Abstract
Maintaining the appropriate complement and content of lipids in cellular membranes is critical for normal neural function. Accumulating evidence suggests that even subtle perturbations in the lipid content of neurons and myelin can disrupt their function and may contribute to myelin and axonal degradation. In this study, we determined the composition and quantified the content of lipids and sterols in normal appearing white matter (NAWM) and normal appearing grey matter (NAGM) from control and multiple sclerosis brain tissues by electrospray ionization tandem mass spectrometry. Our results suggest that in active-multiple sclerosis, there is a shift in the lipid composition of NAWM and NAGM to a higher phospholipid and lower sphingolipid content. We found that this disturbance in lipid composition was reduced in NAGM but not in NAWM of inactive-multiple sclerosis. The pattern of disturbance in lipid composition suggests a metabolic defect that causes sphingolipids to be shuttled to phospholipid production. Modelling the biophysical consequence of this change in lipid composition of NAWM indicated an increase in the repulsive force between opposing bilayers that could explain decompaction and disruption of myelin structure.
Keywords: multiple sclerosis, sphingolipid, phospholipid, myelin
Introduction
Multiple sclerosis is an inflammatory neurodegenerative condition that is characterized by the demyelination of axons in discrete regions of the brain and spinal cord. There is however, ample evidence of more wide spread damage in the CNS of patients with multiple sclerosis. Brain imaging studies using diffusion tensor imaging-based histogram analysis have shown alterations in normal appearing white matter (NAWM) and normal appearing grey matter (NAGM) of patients with clinically isolated syndrome, and even greater disruptions apparent in patients with relapsing remitting multiple sclerosis (RRMS) (Chen et al., 2005; Narayanan et al., 2006; Cudrici et al., 2007; Reich et al., 2007; Yu et al., 2007). Spectroscopic studies have shown decreased N-acetylaspartate and N-acetyl aspartylglutamate containing compounds in both clinically isolated syndrome and RRMS and increased choline and myo-inositol in RRMS (De Stefano et al., 2002; Tartaglia et al., 2002; Gustafsson et al., 2007; Wattjes et al., 2007, 2008). These findings suggest that widespread disruptions in brain biochemistry may contribute to myelin and axonal damage in tissues that appear normal on gross examination. Multiple lines of evidence corroborate findings from brain imaging studies including findings of cytokine imbalance, oxidative stress, cytoskeletal damage and neurotransmitter abnormalities (Glabinski et al., 1993; Semra et al., 2002; Bartosik-Psujek and Stelmasiak, 2006; Skundric et al., 2006; Bartos et al., 2007a, b).
Considering the striking asymmetric distribution of lipids in myelin bilayers and the importance of lipid content to biophysical and biochemical properties of membranes, there has been little research on the content or metabolic processing of lipids in NAWM and NAGM of multiple sclerosis. There is a single report from over 30 years ago suggesting that the sphingosine content of NAWM in multiple sclerosis may be increased (Moscatelli and Isaacson, 1969) and a more recent report of reduced sulphatide and increased hydroxylated sulphatide (h24:0-sulphatide; hydroxy-lignoceroyl sulphatide) in NAWM (Marbois et al., 2000). In an experimental allergic encephalomyelitis model of multiple sclerosis there is evidence of increased polyunsaturated lipid content and phosphatidylserine (PS) (Ohler et al., 2004). These findings suggest that there may be widespread disruptions of lipid metabolism in the multiple sclerosis brain that perturbs membrane asymmetry and myelin structure. To determine if lipid metabolism is altered, we measured the sphingolipid, phospholipid and sterol content in NAWM and NAGM of multiple sclerosis and control brains, and modelled how these changes would affect the biophysical properties of myelin.
Materials and Methods
Brain tissues
Fresh frozen brain tissues from multiple sclerosis patients and control brain tissues were obtained from the Rocky Mountain Brain Bank. Detailed pathological examinations of these tissues were conducted at the University of Colorado Health Sciences Center, Department of Pathology. We categorized the multiple sclerosis tissues used in our studies based on the findings of pathological exams as active, based on evidence of monocyte infiltrates and inactive, if there were no infiltrates present. The fresh frozen tissues used in our biochemical analysis were obtained from middle frontal gyrus and were adjacent to the regions used for pathological examinations. All fresh frozen brain tissues used in our analysis were normal in appearance by gross pathological examination and did not contain demyelinating lesions. Although we cannot entirely rule out the possibility that subtle demyelination was present in the tissues used for our analysis, we did not observe differences in the total lipid content of multiple sclerosis compared with control tissues. Tissue samples contained both grey and white matter and were dissected microscopically into white and grey matter before extraction of lipids for analysis. Patient demographics were similar in each group: the active-multiple sclerosis group consisted of six males and six females with a median age of 48 years (range 24–62 years). The inactive-multiple sclerosis group consisted of six males and six females with a median age of 46 years (range 24–60 years). The control group consisted of five females and six males with a median age of 49 years (range 33–63 years). Cause of death in the control group consisted of heart attack (n = 4), chronic pulmonary obstruction (n = 4), abdominal aortic aneurism (n = 1) and liver failure (n = 2).
