Abstract
The developing brain has the capacity for a great deal of plasticity. A number of investigators have demonstrated that intellectual and language skills may be in the normal range in children following unilateral perinatal stroke. Questions have been raised, however, about whether these skills can be maintained at the same level as the brain matures. This study aimed to examine the stability of intellectual, academic and language functioning during development in children with perinatal stroke, and to resolve the inconsistencies raised in previous studies. Participants were 29 pre-school to school-age children with documented unilateral ischaemic perinatal stroke and 24 controls. Longitudinal testing of intellectual and cognitive abilities was conducted at two time points. Study 1 examined IQ, academic skills and language functions using the same test version over the test–retest interval. Study 2 examined IQ over a longer test–retest interval (pre-school to school-age), and utilized different test versions. This study has resulted in important new findings. There is no evidence of decline in cognitive function over time in children with perinatal unilateral brain damage. These results indicate that there is sufficient ongoing plasticity in the developing brain following early focal damage to result in the stability of cognitive functions over time. Also, the presence of seizures limits plasticity such that there is not only significantly lower performance on intellectual and language measures in the seizure group (Study 1), but the course of cognitive development is significantly altered (as shown in Study 2). This study provides information to support the notion of functional plasticity in the developing brain; yields much-needed clarification in the literature of prognosis in children with early ischaemic perinatal stroke; provides evidence that seizures limit plasticity during development; and avoids many of the confounds in prior studies. A greater understanding of how children with ischaemic perinatal stroke fare over time is particularly important, as there has been conflicting information regarding prognosis for this population. It appears that when damage is sustained very early in brain development, cerebral functional reorganization acts to sustain a stable rate of development over time.
Keywords: ischaemic perinatal stroke (IPS), brain development, plasticity, cognitive development, focal lesion, intelligence, language
Evidence for stability of intellectual and cognitive functions in children with pre- or peri-natal focal brain damage
The developing brain has the capacity for a great deal of plasticity. In the face of an early insult, there is the potential for functional reorganization. Plasticity has been demonstrated numerous times in both animal (see review by Stiles, 2000; Goldman, 1972; Merzenich and Kaas, 1982; Kilgard and Merzenich, 1998; Xerri et al., 1998) and human studies (Lennenberg, 1967; Thal et al., 1991; Dall’Oglio et al., 1994; Stiles, 1995, 2000; Bates, 1998, 1999). A number of investigators have demonstrated that intellectual and language skills may be in the normal range in children following perinatal stroke and similar focal brain lesions (Aram et al., 1985; Aram and Ekelman, 1986; Riva and Cazzaniga, 1986; Riva et al., 1986; Aram, 1988; Trauner et al., 1993; Ballantyne et al., 1994; Stiles, 1995; Isaacs et al., 1996; Reilly et al., 2004). Questions have been raised, however, about whether these skills can be maintained at the same level as the brain matures (Banich et al., 1990; Aram and Eisele, 1994; Muter et al., 1997; Levine et al., 2005; Stiles et al., 2005). Since different parts of the brain develop at different ages, it is possible that problems not present on initial testing early in life might become apparent as pruning and other ‘refinements’ in brain development occur.
There have been few longitudinal studies of cognitive development in children with early stroke, and results of those studies have been conflicting. Aram and Eisele (1994) studied the longitudinal effects of focal brain damage sustained in childhood on the stability of IQ in children with unilateral brain damage and no ongoing seizure disorder. They concluded that children with congenital or acquired unilateral lesions have relatively stable patterns of intellectual performance over time. Muter et al. (1997) conducted a longitudinal study of IQ in young children (3–6 years at first data point) with presumed pre- or peri-natal unilateral lesions. Their results showed that IQ was stable over the 2-year time-period of the study. In contrast, a correlational study by Banich et al. (1990) found that in children with congenital hemiplegia of ‘mostly uncertain’ aetiology, age at testing (i.e. time since lesion) was a significant predictor of IQ score, such that children under age 6 years performed at near-age appropriate levels, but after age 6 performance declined, suggesting a slowed rate of cognitive development. Levine et al. (2005) conducted a longitudinal study of IQ in children and young adults with congenital hemiparesis and unilateral brain lesions. Their results indicated that in children with early unilateral brain injury, IQ scores decreased over the course of development, and that this decline occurred in both the verbal and performance domains and occurred regardless of seizure status or lesion laterality. Levine et al. (2005) speculated that, in children with early unilateral brain damage, functional plasticity is not sufficient to sustain a normal rate of development over time.
In existing studies of longitudinal outcome after early stroke, there are multiple factors that may have lead to the inconsistent findings. For example, the inclusion of participants with presumed rather than documented early unilateral brain lesions (Banich et al., 1990; Muter et al., 1997) or the use of participants with both congenital and acquired lesions (Aram and Eisele, 1994); the use of numerous different assessment measures (Banich et al., 1990; Aram and Eisele, 1994; Levine et al., 2005); the lack of a control group (Banich et al., 1990; Aram and Eisele, 1994; Levine et al., 2005); a short test–retest interval with very young subjects (Muter et al., 1997); and a cross-sectional rather than a longitudinal design (Banich et al., 1990).
Thus, the studies conducted to date offer no clear determination of whether children with early-onset unilateral brain damage have sufficient capacity for brain reorganization to allow cognitive function to remain stable over the course of development. In typically developing children, stability of cognitive functions over time on standardized tests would be expected (i.e. no significant increase or decrease in cognitive ability would be expected during typical development). Based on the literature, however, there are two competing hypotheses regarding longitudinal cognitive outcome in children with early focal brain damage. Either cognitive functions are stable over time, or alternatively, cognition may decline due to increasing task demands in a brain that is compromised. The present study was designed to examine stability of intellectual, academic and language functioning during development in children with perinatal ischaemic stroke, and to resolve the inconsistencies raised in the previous studies. Lesions were documented by Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) scan, and timing of the lesion was based on review of neonatal and infant medical records. Standardized tests of intelligence, language and academic functioning were administered to children at two time points during childhood in order to determine whether abilities in any or all of these cognitive domains declined or were stable over time. It was hypothesized that in children with early focal brain damage, intelligence, academic and language functioning would remain stable over time and that these functions would follow a similar trajectory as in typically developing children.
