerebellar and basal ganglia related motor disorder. Dev Med Child Neurol. 2006b;48:819–24. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry. 1986;143:862–6. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Rogers S, Bennetto L, McEvoy R, Pennington B. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Dev. 1996;67:2060–73. [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44:763–81. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Sanes J, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain. 1990;113:103–20. doi: 10.1093/brain/113.1.103. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brandt J, Corkin S. Time-dependent motor memory processes in amnesic subjects. J Neurophysiol. 1998;80:1590–7. doi: 10.1152/jn.1998.80.3.1590. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–81. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? J Child Psychol Psychiatry. 1993;34:1351–64. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Smith IM, Bryson SE. Imitation and action in autism: a critical review. Psychol Bull. 1994;116:259–73. doi: 10.1037/0033-2909.116.2.259. [DOI] [PubMed] [Google Scholar]
- Smith M, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Hermann A, Rosen J. Prism adaptation in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:1584–7. doi: 10.1136/jnnp.51.12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–7. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilensky JA, Damasio AR, Maurer RG. Gait disturbances in patients with autistic behavior: a preliminary study. Arch Neurol. 1981;38:646–9. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, Gidley Larson JC, Ullman MT. Brief report: enhanced picture naming in autism. J Autism Dev Disord. 2008;38:1395–9. doi: 10.1007/s10803-007-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 3rd edn. San Antonio, TX: The Psychological Corporation; 1991. Manual for the Wechsler Intelligence Scale for children. [Google Scholar]
- Wechsler D. 4th edn. San Antonio, TX: The Psychological Corporation; 2003. Manual for the Wechsler Intelligence Scale for children. [Google Scholar]
- Weiner M, Hallett M, Funkenstein H. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–72. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Welch R, Goldstein G. Prism adaptation and brain damage. Neuropsychologia. 1972;10:387–94. doi: 10.1016/0028-3932(72)90001-2. [DOI] [PubMed] [Google Scholar]
- Williams RS, Hauser SL, Purpura DP, DeLong GR, Swisher CN. Autism and mental retardation: neuropathologic studies performed in four retarded persons with autistic behavior. Arch Neurol. 1980;37:749–53. doi: 10.1001/archneur.1980.00500610029003. [DOI] [PubMed] [Google Scholar]
- Williams J, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J Autism Dev Disord. 2004;34:285–99. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25:287–95. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]