Lipid extraction of tissues
Total lipids were then extracted using a modified Bligh and Dyer procedure as previously described (Haughey et al., 2004). Purified standards of sphingomyelin C12:0, ceramide C12:0 and deuterated cholesterol (10 nM each; Avanti Polar Lipids, Alabaster, AL, USA) were added directly to homogenates. Three volumes of 100% methanol containing 53 mM ammonium formate was added, and the mixture vortexed. Four volumes of chloroform then were added, the mixture vortexed and centrifuged at 1000g for 10 min. The chloroform layer was removed into a glass storage vial, flushed with nitrogen and vial was sealed to be stored at −80°C.
Measurement of sphingolipids, phospholipids, cholesterol and lipid peroxides
Mass spectrometry analyses were performed using a Sciex API 3000 triple stage quadrupole tandem mass spectrometer (ESI/MS/MS) from Sciex Inc. (Thornhill, Ontario, Canada), using methods similar to those described in previous studies (Haughey et al., 2004; Bandaru et al., 2007). Counts were standardized to internal controls described in the preceding section. Cholesterol and cholesterol ester standards C16:0, C18:0 and C18:1 were purchased from Sigma (St Louis, MO, USA). Sphingomyelin and ceramides C16:0, C18:0, C20:0, C22:0, C24:0, C24:1 were purchased from Avanti Polar Lipids (Alabaster, AL, USA). 4-Hydroxynonenol and adducts were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Immunoblot analyses
Brain tissues were homogenized in ice-cold RIPA buffer consisting of 62 mM Tris, 2 mM EDTA, 2 mM EGTA, 2% SDS, 10% glycerol and a protease inhibitor cocktail (Sigma, St Louis, MO, USA), pH 6.0. Proteins (50 μg/lane) were separated by SDS–polyacrylamide gel electrophoresis (10% acrylamide) and transferred to a nitrocellulose membrane. Membranes were incubated overnight at 4°C with the primary antibodies proteolipid protein (PLP) (1:200; Millipore, Temecula, CA, USA) or NeuN (1:500; Millipore, Temecula, CA, USA). Membranes were then exposed to the appropriate HRP-conjugated secondary antibody (1:3000; Jackson Immunological Research Laboratories Inc., West Grove, PA, USA) and immunoreactive proteins were visualized using a chemiluminescene-based detection kit according to the manufacture's protocol (ECL kit; Amersham Corp., Arlington Heights, IL, USA).
Data analysis
Heat maps were constructed by a global alignment of the Q3 spectra for all samples. This procedure was used to maximize peak overlap and correct for small fluctuations in the masses reported by the instrument. Peaks with average intensities in all three groups (control, active- and inactive-multiple sclerosis) <1 × 106 were considered below the threshold for noise and removed from analysis. The remaining peaks were evaluated with non-parametric one-way ANOVA (Kruskal–Wallis test). Peaks with a P-value > 0.05 were arbitrarily assigned active-multiple sclerosis: C and inactive-multiple sclerosis: C ratio = 1 (coded as black). Peaks with a P-value <0.05 were assigned active-multiple sclerosis: C and inactive-multiple sclerosis: C ratios equal to the fold change between the active-multiple sclerosis or inactive-multiple sclerosis and control. Using the program Image J (NIH freeware), a green colour was used to indicate a ratio >1, red if the ratio was < 1 and increasing colour intensity to indicate a greater fold change.
Modelling of membrane energetics
A modified Derjaguin–Landau–Verwey–Overbeek (DLVO) theory was used to model the forces that govern interactions of opposing bilayers (Derjaguin and Landau, 1941; Verwey and Overbeek, 1948). The DLVO model assumes that the energy of adhesion can be considered as the sum of three independent energies: van der Waals interactions (lipid species independent), electrostatics (phosphatydylserine and ceramide dependent) and thermal undulations (cholesterol and chain length dependent; see Supplementary Appendix equation 1).
Results
Sphingolipid content of NAWM is decreased in multiple sclerosis
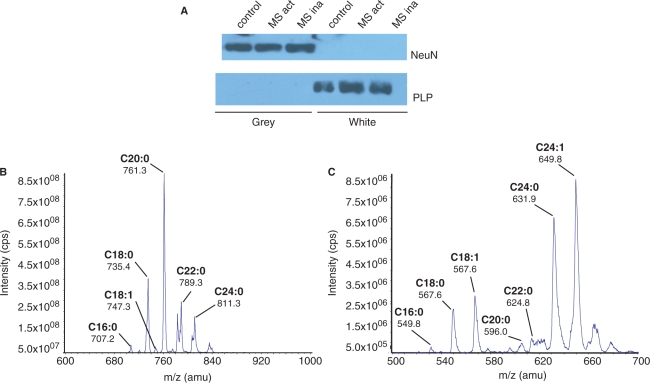
Cortical tissues from control and multiple sclerosis brains (healthy appearing tissues) were first separated into white and grey matter before analysis of lipid content by mass spectrometry. Western blot results show that after dissection, white matter samples were immunopositive for the oligodendrocyte marker PLP, and negative for the neuronal marker NeuN (Fig. 1A). Grey matter samples were negative for PLP and positive for NeuN (Fig. 1A). ESI/MS/MS analysis of grey and white matter was conducted to quantify individual lipid species (Fig. 1B and C). Although myelin is also found in grey matter (Moll et al., 2008), the amount of PLP in grey matter is likely insufficient for detection by Western blot in a side-by-side comparison with white matter. Qualitative analysis of grey matter showed an overall loss of sphingolipids in both active- and inactive-multiple sclerosis. In white matter, there was a loss of select sphingolipids in active- and inactive-multiple sclerosis and evidence for increased levels of some sphingolipid species in active- and inactive-multiple sclerosis (Fig. 2A).