General methodology of Studies 1 and 2
We conducted longitudinal testing of intellectual and cognitive abilities in children with documented unilateral ischaemic perinatal stroke (IPS). Data were collected as part of a 20-year longitudinal study of language and learning in children with IPS. Subjects were administered the Wechsler Intelligence Scale for Children-Revised (WISC-R) (Wechsler, 1974), the Wide Range Achievement Test-Revised (WRAT-R) (Jastak and Wilkinson, 1984), the Clinical Evaluation of Language Fundamentals-Revised (CELF-R) (Semel et al., 1987) and/or the Peabody Picture Vocabulary Test-Revised (PPVT-R) (Dunn and Dunn, 1981) at two time points, and the data were examined longitudinally (Study 1). Additionally, in order to examine IQ change over time during a longer interval, longitudinal analyses were conducted on children receiving a WPPSI/WPPSI-R at preschool age and a WISC-R/WISC-III at school-age (Study 2).
General participant information
Twenty-nine preschool to school-age children with unilateral IPS (16 male, 13 female) and 38 control participants (16 male, 22 female) were included in Studies 1 and 2 combined. In the IPS group, 20 had left hemisphere (LH) damage and nine had right hemisphere (RH) damage. Only subjects with single, unilateral IPS [‘a group of heterogeneous conditions in which there is a focal disruption of cerebral blood flow secondary to arterial or cerebral venous thrombosis or embolization, between 20 weeks of fetal life through the 28th post-natal day, confirmed by neuroimaging or neuropathologic studies’ (Raju et al., 2007, p. 610)] were included in the IPS group. IPS participants were identified prospectively either in the newborn period due to seizures or lethargy, or by 6 months of age when they were showing lack of use of one hand. Children with bilateral, generalized or evolving (e.g. tumour) brain damage, as well as those with traumatic brain injuries, were excluded from the study. Each child was identified as having a unilateral IPS by review of medical history and neuroimaging results, including MRI (n = 12), CT (n = 2) or both CT and MRI (n = 15). A clinical neuroradiologist (J.H.), blinded to subject status, performed a clinical assessment of each neuroimaging study, providing documentation of lesion location and lesion severity. Severity was rated qualitatively on a five-point scale (adapted from Vargha-Khadem et al., 1985), with one being the smallest lesion and five being a lesion involving multiple lobes (Table 1).
Table 1.
Grading system for rating severity of the unilateral IPS
| Rating | Lesion description |
|---|---|
| 1 | Focal ventricular dilation or atrophy seen on <3 cuts on CT or MRI |
| 2 | Focal ventricular dilation or atrophy seen on >3 cuts on CT or MRI |
| 3 | Focal porencephaly involving one lobe only, <3 cuts on CT or MRI |
| 4 | Focal porencephaly involving one lobe only, >3 cuts on CT or MRI |
| 5 | Porencephaly or cortical atrophy involving multiple lobes |
See Table 2 for demographic characteristics and lesion/neurological information on the IPS participants. As can be seen in the table, 62% of the IPS participants (n = 18) had a hemiparesis (5 left and 13 right) and 38% (n = 11) did not have a hemiparesis; 24% of the IPS participants (n = 7) had a visual field deficit; 69% (n = 20) did not have a visual field deficit and the visual field status of two IPS participants (7%) was unknown. All of the IPS participants were either in regular class or in a special day class for learning disabilities, and all had undergone some type of rehabilitative therapy in infancy or early childhood, including speech, occupational and/or physical therapy.
Table 2.
IPS participants: demographic characteristics and lesion/neurological information
| Subject/Sex | Study | Ethnicity | SES | Hand preference | Gestational sge (weeks) | Lesion laterality | Lesion location | Severity | Seizures | Hemi-paresis | Visual field deficit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1M | 1 | Caucasian | 1 | R | 40 | L | O-S | 3 | N | N | N |
| 2F | 1 | Caucasian | 2 | L | 38 | L | F-T-P-O | 5 | Y Seizures age 7 years; seizure free on VPA | R | Unknown |
| 3F | 1 | Caucasian | 2 | R | 40 | L | P-O | 5 | N | N | Y |
| 4M | 1 | Caucasian | 1 | L | 40 | L | T-P-S | 5 | N | N | N |
| 5F | 1 | Hispanic | 4 | R | 40 | L | F-S | 4 | Y Ongoing occasional seizures | N | N |
| 6M | 1 | Caucasian | 1 | R | 38 | R | F-P | 5 | N | L | N |
| 7F | 1, 2 | Caucasian | 1 | L | 40 | L | S | 2 | N | R | N |
| 8M | 1, 2 | Caucasian | 2 | L | 40 | L | T-P-O | 5 | Y Two seizures age 2 years; no meds no seizures after | R | Y |
| 9M | 1, 2 | Caucasian | 2 | L | 34 | L | F-T-P-O-S | 5 | N | R | N |
| 10F | 1, 2 | Caucasian | 1 | L | 39 | L | F-T-P-O-S | 5 | N | R | Y |
| 11M | 1, 2 | Caucasian | 2 | L | 40 | L | F-T-P | 5 | Y Ongoing occasional seizures since age 2 months; on CBZ | R | N |
| 12M | 1, 2 | Caucasian | 1 | L | 30 | L | F-S | 3 | N | N | N |
| 13F | 1, 2 | Caucasian | 1 | L | 40 | L | T | 1 | N | R | N |
| 14M | 1, 2 | Caucasian | 1 | L | 40 | L | T | 4 | N | R | N |
| 15M | 1, 2 | Caucasian | 1 | R | 38 | R | F-T-P-O-S | 5 | Y Ongoing occasional seizures | L | Y |
| 16M | 1, 2 | Caucasian | 2 | R | 39 | R | F-T-P-O-S | 5 | Y Occasional seizures on CBZ | L | Unknown |
| 17F | 1, 2 | Caucasian | 2 | R | 40 | R | F-T-P-O | 5 | Y Seizures until age 8 years; none since on VPA | L | Y |
| 18F | 1, 2 | Caucasian | 3 | L | 40 | R | T-P-S | 5 | N | N | N |
| 19M | 1, 2 | Caucasian | 3 | R | 42 | R | F-P-S | 5 | N | L | N |
| 20M | 2 | Caucasian | 5 | L | 40 | L | F-T-P-O-S | 5 | Y Ongoing occasional seizures | R | Y |
| 21M | 2 | Hispanic | 3 | L | 42 | L | F-T-S | 5 | N | R | N |
| 22M | 2 | African-American | 3 | L | 38 | R | F | 3 | N | N | N |
| 23M | 2 | Caucasian | 1 | L | 36 | L | P-S | 3 | N | R | N |
| 24F | 2 | Asian | 1 | L | 37 | L | F-T-P-O-S | 5 | Y Ongoing occasional seizures | R | Y |
| 25F | 2 | Caucasian | 2 | R | 40 | L | F-T-S | 5 | N | N | N |
| 26F | 2 | Caucasian | 1 | L | 39 | L | F-T-P-O | 5 | N | R | N |
| 27F | 2 | Caucasian | 4 | R | 32 | L | S | 2 | N | N | N |
| 28F | 2 | Caucasian | 1 | R | 38 | R | F-S | 4 | Y Seizures started at age 5 years; controlled on CBZ | N | N |
| 29M | 2 | Caucasian | 2 | R | 40 | R | S | 2 | N | N | N |
F = Female; M = Male; SES = Socioeconomic status (1 = highest, 5 = lowest); R = Right; L = Left; F = Frontal; T = Temporal; P = Parietal; O = Occipital; S = Subcortical; Severity (1 = smallest, 5 = largest); Y = Yes; N = No; VPA = Valproic acid; CBZ = Carbamazepine.