Fig. 1.
Brain dissection and detection of lipids by ESI/MS/MS. (A) Western blot results showing the detection of MBP and the neuronal marker NeuN in white and grey matter fractions dissected from control brain (B and C). Example MS/MS spectra showing the identification of sphingomyelin (B) and ceramide (C) from control white matter.
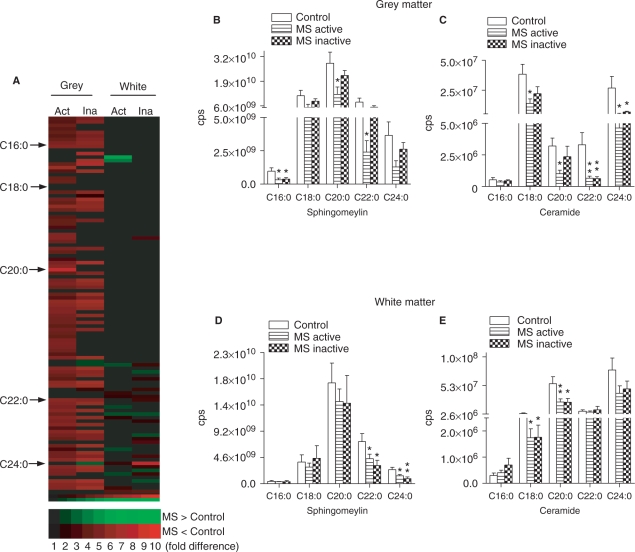
Fig. 2.
Sphingolipid content of control and multiple sclerosis brain tissues. (A) Sphingolipid array of grey and white matter showing individual forms of sphingolipid that are decreased (red) and increased (green) in active-multiple sclerosis (Act) or inactive-multiple sclerosis (Ina) compared with control tissues. (B–E) Quantitative analysis of MRM spectra from grey (B and C) and white (D and E) matter showing levels of sphingomyelin and ceramide (all are species shown contain a sphingosine C18:1 backbone) C16:0–C24:0 in multiple sclerosis and control brains. Data are mean ± SD from 8 control and 12 multiple sclerosis brains. ANOVA with Student–Neuman–Keuls post hoc comparison. *P < 0.05, **P < 0.01 compared with control.
A quantitative analysis of grey matter confirmed that multiple species of sphingolipid were decreased in active-multiple sclerosis and only one of the sphingolipid species we quantified was different from control in inactive-multiple sclerosis. Sphingolipids decreased only in active-multiple sclerosis were sphingomyelin C20:0 (57%), C22:0 (70%), ceramide C18:0 (64%) and C20:0 (69%). Sphingolipids that were decreased in both active- and inactive-multiple sclerosis were sphingomyelin C16:0 (67 and 61%, respectively), ceramide C20:0 (81 and 82%) and C22:0 (83 and 75%) (Fig. 2B and C). In contrast to the findings from grey matter, a quantitative analysis of sphingolipids in white matter showed that only a small number of sphingolipids were decreased in active- or inactive-multiple sclerosis, however, all sphingolipids decreased in white matter of active-multiple sclerosis were also decreased in inactive-multiple sclerosis. Sphingolipids decreased in active- and inactive-multiple sclerosis were sphingomyelin C22:0 (35 and 61%, respectively), C24:0 (37 and 67%), ceramide C18:0 (48 and 56%) and C20:0 (53 and 63%) (Fig. 2E and F). These results suggest that during active-multiple sclerosis, the content of sphingomyelin and ceramide in normal appearing brain tissues are decreased and that during periods of disease inactivity, sphingolipid content of grey matter, but not white matter is similar to control brain.