Control children were recruited from the community through advertisements and from the University of California, San Diego subject pool. All controls included in the study had normal medical and developmental histories, and scored within normal limits on measures of intelligence, language and academic skills.
For all participants, complete medical and family histories were obtained, and information on socioeconomic status (SES) was obtained using the Hollingshead Four Factor Index of Social Status (Hollingshead, 1975). Informed consent was obtained prior to testing each subject in accordance with the Institutional Review Board at the University of California, San Diego.
Study 1—Longitudinal analyses within test versions: data from intelligence (WISC-R), academic (WRAT-R) and language (CELF-R, PPVT-R) tests: Method
Participants and procedures
Nineteen school-age children with IPS (11 male, 8 female) and 24 control participants (11 male, 13 female) were studied. In the IPS group, 13 had LH damage and 6 had RH damage. Given the rare nature of unilateral IPS and the availability of subjects, it was not possible to use only cases for which complete data were available. Although every attempt was made to collect complete data on every subject, due to the time demands of the study and families’ busy schedules, some subjects were unable to complete all testing sessions. Table 3 shows the number of IPS and control participants who received each test, the mean ages of the IPS and control groups on each measure, and the mean test–retest interval. The IPS and control groups were of similar SES based on the Hollingshead Index of SES (Hollingshead, 1975) (IPS = 1.74, Control = 1.38, P = NS).
Table 3.
Number of IPS and control participants who received each test, mean ages of the IPS and control groups on each measure and mean test–retest interval
| IPS |
Control |
|||||||
|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2 | Test–retest interval | Time 1 | Time 2 | Test–retest interval | |||
| Measure | n | Mean age ± SD | Mean age ± SD | Min. and Max. | n | Mean age ± SD | Mean age ± SD | Min. and Max. |
| WISC-R | 15 | 7y 3m ± 1y 0m | 11y 4m ± 1y 2m | 1y 10m–10y 1m | 12 | 8y 8m ± 2y 8m | 12y 0m ± 2y 11m | 1y 5m–7y 3m |
| WRAT-R | 13 | 8y 6m ± 1y 11m | 11y 7m ± 2y 2m | 1y 0m–5y 6m | 17 | 8y 1m ± 2y 3m | 11y 3m ± 3y 0m | 0y 11m–6y 0m |
| CELF-R | 17 | 7y 6m ± 2y 3m | 10y 6m ± 2y 11m | 1y 10m–5y 7m | 20 | 7y 7m ± 2y 2m | 11y 1m ± 3y 4m | 1y 0m–6y 0m |
| PPVT-R | 15 | 7y 7m ± 2y 7m | 10y 10m ± 2y 7m | 1y 10m–5y 6m | 19 | 7y 8m ± 2y 5m | 11y 1m ± 2y 10m | 1y 3m–6y 0m |
y = years; m = months.
Subjects were administered the Wechsler Intelligence Scale for Children-Revised (WISC-R) (Wechsler, 1974), the Wide Range Achievement Test-Revised (WRAT-R) (Jastak and Wilkinson, 1984), the Clinical Evaluation of Language Fundamentals-Revised (CELF-R) (Semel et al., 1987) and/or the Peabody Picture Vocabulary Test-Revised (PPVT-R) (Dunn and Dunn, 1981) as part of a larger study of cognitive development in normal and neurologically impaired children. The tests yielded standard scores with a mean of 100 and a standard deviation of 15. IPS and control participants were tested on the same set of measures on two occasions thus allowing for longitudinal analyses of IQ, academic functioning and language abilities within-test over time.
Study 1 analyses
SPSS 8.0 for Windows was used to screen and analyze all data. Longitudinal data for the WISC-R [Verbal IQ (VIQ), Performance IQ (PIQ), Full Scale IQ (FSIQ)], WRAT-R (Reading, Spelling, Arithmetic), CELF-R [Receptive Language Score (RLS), Expressive Language Score (ELS), Total Language Score (TLS)] and PPVT-R were analysed using repeated measures analysis of variance (ANOVA). For each cognitive index [dependent variable (DV)], separate repeated measures analyses were conducted for the following four independent variables (IVs): Neurological Status (IPS versus control), Laterality (LH versus RH lesion), Seizure Status (no seizures versus seizures) and Severity (single lobe versus multiple lobe involvement). Any significant main effects and interactions were examined using follow-up t-tests. The four predictor variables and four outcome measures were chosen specifically a priori; therefore, corrections for multiple comparisons within the framework of this study were not appropriate (for a thorough discussion of this issue, see, e.g. Miller, 1981; Rothman, 1990; Cook and Farewell, 1996; O'Keefe, 2003; Dallal, 2007; Matsunaga, 2007).
Study 1 results
Tables 4–7 show the mean standard score for each cognitive index of the WISC-R, WRAT-R, CELF-R and PPVT-R, respectively, for Time 1 and Time 2 datapoints. In addition, the tables show the repeated measures ANOVA results for the IVs of Neurological Status (IPS versus Control), Laterality (LH versus RH), Seizure Status (no seizures versus seizures) and Severity (single lobe versus multiple lobes).
Table 4.
WISC-R mean VIQ, PIQ and FSIQ scores for Time 1 and Time 2, and repeated measures ANOVA results for the IVs of Neurological Status, Laterality, Seizure Status and Severity
| Index | IV (Comparison) | Time 1 | Time 2 | Time × group interaction | Time main effect | Group main effect |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P | P | P | ||
| VIQ | Neurological Status | |||||
| IPS | 96.6 ± 20.5 | 98.7 ± 20.0 | ||||
| CTL | 126.1 ± 16.0 | 123.6 ± 13.1 | NS | NS | <0.0001 | |
| Laterality | ||||||
| LH | 96.2 ± 16.8 | 97.9 ± 19.4 | ||||
| RH | 97.4 ± 28.9 | 100.2 ± 23.3 | NS | NS | NS | |
| Seizure Status | ||||||
| No seizures | 105.4 ± 16.9 | 108.4 ± 14.7 | ||||
| Seizures | 79.0 ± 16.0 | 79.2 ± 14.2 | NS | NS | 0.005 | |
| Severity | ||||||
| Single lobe | 94.7 ± 18.8 | 95.0 ± 20.2 | ||||
| Multiple lobes | 98.3 ± 23.1 | 101.9 ± 20.6 | NS | NS | NS | |
| PIQ | Neurological Status | |||||
| IPS | 92.8 ± 19.9 | 93.5 ± 20.0 | ||||
| CTL | 115.2 ± 13.8 | 116.0 ± 10.5 | NS | NS | 0.002 | |
| Laterality | ||||||
| LH | 90.5 ± 18.7 | 93.5 ± 23.0 | ||||
| RH | 97.4 ± 23.7 | 93.6 ± 14.8 | NS | NS | NS | |
| Seizure Status | ||||||
| No seizures | 101.0 ± 19.5 | 99.3 ± 22.1 | ||||
| Seizures | 76.4 ± 5.6 | 82.0 ± 7.4 | NS | NS | 0.041 | |
| Severity | ||||||
| Single lobe | 95.3 ± 20.6 | 99.4 ± 24.2 | ||||
| Multiple lobes | 90.6 ± 20.5 | 88.4 ± 15.4 | NS | NS | NS | |
| FSIQ | Neurological Status | |||||
| IPS | 94.7 ± 20.4 | 96.1 ± 19.1 | ||||
| CTL | 123.0 ± 15.0 | 122.3 ± 10.2 | NS | NS | <0.0001 | |
| Laterality | ||||||
| LH | 93.2 ± 17.8 | 95.5 ± 19.9 | ||||
| RH | 97.6 ± 26.9 | 97.2 ± 19.7 | NS | NS | NS | |
| Seizure Status | ||||||
| No seizures | 103.8 ± 18.0 | 104.6 ± 16.7 | ||||
| Seizures | 76.4 ± 9.9 | 79.0 ± 10.5 | NS | NS | 0.007 | |
| Severity | ||||||
| Single lobe | 94.9 ± 20.6 | 97.1 ± 22.7 | ||||
| Multiple lobes | 94.5 ± 21.6 | 95.1 ± 17.0 | NS | NS | NS |
CTL = control.
Table 5.
WRAT-R mean Reading, Spelling and Arithmetic scores for Time 1 and Time 2, and repeated measures ANOVA results for the IVs of Neurological Status, Laterality, Seizure Status, and Severity
| Index | IV (Comparison) | Time 1 | Time 2 | Time × group interaction | Time main effect | Group main effect |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P | P | P | ||
| Reading | Neurological Status | |||||
| IPS | 85.0 ± 16.1 | 89.4 ± 13.3 | ||||
| CTL | 113.0 ± 13.3 | 108.9 ± 13.8 | 0.045 | NS | <0.0001 | |
| Laterality | ||||||
| LH | 87.4 ± 15.8 | 91.0 ± 16.0 | ||||
| RH | 80.8 ± 18.2 | 86.5 ± 7.9 | NS | NS | NS | |
| Seizure Statusa | ||||||
| No seizures | 88.3 ± 15.8 | 91.4 ± 14.0 | ||||
| Seizures | 70.0 ± 7.1 | 80.0 ± 1.4 | – | – | – | |
| Severity | ||||||
| Single lobe | 78.6 ± 12.4 | 84.0 ± 14.8 | ||||
| Multiple lobes | 90.3 ± 18.0 | 93.8 ± 11.3 | NS | NS | NS | |
| Spelling | Neurological Status | |||||
| IPS | 82.5 ± 18.2 | 87.0 ± 16.8 | ||||
| CTL | 106.2 ± 15.9 | 104.6 ± 13.1 | NS | NS | 0.001 | |
| Laterality | ||||||
| LH | 81.6 ± 21.7 | 86.3 ± 19.3 | ||||
| RH | 84.0 ± 12.4 | 88.3 ± 13.8 | NS | NS | NS | |
| Seizure Statusa | ||||||
| No seizures | 84.0 ± 19.5 | 89.3 ± 17.8 | ||||
| Seizures | 75.5 ± 12.0 | 76.5 ± 3.5 | – | – | – | |
| Severity | ||||||
| Single lobe | 78.6 ± 12.4 | 80.6 ± 16.2 | ||||
| Multiple lobes | 85.7 ± 22.6 | 92.3 ± 16.6 | NS | NS | NS | |
| Arithmetic | Neurological Status | |||||
| IPS | 91.5 ± 10.2 | 94.2 ± 18.7 | ||||
| CTL | 111.9 ± 11.2 | 113.1 ± 16.2 | NS | NS | <0.0001 | |
| Laterality | ||||||
| LH | 90.6 ± 10.3 | 94.1 ± 18.9 | ||||
| RH | 93.5 ± 11.1 | 94.5 ± 21.3 | NS | NS | NS | |
| Seizure Statusa | ||||||
| No seizures | 93.3 ± 11.0 | 98.1 ± 19.3 | ||||
| Seizures | 85.3 ± 2.5 | 81.3 ± 10.0 | – | – | – | |
| Severity | ||||||
| Single lobe | 93.5 ± 13.2 | 94.5 ± 24.4 | ||||
| Multiple lobes | 89.7 ± 7.4 | 94.0 ± 14.4 | NS | NS | NS |
aWhen the group of IPS participants who received the WRAT-R was divided into No Seizure and Seizure subgroups, only 2–3 participants had seizures, depending on the subtest. Therefore, statistical analyses were not performed on this measure. CTL = control
Table 6.