Phospholipid content of NAWM and NAGM is increased in multiple sclerosis
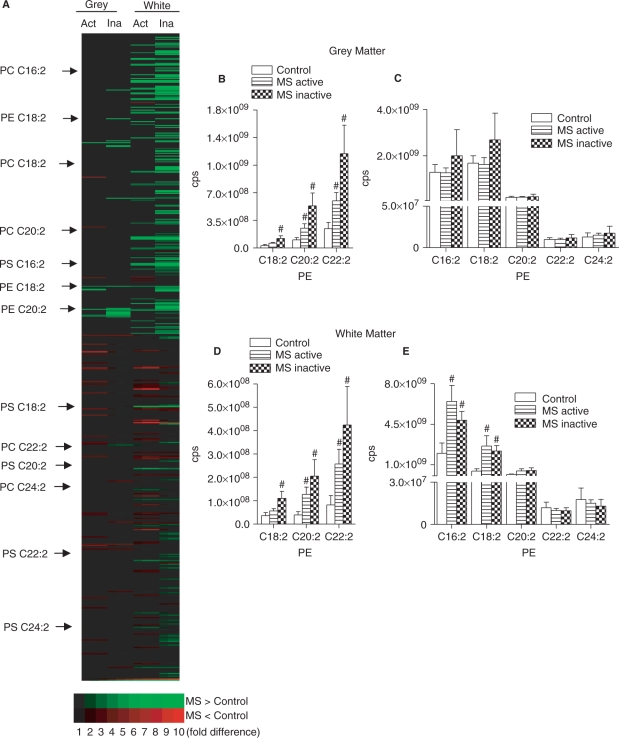
We next determined the phospholipid content of grey and white matter dissected from normal appearing multiple sclerosis and control brains. Qualitative analysis of all phospholipid species present in the precursor scan showed an overall increase in the phospholipid content in multiple sclerosis, with the greatest accumulations in the white matter of active-multiple sclerosis (Fig. 3A). In grey matter of active- and inactive-multiple sclerosis, there were both increased and decreased phospholipid species. In white matter, there was a dramatic increase of multiple phospholipids in active- and inactive-multiple sclerosis, with comparatively few phospholipids species decreased. Quantitative analysis of individual phospholipids in grey matter showed a selective increase of phosphatidylethanolamine (PE) in active-multiple sclerosis (from 197% to 225%) that trended toward greater increases in inactive-multiple sclerosis (388–521%; Fig. 2B and C). In multiple sclerosis white matter there was increased content of all PE (152–326% in active-multiple sclerosis and 299–524% in inactive-multiple sclerosis) and two of five PS species (327–578% in active-multiple sclerosis and 245–483% in inactive-multiple sclerosis). Similar to the findings in grey matter, PE trended toward greater accumulations in inactive- compared with active-multiple sclerosis (Fig. 3D and E). The phosphtidylcholine (PC) content of grey and white matter were similar in multiple sclerosis and control brains with the exception of a single PC analyte that was increased in grey matter of inactive-multiple sclerosis (Fig. 1 in Supplementary Appendix).
Fig. 3.
Phospholipid content of control and multiple sclerosis brain. (A) Phospholipid array of grey and white matter showing individual phospholipids that are decreased (red) and increased (green) in active-multiple sclerosis (Act) or inactive-multiple sclerosis (Ina) compared with control tissues. (B–E) Quantitative analysis of MRM spectra from grey (B and C) and white (D and E) matter showing levels of PE and PS in multiple sclerosis and control brains (all phospholpid species shown contain either C18:0 or C18:1 at the sn-1 position). Data are mean ± SD from 8 control and 12 multiple sclerosis brains. ANOVA with Student–Neuman–Keuls post hoc comparison. #P < 0.05 compared with control.
Cholesterol content of NAWM and NAGM is reduced in multiple sclerosis
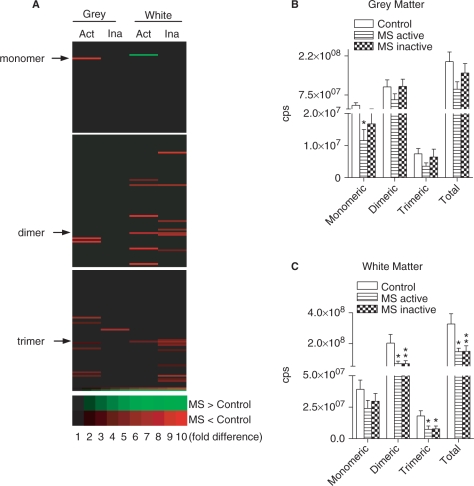
A qualitative analysis of sterols in grey and white matter of multiple sclerosis showed an overall decrease in the sterol content of cellular membranes (Fig. 4A). In grey matter, a number of sterols were decreased in active-multiple sclerosis, but only one sterol was decreased compared with control in inactive-multiple sclerosis. In white matter, a number of sterols were decreased in active- and inactive-multiple sclerosis. Quantitative analysis of grey matter spectra showed that the monomeric form of cholesterol was decreased (67%) in active-multiple sclerosis, but was not different from control in inactive-multiple sclerosis (Fig. 4B). In white matter, dimeric (66% decrease) and trimeric (59% decrease) forms of cholesterol were depleted in both active- and inactive-multiple sclerosis (66 and 58% decrease, respectively). Thus, during active-multiple sclerosis there is a depletion of cholesterol in white and grey matter. During periods of disease inactivity, sterol levels of grey matter are not different from control tissues, while sterol levels in white matter remain lower than controls.
Fig. 4.
Cholesterol content of control and multiple sclerosis brain. (A) Sterol array of grey and white matter showing individual sterols that are decreased (red) and increased (green) in active-multiple sclerosis (Act) or inactive-multiple sclerosis (Ina) compared with control tissues. (B–E) Quantitative analysis of MRM spectra from grey (B) and white (C) matter showing levels of monomeric, dimeric, trimeric and total cholesterol in multiple sclerosis and control brains. Data are mean ± SD from 8 control and 12 multiple sclerosis brains. ANOVA with Student–Neuman–Keuls post hoc comparison. *P < 0.05, **P < 0.01 compared with control.
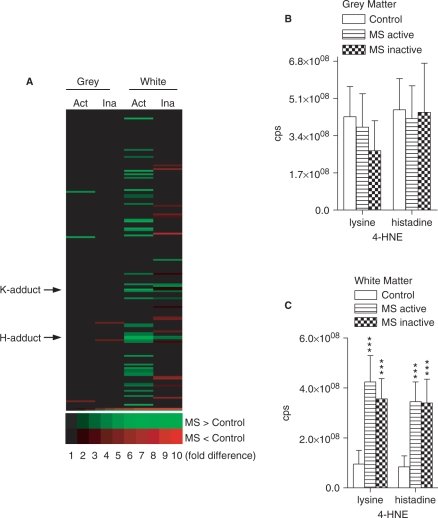
Reactive aldehyde levels are increased in NAWM of multiple sclerosis
We next sought to determine if lipid peroxidation products accumulate in normal appearing brain tissues of multiple sclerosis. Qualitative analysis of the entire spectra clearly showed that lipid peroxidation is increased primarily in the white matter of multiple sclerosis (Fig. 5A). Quantitative analysis of two 4-hydroxynonenal (4-HNE) species showed no difference in grey matter of active- or inactive-multiple sclerosis compared with controls (Fig. 5B). However, in white matter of active- and inactive-multiple sclerosis, there were large accumulations of both the lysine and histadine adducts of 4-HNE (443 and 406%, respectively).
Fig. 5.
Reactive aldehyde levels of control and multiple sclerosis brain. (A) Reactive aldehyde array of grey and white matter showing individual hydroxynoneals that are decreased (red) and increased (green) in active-multiple sclerosis (Act) or inactive-multiple sclerosis (Ina) compared with control tissues. (B–C) Quantitative analysis of MRM spectra from grey (B) and white (C) matter showing levels of the lysine and histadine adducts of 4-HNE in multiple sclerosis and control brains. Data are mean ± SD from 8 control and 12 multiple sclerosis brains. ANOVA with Student–Neuman–Keuls post hoc comparison. ***P < 0.001 compared with control.
Modelling of membrane energetics
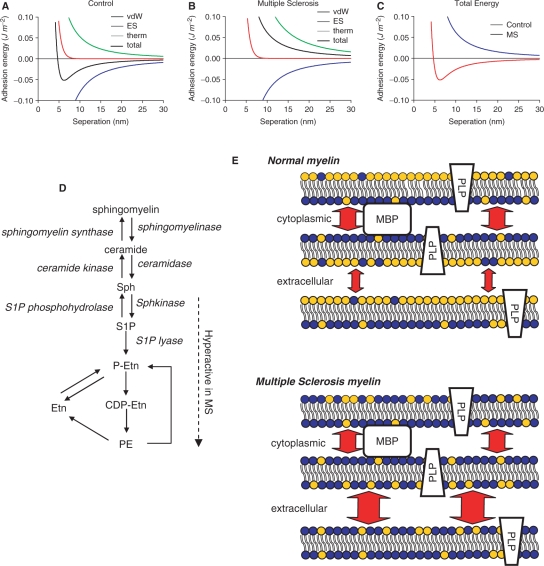
Alterations in the lipid content of cellular membranes can have dramatic effects on cellular functions by altering in the biophysical properties of the membrane. We therefore determined how changes in the sphingolipid, phospholipid and sterol content of multiple sclerosis white matter could alter the biophysical properties of myelin. To accomplish this, we used the DLVO theory to model the forces that govern interactions of opposing bilayers. The DLVO model assumes that the energy of adhesion can be considered as the sum of three independent energies: van der Waals interactions (lipid species independent), electrostatics (phosphatydylserine and ceramide dependent) and thermal undulations (cholesterol and chain length dependent) (Fig. 6A–C). Because van der Waals energy is independent of the lipid species it was identical in control and multiple sclerosis white matter (Fig. 6A and B). The electrostatic potential decreased from y = −44.1 mV in control to y = −48.3 mV in multiple sclerosis, due to a decreased content of ceramide and increased content of PS in multiple sclerosis white matter (Fig. 6A and B). Although the change in electrostatics raised the adhesion energy, the dominant destabilizing force resulted from an increased thermal undulation force; thermal energy in lipid bilayers is primarily dependent on the degree of chain saturation (Fig. 6A and B). The bending modulus of lipid bilayer kc = 0.69 × 10−19 J in control white matter, decreased to kc = 0.39 × 10−19 J in multiple sclerosis white matter. These calculations imply that in multiple sclerosis (NAWM) there is an increase in the magnitude of total repulsive pressure between myelin bilayers, particularly in the long-range component of the repulsive pressure that is thought to be due primarily to thermally induced bilayer undulations (Fig. 6C) (Servuss et al., 1976; Evans and Parsegian, 1986; Bloom et al., 1991). We next calculated the natural repulsion created by head-to-head arrangement of polar groups on opposing bilayers of myelin. Assuming a displacement distance of 4 nm, the energy of repulsions for control tissue was 0.112 mJ/m2 and for multiple sclerosis tissue 0.869 mJ/m2. Because our model does not account for protein–lipid interactions, we also calculated the energy of repulsion using a more conservative displacement distance of 5 nm. When we examined the energetics at 5 nm, control tissue demonstrated an attractive force of 0.0268 mJ/m2 while multiple sclerosis myelin still showed a repulsive force of 0.385 mJ/m2, consistent with calculations from other groups (Hu et al., 2004). This additional repulsive energy would decrease the stability of bonds between layers and destabilize the structure of myelin.
Fig. 6.