CELF-R mean RLS, ELS and TLS scores for Time 1 and Time 2, and repeated measures ANOVA results for the IVs of Neurological Status, Laterality, Seizure Status and Severity
| Index | IV (Comparison) | Time 1 | Time 2 | Time × group interaction | Time main effect | Group main effect |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P | P | P | ||
| RLS | Neurological Status | |||||
| IPS | 84.2 ± 10.9 | 82.3 ± 20.1 | ||||
| CTL | 109.1 ± 12.2 | 111.4 ± 13.7 | NS | NS | <0.0001 | |
| Laterality | ||||||
| LH | 84.5 ± 10.3 | 81.3 ± 20.9 | ||||
| RH | 83.5 ± 12.9 | 84.2 ± 20.3 | NS | NS | NS | |
| Seizure Status | ||||||
| No seizures | 88.7 ± 8.8 | 90.5 ± 19.0 | ||||
| Seizures | 75.8 ± 9.8 | 67.3 ± 12.3 | NS | NS | 0.010 | |
| Severity | ||||||
| Single lobe | 88.0 ± 12.0 | 82.9 ± 26.3 | ||||
| Multiple lobes | 81.5 ± 9.8 | 81.9 ± 16.0 | NS | NS | NS | |
| ELS | Neurological Status | |||||
| IPS | 72.5 ± 12.0 | 78.4 ± 16.0 | ||||
| CTL | 101.0 ± 17.5 | 105.8 ± 11.9 | NS | 0.017 | <0.0001 | |
| Laterality | ||||||
| LH | 73.1 ± 14.0 | 78.5 ± 17.9 | ||||
| RH | 71.3 ± 8.1 | 78.0 ± 13.4 | NS | 0.016 | NS | |
| Seizure Status | ||||||
| No seizures | 77.4 ± 11.1 | 85.0 ± 15.4 | ||||
| Seizures | 63.5 ± 8.0 | 66.2 ± 8.4 | NS | 0.030 | 0.011 | |
| Severity | ||||||
| Single lobe | 74.0 ± 16.7 | 80.7 ± 21.4 | ||||
| Multiple lobes | 71.4 ± 8.1 | 76.7 ± 11.9 | NS | 0.014 | NS | |
| TLS | Neurological Status | |||||
| IPS | 76.9 ± 11.1 | 79.1 ± 18.3 | ||||
| CTL | 105.6 ± 14.2 | 109.8 ± 14.0 | NS | NS | <0.0001 | |
| Laterality | ||||||
| LH | 77.6 ± 11.8 | 78.6 ± 19.6 | ||||
| RH | 75.5 ± 10.6 | 79.8 ± 17.6 | NS | NS | NS | |
| Seizure Status | ||||||
| No seizures | 81.8 ± 9.7 | 86.9 ± 17.2 | ||||
| Seizures | 67.8 ± 7.4 | 64.7 ± 10.1 | NS | NS | 0.007 | |
| Severity | ||||||
| Single lobe | 80.3 ± 13.7 | 80.9 ± 25.0 | ||||
| Multiple lobes | 74.5 ± 8.9 | 77.8 ± 13.3 | NS | NS | NS |
CTL = control.
Table 7.
PPVT-R mean scores for Time 1 and Time 2, and repeated measures ANOVA results for the IVs of Neurological Status, Laterality, Seizure Status and Severity
| Index | IV (Comparison) | Time 1 | Time 2 | Time × group interaction | Time main effect | Group main effect |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P | P | P | ||
| PPVT-R | Neurological Status | |||||
| IPS | 97.5 ± 19.7 | 99.9 ± 20.0 | ||||
| CTL | 117.1 ± 17.0 | 118.9 ± 13.9 | NS | NS | 0.002 | |
| Laterality | ||||||
| LH | 97.8 ± 22.2 | 100.2 ± 22.2 | ||||
| RH | 96.5 ± 12.8 | 99.3 ± 14.9 | NS | NS | NS | |
| Seizure Status | ||||||
| No seizures | 107.1 ± 15.5 | 108.8 ± 17.9 | ||||
| Seizures | 78.2 ± 10.9 | 82.2 ± 9.5 | NS | NS | 0.004 | |
| Severity | ||||||
| Single lobe | 98.3 ± 22.8 | 99.3 ± 21.7 | ||||
| Multiple lobes | 96.8 ± 18.1 | 100.5 ± 19.8 | NS | NS | NS |
CTL = control.
Analysis of interactions
For each cognitive index of the WISC-R, WRAT-R, CELF-R and PPVT-R, there was no Time × Group interaction for any of the four IVs (Neurological Status, Laterality, Seizure Status, Severity), with the exception of an interaction between Neurological Status and Time on the WRAT-R Reading subtest. Analysis of this interaction indicated that Reading scores for the IPS group increased slightly over time (not significantly) and the Reading scores for the control group slightly decreased over time (not significantly) resulting in this interaction.
Analysis of time main effects
With respect to performance over time on the measures of intelligence (DVs: VIQ, PIQ, FSIQ), academic (DVs: Reading, Spelling, Arithmetic) and language functioning (DVs: RLS, ELS, TLS; PPVT-R Standard Score), there were no main effects (Time 1 versus Time 2) for the IVs of Neurological Status, Laterality, Seizure Status or Severity. The only exception to this was a significant main effect of Time for the CELF-R ELS on the four IVs. Examination of the data revealed that the ELS main effects represent significant increases from Time 1 to Time 2. Follow-up analyses on the ELS indicated that the IPS group significantly improved from Time 1 to Time 2 on the ELS (t = −2.834, P = 0.012), whereas the control group did not significantly improve. Within the IPS group on the ELS, follow-up analyses of Lesion Side indicated that the RH group significantly improved (t = −2.892, P = 0.034), and there was a trend for the LH group to improve as well (t = −1.803, P = 0.102). Follow-up analyses of Seizure Status on the ELS indicated that both the Non-seizure and Seizure groups improved over time (t = −2.459, P = 0.034; t = −3.730, P = 0.014, respectively). Follow-up analyses of Lesion Severity on the ELS indicated that IPS participants with single lobe involvement did not significantly improve, whereas participants with multiple lobe involvement did significantly improve (t = −2.759, P = 0.022).
Analysis of group main effects
On the WISC-R, WRAT-R, CELF-R and PPVT-R indices, there were significant main effects of Group on the IVs of Neurological Status and Seizure Status, with the control group scoring significantly higher on the cognitive indices than the IPS group, and the Non-seizure group scoring significantly higher than the Seizure group. (On the WRAT-R, only two or three IPS participants had seizures, depending on the subtest. Therefore, statistical analyses were not performed on this IV for this measure.) There was no difference in performance on each index of the WISC-R, WRAT-R, CELF-R and PPVT-R for the IVs of Laterality or Severity.
Study 2—Longitudinal analyses of IQ from preschool to school-age: WPPSI/WPPSI-R compared with WISC-R/WISC-III: Methods
Participants and procedures
In order to examine IQ over a broader age range, analyses comparing Wechsler Preschool and Primary Scales of Intelligence (WPPSI or WPPSI-R) (Wechsler, 1967, 1989) IQ scores to WISC-R or WISC-3 (Wechsler, 1991) IQ scores were conducted on a group of 23 IPS participants and 24 control participants. To be included in this analysis, children were required to have WPPSI or WPPSI-R test scores by the age of 6 years 6 months, and WISC-R or WISC-III scores after age 6 years 6 months. The 23 IPS participants in Study 2 were comprised of 13 participants from Study 1 who also had a WPPSI/WPPSI-R, and 10 additional IPS participants who were not included in Study 1. Fifteen had LH damage and eight had RH damage. The 24 control participants in Study 2 were comprised of 10 participants from Study 1 who also had a WPPSI/WPPSI-R, and 14 additional control participants who were not included in Study 1. See Table 8 for demographic information including sex, SES and test–retest intervals for Study 2 participants.
Table 8.
Number of participants, mean ages at testing, sex, mean SES, mean test–retest interval and test–retest range for the IPS group, Non-seizure and Seizure subgroups, and Control group
| Group/subgroup | N | Mean age Time 1 | Mean age Time 2 | Sex | Mean SES | Mean test–retest interval | Test–retest range |
|---|---|---|---|---|---|---|---|
| IPS | 23 | 4y 10m | 10y 8m | 13 M; 10 F | 1.96 ± 1.1 | 5y 10m | 1y 9m–11y 9m |
| Non-seizure | 15 | 4y 8m | 10y 5m | 8 M; 7 F | 1.93 ± 1.0 | 5y 8m | 1y 9m–11y 9m |
| Seizure | 8 | 5y 0m | 11y 3m | 5 M; 3 F | 2.00 ± 1.3 | 6y 3m | 2y 7m–10y 10m |
| Control | 24 | 4y 8m | 8y 7m | 9 M; 15 F | 1.79 ± 1.1 | 4y 0m | 1y 5m–9y 7m |
y = years; m = months; M = male; F = female.
Study 2 analyses
SPSS 8.0 for Windows was used to analyze all data. Trajectories of the longitudinal Full Scale IQ (FSIQ) data from preschool to school-age (WPPSI or WPPSI-R compared to WISC-R or WISC-III) for the IPS and Control groups were analyzed using a 2 × 2 split-plot design with a within-subject factor (Time 1 versus Time 2) and a between-subjects factor (IPS versus Control). Additionally, the IPS group was divided into Seizure and Non-seizure subgroups and analyzed separately due to a priori hypotheses regarding the differential performance between the two groups. Again, 2 × 2 split-plot designs were utilized to analyse trajectories of FSIQ performance with the within-subject factor being Time (Time 1 versus Time 2) and the between-subject factors being group (Seizure versus Control; Non-seizure versus Control).
Examination of the relationship between the length of time between test administrations (WPPSI/WPPSI-R to WISC-R/WISC-III) and amount of FSIQ change over time was examined using correlational analyses.
Study 2 results
In Study 2, at Time 1, all subjects were under age 6.5 years and received the WPPSI or WPPSI-R; at Time 2, all subjects were older than age 6.5 years and received the WISC-R or WISC-3.
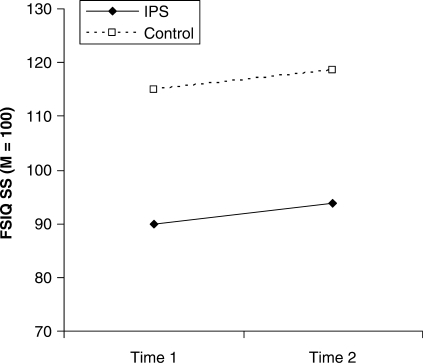
Analyses between the IPS and Control groups on FSIQ revealed that there was no Time × Group interaction (f = 0.004, P = NS), indicating parallel trajectories of performance over time (Fig. 1). Both groups showed a small but significant increase from T1 to T2 (f = 5.18, P = 0.028) that is likely to reflect test version differences. Furthermore, the length of time between test administrations (WPPSI/WPPSI-R to WISC-R/WISC-III) was not significantly correlated with the amount of FSIQ change over time in either the IPS or Control groups.
Fig. 1.
Mean Time 1 (WPPSI/WPPSI-R) and Time 2 (WISC-R/WISC-3) Full Scale IQ for the IPS and Control groups. IPS and Control group trajectories are statistically parallel.
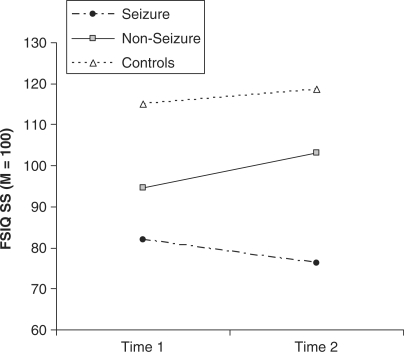
Subgroup analyses demonstrated differential trajectories of cognitive performance (FSIQ) over time for the Seizure group, as compared with both the Non-seizure and Control groups. Specifically, there was a significant Time × Group interaction between the Seizure group and both the Control (f = 4.75, P = 0.037) and Non-seizure groups (f = 8.25, P = 0.009). This significant interaction indicates that the Seizure group follows a different developmental course than the Non-seizure and Control groups. In contrast, there was no Time × Group interaction (f = 2.44, P = NS) between the Non-seizure and Control groups, indicating that there was no difference in the trajectory of the Non-seizure and Control groups. See Fig. 2 for the longitudinal trajectories of performance in the Seizure, Non-seizure and Control groups.
Fig. 2.
Mean Time 1 (WPPSI/WPPSI-R) and Time 2 (WISC-R/WISC-3) Full Scale IQ for the Seizure and Non-seizure subgroups, and Control group. Non-seizure subgroup and Control group trajectories are statistically parallel, whereas the Seizure subgroup has a significantly different trajectory than the Non-seizure subgroup and Control group.
Discussion
This study has resulted in important new findings. There is no evidence of decline in cognitive function over time in children with perinatal unilateral brain damage. These results indicate that there is sufficient ongoing plasticity in the developing brain following early focal damage to result in the stability, and in some cases improvement, of cognitive functions over time. Also, the presence of seizures limits plasticity such that there is not only significantly lower performance on intellectual and language measures in the seizure group (Study 1), but the course of cognitive development is significantly altered (as shown in Study 2).
The findings of cognitive stability over time in the IPS group were demonstrated both using the same task over time, and using different tasks over longer periods of time. Results of longitudinal studies using the same test (Study 1) indicate that for children with early IPS, intellectual (WISC-R), academic (WRAT-R) and language (CELF-R, PPVT-R) indices remain stable over an average 3-year interval during the school-age years. Interestingly, the one exception to this finding was a significant increase from Time 1 to Time 2 in the Expressive Language Score of the CELF-R for IPS subjects, independent of side of lesion, while controls remained stable over time. These results suggest that functional plasticity is not only sufficient to sustain a stable rate of development, but actually may lead to increased compensation over time. Similar results were demonstrated in Study 2, which extended the time period between test administrations utilizing age-appropriate versions of the Wechsler Intelligence Scales and permitted longitudinal testing of children from preschool age to well into school age. The IQ in the IPS group followed a parallel trajectory to that of controls.