Bioenergetic modelling of lipid membranes in multiple sclerosis and control white matter. This DLVO model assumes that energy of adhesion can be considered the sum of three independent energies: van der Waals interactions (vdW; lipid species independent), electrostatics (ES; phosphatydylserine and ceramide dependent) and thermal undulations (therm; cholesterol and unsaturated lipid dependent). (A and B) vdW, ES, therm energies and their sum (total adhesion energy) have been plotted for control (A) white matter and white matter from multiple sclerosis (B) brain. Since vdW energy is lipid species independent it is identical in control and multiple sclerosis. The increase in total PS results in a decrease of the surface charge density and therefore a decrease in the surface potential (y = –44 mV for control and y = –48 mV for multiple sclerosis). While this does slightly raise adhesion energy, the dominant destabilizing force is the increase in thermal undulation force (thermal energy) due to decrease average lipid length (kc = 0.69 × 10−19 J in control to kc = 0.39 × 10−19 J in multiple sclerosis tissue). (C) Total energy (sum of vdW, ES and therm) for control and multiple sclerosis white matter are plotted. (D) Pathways of sphingolipid and phospholipid metabolism. Arrows indicate direction of reactions and enzymes involved are shown in italics. The portion of the pathway proposed to be hyperactive in multiple sclerosis is indicated. (E) Schematic representation of the mechanisms by which disordered lipid content perturbs the compacting of myelin. In normal myelin there is a greater repulsive energy (degree of repulsive energy is depicted by arrow size) at the major dense lines (extracellular interface) that is largely due to a greater abundance of phospholipids in these opposing bilayers (phospholipids are shown in blue and sphingolipids in gold). Proteins such as MBP help to stabilize myelin at major dense lines through interactions with headgroups from both opposing bilayers. There is a greater content of sphingolipid and less repulsive energy at the intraperiod line (extracellular interface) compared with major dense lines. In multiple sclerosis, a decrease in sphingolipid and increase in phospholipid content would increase the repulsive energy at the intraperiod line and destabilize myelin.
Discussion
Although the exact causes of multiple sclerosis are not known, there is increasing evidence of global disruptions in brain biochemistry that begin early in the course of the disease and worsen with disease progression. We found that there is not an overall loss of lipid mass in NAWM or NAGM, but a reduction in sphingolipid and gain in phospholipid content that was most prominent during active disease. The lipid content normalized to a greater extent in NAGM than NAWM suggesting there may be a selective defect in the ability of myelin to normalize lipid content during periods of disease inactivity.
At the core of sphingolipid structure is the long chain amino alcohol sphingosine. N-acetyl-sphingosine (ceramide) is converted to sphingomyelin by sphingomyelin synthase that adds a phosphorylcholine head group to ceramide. This is a bi-directional reaction in which sphingomyelin can be converted back to ceramide by sphingomyelinase-mediated hydrolysis of sphingomyelin (see Clarke et al., 2006 for a review). Ceramide can be further metabolized to sphingosine (by sphingosine kinase), to sphingosine-1 phosphate (by S1P-phosphohydrolase), to phosphoethanolamine (by S1P-lyase), to CDP-ethanolamine and finally PE (by the Kennedy pathway; Fig. 6D). A hyperactivation of this pathway in multiple sclerosis is consistent with our findings of decreased sphingomyelin and ceramide with increased PE in NAGM, increased PE and PS in NAWM and no change of PC in either tissue. Although we can make no definite conclusions on the enzyme(s) involved, based on our findings of oxidative damage in the form of 4-HNE modified lysine and histadine residues in NAWM, it is likely that oxidative modifications of one or multiple enzymes in this pathway play roles in shuttling ceramide to the PE/PS pathway. This conclusion is supported by a lack of evidence for 4-HNE modified proteins and an absence of excess PS in NAGM of either active- or inactive-multiple sclerosis, suggesting that ceramide is not as efficiently shuttled to PE and PS in the absence of oxidative stress.
If the sphingomyelin–ceramide–sphingosine–S1P pathway is hyperactive in multiple sclerosis, then it is likely that S1P levels would also be reduced in NAWM and NAGM. S1P is a potent endogenous lipid mediator involved in cell survival, proliferation and cell–cell interactions (see Hait et al., 2006 for a review). S1P-signalling has recently received a great deal of attention in multiple sclerosis therapy due to the promise of FTY720 [2-amino-2-[2-(4-octylphenyl) ethyl]propane-1,3-diol hydrochloride]. This orally available S1P-receptor modulator has shown striking efficacy in experimental allergic encephalomyelitis models of multiple sclerosis (Brinkmann et al., 2002; Fujino et al., 2003; Webb et al., 2004 Kataoka et al., 2005; Balatoni et al., 2007) and recent results from phase II trials with RRMS patients are promising (Kappos et al., 2006). There is additional evidence that suggests the effectiveness of FTY720 in the CNS extends beyond immunomodulation to include an influence on neuronal function, blood–brain barrier and glial repair mechanisms that could contribute to preservation or restoration of nerve function (Baumruker et al., 2007; Jung et al., 2007; Osinde et al., 2007). These results are consistent with evidence that the bioactive metabolite FTY720-P widely distributes to the CNS white matter, supporting the likelihood for functional interaction with glial cells bearing S1P receptors in the brain and spinal cord (Foster et al., 2007). Thus, our data are consistent with the notion that FTY720 may restore S1P-receptor signalling that is lost in multiple sclerosis due to a hyperactive metabolism of S1P to phosphoethanolamine, to CDP-ethanolamine and finally PE.