The presence or absence of seizures played an important role in cognitive outcome in the IPS groups. A history of seizures during infancy or childhood resulted in lower scores on language and intelligence measures in Study 1, although expressive language still improved over time in the seizure group. In Study 2, the role of seizure status is further elucidated by examining the trajectory of IQ development over time, with the seizure group following a significantly different developmental course than both the Non-seizure and Control groups. The latter groups both showed a small but significant increase from Time 1 to Time 2 that is likely to reflect test version differences between the WPPSI/WPPSI-R and the WISC-R/WISC-III, while the seizure group did not. This exemplifies the need for a well-matched control group when utilizing data across different tests.
It is difficult to determine with certainty whether the observed differences between seizure and Non-seizure groups are the result of the seizures or of anti-epileptic drugs (AED). However, some of these children were not on medications at the time of testing, and of the ones who were taking an AED, there was no consistent medication that the majority were taking, and most were taking one of the newer AEDs that have few if any reported cognitive complications. Thus, we believe that the differences noted in the present study are more likely related to the seizures than to medications used for treatment of seizures.
The results of this study indicate that the brain is able to compensate after early injury, and maintain a steady rate of development (albeit at a lower level than SES-matched controls). We do not find evidence of these children falling further behind over time. An important finding that differentiates outcome in early childhood stroke in comparison to adult-onset stroke is that there were no differences in cognitive performance based on hemispheric side of the lesion, nor were there differences in performance based on size of the lesion (i.e. single lobe versus multiple lobe lesions). The most influential variable appeared to be seizure status, with IPS children who experienced seizures performing significantly more poorly on all IQ and language indices than those who did not experience seizures beyond the neonatal period. The detrimental effect of seizures (or some associated variable such as medication) on the cognitive performance of IPS children has been noted in other studies (Vargha-Khadem et al., 1992, 1994; Isaacs et al., 1996; Muter et al., 1997; Ballantyne et al., 2007).
The results of the present study are in contrast to studies by both Banich et al. (1990) and Levine et al. (2005), who found evidence of cognitive decline over the school years. The earlier (Banich) study was cross-sectional in nature, which does not adequately address true ‘change over time’. Levine et al.'s participants were said to have ‘early unilateral brain injury’ but appeared to have been more heterogeneous than our group (e.g. asphyxia, lesion at 7 months, normal MRI but diagnosed with infantile hemiparesis). The IPS population in the present study was very homogeneous, with all lesions incurred in the perinatal period, and all secondary to ischaemic or haemorrhagic lesions. Finally, neither of the earlier studies utilized a control group, which is important for making accurate inferences.
This is the first longitudinal study to utilize a homogenous group of children with documented early IPS to sample a variety of intellectual and cognitive skills using the same tests across the longitudinal interval. The major advantages of the present study were the inclusion of children with documented unilateral focal brain lesions; the fact that the lesions were all acute and confined to the pre- or peri-natal period; the large number of children studied (for such a rare population); and the use of well-matched control groups. By excluding children with brain lesions of diverse causes, such as closed head trauma and tumours, and differing ages of lesion acquisition, we were able to study a relatively pure sample of individuals with early, acute, unilateral brain damage. In this way, confounding issues such as more diffuse damage, timing of the lesion, or effects of a chronic, evolving lesion were avoided. A strength of Study 1 was the use of the same measures across subjects and across time; however, this resulted in a potential limitation of a restricted test–retest interval. It is possible that a longer interval might have yielded different findings, although we attempted to alleviate this concern by increasing the test–retest interval in Study 2, which yielded the same findings of cognitive stability over time. Another potential limitation is that, while we studied a relatively large group of IPS children for such a rare population, it would be optimal to have an even larger group, especially for the examination of potentially influential variables such as laterality, seizure status and severity. Studies examining these variables are ongoing in our laboratory, and results are forthcoming. Furthermore, future research should include a more socioeconomically diverse IPS sample, as well as outcomes in other important neuropsychological domains, such as executive functioning, visual-spatial skills and memory.
This study is important for several reasons. First, it provides information to support the notion of functional plasticity in the developing brain. Second, this study increases our theoretical understanding of brain–behaviour relationships. Third, it provides additional evidence that seizures limit plasticity during development. Fourth, this study provides important information on prognosis in children with unilateral IPS and avoids many of the confounds in prior studies. A greater understanding of how children with IPS fare over time is particularly important, as there has been conflicting information regarding prognosis for this population. The information provided by this study will benefit parents, physicians and teachers in generating appropriate expectations and realistic goals. Moreover, this information can lead to the development and implementation of appropriate interventions for these children.
Acknowledgements
Our sincerest appreciation is extended to all of the children and their parents whose participation was vital to this research. We would like to thank Dr Georg Matt for his statistical guidance on Study 2. This project was funded by the National Institutes of Health (NINDS 5-P50-NS22343, NINDS 1 R01 NS042584 and NIDCD P50 DC01289).