Intramembrane adhesion is critical for myelin to maintain its compact structure. The head-to-head arrangement of lipids in myelin creates a repulsive energy between layers that is overcome at the extracellular surfaces (major dense line) primarily by the stabilizing actions of myelin basic protein (MBP). Electrostatic interactions between positively charged surface groups on MBP and negatively charged PS, combined with attractive van der Waals force create a highly stable interface. Indeed, the major dense line is exceptionally stable, even when deliberate attempts are made to destabilize the structure (Sedzik and Blaurock, 1995). Adhesion at the extracellular surface (intraperiod line) is less stable and primarily relies on the interactions of PLP with lipids through van der Waals force to overcome repulsive energies originating from thermal undulations. Thus, a change in repulsive energy is more likely to disrupt adhesion at the intraperiod line, consistent with reports that this interface is more often disrupted in demyelinating disease (Bornstein and Raines, 1976; Genain et al., 1999). Our calculations based on the lipid content of NAWM indicate that the repulsive energy between bilayers is increased by an order of magnitude in multiple sclerosis (Fig. 6E), suggesting that myelin which appears intact on gross morphological examination has a reduced stability that would affect its ability to insulate axons. These conclusions are consistent with evidence for widespread axonal damage early in multiple sclerosis (De Stefano et al., 2002; Tartaglia et al., 2002; Chen et al., 2005; Narayanan et al., 2006; Cudrici et al., 2007; Gustafsson et al., 2007; Reich et al., 2007; Wattjes et al., 2007, 2008; Yu et al., 2007).
We conclude from our findings that abnormal lipid metabolism in normal appearing brain tissues may contribute to the pathology of multiple sclerosis by perturbing the biophysical properties of cellular membranes. The patterns of change in lipid content that we observed suggest that deficits in lipid metabolism worsen during active-multiple sclerosis and modify the lipid composition of axonal and myelin membranes at sites distant from lesions. During periods of disease inactivity, axons may partially normalize lipid content, but a defect in the ability of myelin to normalize content likely contributes to progressive widespread damage in multiple sclerosis.
Supplementary material
Supplementary material is available at BRAIN online.
Funding
National Institutes of Health (AG023471, MH077542, AA017408) to N.J.H.; National Multiple Sclerosis Society (P.A.C.).
Supplementary Material
Glossary
Abbreviations:
- 4-HNE
4-hydroxynonenal
- DLVO
Derjaguin–Landau–Verwey–Overbeek
- FTY720
2-amino-2-[2-(4-octylphenyl) ethyl]propane-1,3-diol hydrochloride
- MBP
myelin basic protein
- NAGM
normal appearing grey matter
- NAWM
normal appearing white matter
- PC
phosphtidylcholine
- PE
phosphatidylethanolamine
- PI
phosphitidylinositol
- PLP
proteolipid protein
- PS
phosphatidylserine
- RRMS
relapsing remitting multiple sclerosis
- S1P
sphingosine-1 phosphate
References
- Balatoni B, Storch MK, Swoboda EM, Schonborn V, Koziel A, Lambrou GN, et al. FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain Res Bull. 2007;74:307–16. doi: 10.1016/j.brainresbull.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–7. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos A, Fialova L, Soukupova J, Kukal J, Malbohan I, Pitha J. Elevated intrathecal antibodies against the medium neurofilament subunit in multiple sclerosis. J Neurol. 2007a;254:20–5. doi: 10.1007/s00415-006-0185-0. [DOI] [PubMed] [Google Scholar]
- Bartos A, Fialova L, Soukupova J, Kukal J, Malbohan I, Pitha J. Antibodies against light neurofilaments in multiple sclerosis patients. Acta Neurol Scand. 2007b;116:100–7. doi: 10.1111/j.1600-0404.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Stelmasiak Z. The CSF levels of total-tau and phosphotau in patients with relapsing-remitting multiple sclerosis. J Neural Transm. 2006;113:339–45. doi: 10.1007/s00702-005-0327-z. [DOI] [PubMed] [Google Scholar]
- Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin Invest Drugs. 2007;16:283–9. doi: 10.1517/13543784.16.3.283. [DOI] [PubMed] [Google Scholar]
- Bloom M, Evans E, Mouritsen OG. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991;24:293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Bornstein MB, Raines CS. The initial structural lesion in serum-induced demyelination in vitro. Lab Invest. 1976;35:391–401. [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Chen JT, Collins DL, Freedman MS, Atkins HL, Arnold DL. Local magnetization transfer ratio signal inhomogeneity is related to subsequent change in MTR in lesions and normal-appearing white-matter of multiple sclerosis patients. Neuroimage. 2005;25:1272–8. doi: 10.1016/j.neuroimage.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–56. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- Cudrici C, Ito T, Zafranskaia E, Niculescu F, Mullen KM, Vlaicu S, et al. Dendritic cells are abundant in non-lesional gray matter in multiple sclerosis. Exp Mol Pathol. 2007;83:198–206. doi: 10.1016/j.yexmp.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano N, Narayanan S, Francis SJ, Smith S, Mortilla M, Tartaglia MC, et al. Diffuse axonal and tissue injury in patients with multiple sclerosis with low cerebral lesion load and no disability. Arch Neurol. 2002;59:1565–71. doi: 10.1001/archneur.59.10.1565. [DOI] [PubMed] [Google Scholar]
- Derjaguin BV, Landau L. Theory of the stability of strongly charged hydrophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim URSS. 1941;14:633–62. [Google Scholar]
- Evans EA, Parsegian VA. Thermal–mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci USA. 1986;83:7132–6. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–75. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–7. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–5. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Glabinski A, Tawsek NS, Bartosz G. Increased generation of superoxide radicals in the blood of MS patients. Acta Neurol Scand. 1993;88:174–7. doi: 10.1111/j.1600-0404.1993.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson MC, Dahlqvist O, Jaworski J, Lundberg P, Landtblom AM. Low choline concentrations in normal-appearing white matter of patients with multiple sclerosis and normal MR imaging brain scans. Am J Neuroradiol. 2007;28:1306–12. doi: 10.3174/ajnr.A0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–26. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–67. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Hu Y, Doudevski I, Wood D, Moscarello M, Husted C, Genain C, et al. Synergistic interactions of lipids and myelin basic protein. Proc Natl Acad Sci USA. 2004;101:13466–71. doi: 10.1073/pnas.0405665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, et al. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–7. doi: 10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–48. [PubMed] [Google Scholar]
- Marbois BN, Faull KF, Fluharty AL, Raval-Fernandes S, Rome LH. Analysis of sulfatide from rat cerebellum and multiple sclerosis white matter by negative ion electrospray mass spectrometry. Biochim Biophys Acta. 2000;1484:59–70. doi: 10.1016/s1388-1981(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Moll NM, Rietsch AM, Ransohoff AJ, Cossoy MB, Huang D, Eichler FS, et al. Cortical demyelination in PML and MS: similarities and differences. Neurology. 2008;70:336–43. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- Moscatelli EA, Isaacson E. Gas liquid chromatographic analysis of sphingosine bases in sphingolipids of human normal and multiple sclerosis cerebral white matter. Lipids. 1969;4:550–5. doi: 10.1007/BF02531040. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Francis SJ, Sled JG, Santos AC, Antel S, Levesque I, et al. Axonal injury in the cerebral normal-appearing white matter of patients with multiple sclerosis is related to concurrent demyelination in lesions but not to concurrent demyelination in normal-appearing white matter. Neuroimage. 2006;29:637–42. doi: 10.1016/j.neuroimage.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Ohler B, Graf K, Bragg R, Lemons T, Coe R, Genain C, et al. Role of lipid interactions in autoimmune demyelination. Biochim Biophys Acta. 2004;1688:10–7. doi: 10.1016/j.bbadis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Osinde M, Mullershausen F, Dev KK. Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology. 2007;52:1210–8. doi: 10.1016/j.neuropharm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Reich DS, Zackowski KM, Gordon-Lipkin EM, Smith SA, Chodkowski BA, Cutter GR, et al. Corticospinal tract abnormalities are associated with weakness in multiple sclerosis. Am J Neuroradiol. 2007;29:333–339. doi: 10.3174/ajnr.A0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzik J, Blaurock AE. Myelin vesicles: what we know and what we do not know. J Neurosci Res. 1995;41:145–52. doi: 10.1002/jnr.490410202. [DOI] [PubMed] [Google Scholar]
- Semra YK, Seidi OA, Sharief MK. Heightened intrathecal release of axonal cytoskeletal proteins in multiple sclerosis is associated with progressive disease and clinical disability. J Neuroimmunol. 2002;122:132–9. doi: 10.1016/s0165-5728(01)00455-6. [DOI] [PubMed] [Google Scholar]
- Servuss RM, Harbich W, Helfrich W. Measurement of the curvature-elastic modulus of egg lecithin bilayers. Biochim Biophys Acta. 1976;436:900–3. doi: 10.1016/0005-2736(76)90422-3. [DOI] [PubMed] [Google Scholar]
- Skundric DS, Cai J, Cruikshank WW, Gveric D. Production of IL-16 correlates with CD4+ Th1 inflammation and phosphorylation of axonal cytoskeleton in multiple sclerosis lesions. J Neuroinflammation. 2006;3:13. doi: 10.1186/1742-2094-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia MC, Narayanan S, De Stefano N, Arnaoutelis R, Antel SB, Francis SJ, et al. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol. 2002;249:1382–90. doi: 10.1007/s00415-002-0846-6. [DOI] [PubMed] [Google Scholar]
- Verwey EJW, Overbeek JTG. The interaction of particles having an electric double layer. New York, Amsterdam: Elsevier; 1948. Theory of the stability of lyophobic colloids. [Google Scholar]
- Wattjes MP, Harzheim M, Lutterbey GG, Bogdanow M, Schild HH, Traber F. High field MR imaging and (1)H-MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis: correlation between metabolic alterations and diagnostic MR imaging criteria. J Neurol. 2008;255:56–63. doi: 10.1007/s00415-007-0666-9. [DOI] [PubMed] [Google Scholar]
- Wattjes MP, Harzheim M, Lutterbey GG, Klotz L, Schild HH, Traber F. Axonal damage but no increased glial cell activity in the normal-appearing white matter of patients with clinically isolated syndromes suggestive of multiple sclerosis using high-field magnetic resonance spectroscopy. Am J Neuroradiol. 2007;28:1517–22. doi: 10.3174/ajnr.A0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–21. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Yu CS, Zhu CZ, Li KC, Xuan Y, Qin W, Sun H, et al. Relapsing neuromyelitis optica and relapsing-remitting multiple sclerosis: differentiation at diffusion-tensor MR imaging of corpus callosum. Radiology. 2007;244:249–56. doi: 10.1148/radiol.2441060930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.