Glossary
Abbreviations:
- IPS
ischaemic perinatal stroke
- LH
Left hemisphere
- RH
Right hemisphere
- CT
Computed Tomography
- MRI
Magnetic Resonance Imaging
- WISC-R
Wechsler Intelligence Scale for Children-Revised
- WISC-III
Wechsler Intelligence Scale for Choldren-Third Edition
- VIQ
Verbal IQ
- PIQ
Performance IQ
- FSIQ
Full Scale IQ
- WRAT-R
Wide Range Achievement Test-Revised
- CELF-R
Clinical Evaluation of Language Fundamentals-Revised
- ELS
Expressive Language Score
- RLS
Receptive Language Score
- TLS
Total Language Score
- PPVT-R
Peabody Picture Vocabulary Test-Revised
- WPPSI
Wechsler Preschool and Primary Scales of Intelligence
- WPPSI-R
Wechsler Preschool and Primary Scales of Intelligence-Revised
References
- Aram D. Language sequelae of unilateral brain lesions in children. In: Plum F, editor. Language, communication, and the brain. New York: Raven; 1988. pp. 171–97. [PubMed] [Google Scholar]
- Aram D, Eisele JA. Intellectual stability in children with unilateral brain lesions. Neuropsychologia. 1994;32:85–95. doi: 10.1016/0028-3932(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Aram DM, Ekelman BL. Cognitive profiles of children with early onset of unilateral lesions. Dev Neuropsychol. 1986;2:155–72. [Google Scholar]
- Aram D, Ekelman BL, Rose DF, Whitaker HA. Verbal and cognitive sequelae following unilateral lesions acquired in early childhood. J Clin Exp Neuropsychol. 1985;7:55–78. doi: 10.1080/01688638508401242. [DOI] [PubMed] [Google Scholar]
- Ballantyne AO, Scarvie KM, Trauner DA. Verbal and performance IQ patterns in children after perinatal stroke. Dev Neuropsychol. 1994;10:39–50. [Google Scholar]
- Ballantyne AO, Spilkin AM, Trauner DA. Language outcome after perinatal stroke: does side matter? Child Neuropsychol. 2007;13:494–509. doi: 10.1080/09297040601114878. [DOI] [PubMed] [Google Scholar]
- Banich MT, Levine SC, Kim H, Huttenlocher P. The effects of developmental factors on IQ in hemiplegic children. Neuropsychologia. 1990;28:35–47. doi: 10.1016/0028-3932(90)90084-2. [DOI] [PubMed] [Google Scholar]
- Bates E. Plasticity, localization and language development. In: Fletcher SBJM, editor. The changing nervous system: neurobehavioral consequences of early brain disorders. New York: Oxford University Press; 1998. pp. 214–53. [Google Scholar]
- Bates E. Language and the infant brain. J Commun Disord. 1999;32:195–205. doi: 10.1016/s0021-9924(99)00015-5. [DOI] [PubMed] [Google Scholar]
- Cook RJ, Farewell VT. Multiplicity considerations in the design and analysis of clinical trials. J R Stat Soc Ser A. 1996;159:93–110. [Google Scholar]
- Dall’Oglio AM, Bates E, Volterra V, DiCapua M, Pezzini G. Early cognition, communication and language in children with focal brain injury. Dev Med Child Neurol. 1994;36:1076–98. doi: 10.1111/j.1469-8749.1994.tb11810.x. [DOI] [PubMed] [Google Scholar]
- Dallal GE. The little handbook of statistical practice. 2007 Available at http://www.jerrydallal.com/LHSP/mc.htm (16 April 2008, date last accessed)
- Dunn LM, Dunn LM. Circle Pines. MN: American Guidance Service; 1981. Peabody Picture Vocabulary Test – Revised (PPVT-R) [Google Scholar]
- Goldman PS. Developmental determinants of cortical plasticity. Acta Neurobiol Exp. 1972;32:495–511. [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- Isaacs E, Christie D, Vargha-Khadem F, Mishkin M. Effects of hemispheric side of injury, age at injury, and presence of seizure disorder on functional ear and hand asymmetries in hemiplegic children. Neuropsychologia. 1996;34:127–37. doi: 10.1016/0028-3932(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Jastak S, Wilkinson JS. Wide Range Achievement Test – Revised (WRAT-R). Wilmington, DE: Jastak Associates; 1984. [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1:727–31. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennenberg EH. Biological foundations of language. New York: Wiley; 1967. [Google Scholar]
- Levine SC, Kraus R, Alexander E, Suriyakaham LW, Huttenlocher PR. IQ decline following early unilateral brain injury: a longitudinal study. Brain Cogn. 2005;59:114–23. doi: 10.1016/j.bandc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Matsunaga M. Familywise error in multiple comparisons: disentangling a knot through a critique of O’Keefe's arguments against alpha adjustment. Commun Methods Meas. 2007;1:243–65. [Google Scholar]
- Merzenich MM, Kaas JH. Reorganization of mammalian somatosensory cortex following peripheral nerve injury. Trends Neurosci. 1982;5:434–6. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Miller RG. Simultaneous statistical inference. 2nd. New York: Springer; 1981. [Google Scholar]
- Muter V, Taylor S, Vargha-Khadem F. A longitudinal study of early intellectual development in hemiplegic children. Neuropsychologia. 1997;35:289–98. doi: 10.1016/s0028-3932(96)00079-6. [DOI] [PubMed] [Google Scholar]
- O’Keefe DJ. Colloquy: should familywise alpha be adjusted? Against familywise alpha adjustment. Hum Commun Res. 2003;29:431–47. [Google Scholar]
- Raju TNK, Nelson KB, Ferriero D, Lynch JK, Participants N-NPSW. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120:609–16. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- Reilly JS, Losh M, Bellugi U, Wulfeck B. ‘Frog, Where are You?’ Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain Lang. 2004;88:229–47. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Riva D, Cazzaniga L. Late effects of unilateral brain lesions sustained before and after age one. Neuropsychologia. 1986;24:423–8. doi: 10.1016/0028-3932(86)90029-1. [DOI] [PubMed] [Google Scholar]
- Riva D, Cazzaniga L, Pantaleoni C, Milani N, Fredrizzi E. Acute hemiplegia in childhood: the neuropsychological prognosis. J Pediatr Neurosci. 1986;2:239–50. [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals-Revised (CELF-R). San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Stiles J. Plasticity and development: evidence from children with early occurring focal brain injury. In: Julesz B, Kovacs I, editors. Maturational windows and adult cortical plasticity. Reading, MA: Addison-Wesley; 1995. pp. 217–37. [Google Scholar]
- Stiles J. Neural plasticity and cognitive development. Dev Neuropsychol. 2000;18:237–72. doi: 10.1207/S15326942DN1802_5. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci. 2005;9:136–42. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Thal DJ, Marchman V, Stiles J, Aram D, Trauner DA, Nass R, et al. Early lexical development in children with early focal brain injury. Brain Lang. 1991;40:491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Trauner DA, Chase C, Walker P, Wulfeck B. Neurologic profiles of infants and children after perinatal stroke. Pediatr Neurol. 1993;9:383–6. doi: 10.1016/0887-8994(93)90107-n. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Muter V. A review of cognitive outcome after unilateral lesions sustained during childhood. J Child Neurol. 1994;9:67–73. [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Van Der Werf S, Robb S, Wilson J. Development of intelligence and memory in children with hemiplegic cerebral palsy. Brain. 1992;115:315–29. doi: 10.1093/brain/115.1.315. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, O’Gorman AM, Watters GV. Aphasia and handedness in relation to hemispheric side, age at injury and severity of cerebral lesion during childhood. Brain Lang. 1985;108:677–96. doi: 10.1093/brain/108.3.677. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence (WPPSI). New York: Psychological Corporation; 1967. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Revised (WISC-R). San Antonio, TX: Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence – Revised (WPPSI-R). San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children – III (WISC-III). San Antonio: Psychological Corporation; 1991. [Google Scholar]
- Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79:2119–48. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]