Abstract
The initiation phase of ethanol self-administration is difficult to study using the well-established, sucrose-fading procedure due to the changing concentrations of ethanol in the first few days. The purpose of this experiment was to test whether a modified sucrose-substitution procedure in which rats are initially exposed to high concentrations of ethanol and sucrose for three days would successfully initiate ethanol self-administration. Male Long-Evans rats were trained to lever-press with a 10% sucrose solution in which 4 or 20 responses allowed 20-min access to the solution. Subsequently, rats were exposed to a three-day period of operant self-administration of 10% sucrose + 10% ethanol. This constant-concentration exposure was followed by the standard procedure in which sucrose is completely faded out. The establishment of ethanol self-administration was determined by ethanol intake, pre- and post-procedure 2-bottle choice preference tests, and extinction trials. The mean ethanol intake was 2.2 times higher on day 2 compared with day 1 on the 10% sucrose + 10% ethanol solution. After fading out the sucrose, the daily intake of 10% ethanol solution over 5 days was stable at approximately 0.57 g/kg. Ethanol preference was approximately 3-fold higher after the modified sucrose-fading procedure. Responding during a single session extinction test was dramatically increased from 4 to 61 ± 13 or 20 to 112 ± 22 responses in 20 min. Similar to the standard sucrose-fading method, we did not observe a significant relationship between extinction responding and ethanol intake. Blood alcohol concentrations were 4.5 mM 20 min after consumption began. We conclude that initiation and establishment of ethanol self-administration will occur using this modified sucrose-fading procedure.
Keywords: sucrose-fading, extinction, preference, operant self-administration
Introduction
The sucrose-fading procedure has been well established as a method of initiating ethanol self-administration (Samson, 1986, Samson et al., 1999, Schwarz-Stevens et al., 1991). This sucrose-substitution paradigm has been used in previous studies to investigate the effects of pharmacological agents on ethanol self-administration, as well as neurochemical events during operant ethanol self-administration (for reviews, see Samson and Czachowski, 2002; Gonzales et al., 2004). For example, dopamine activity has been measured in the nucleus accumbens during oral consumption of ethanol initiated by sucrose-substitution (Doyon et al., 2003) or its variant, saccharin-substitution (Weiss et al., 1993). The effects of the dopamine D2 antagonist, remoxipride, on ethanol seeking and drinking behaviors have been studied after ethanol initiation with the sucrose-fading procedure (Czachowski et al., 2002). Rogowski et al. (2003) also used the sucrose-fading procedure to test the potential regulation of ethanol self-administration by dopamine D2 receptors. Also, drinking behaviors of different strains of rats have been compared after sucrose-substitution was used to initiate ethanol self-administration (Samson et al., 1998, Vacca et al., 2002). In fact, it was shown that in the alcohol-preferring rat, the initiation procedure used affects ethanol reinforced behaviors (Schwarz-Stevens et al., 1991).
Although the sucrose-fading paradigm is useful in establishing stable ethanol self-administration, very little is known about the mechanisms that contribute to the initiation of ethanol self-administration. The constantly varying concentrations of ethanol and sucrose throughout the various phases of the sucrose-fading method make it difficult to determine when the initiation versus maintenance of ethanol self-administration is being expressed. For example, initiation could begin during the early phases of exposure to low concentrations of ethanol, or it could be later in the method during which consumption of high doses of ethanol occurs. One potential critical period in the initiation process could be the earliest times that a rat achieves a dose of ethanol that leads to an intoxicating effect. In the original sucrose-fading method, this may not occur for several days after ethanol has been introduced because, in general, the highest doses of ethanol self-administered will be when the concentration of sucrose and ethanol are highest. A model using a constant concentration of ethanol (10% w/v) and sucrose over several days could enable the study of the earliest phase of initiation. Furthermore, a brief period of exposure to this solution may shorten the entire procedure, since it bypasses the time it takes to fade in the ethanol. In the present study we tested a modification of the standard sucrose-fading procedure in which the animals are initially exposed to 10% sucrose and 10% ethanol (10S10E) and the ethanol concentration was kept constant throughout the study. Specifically, we tested whether this model would lead to the initiation and maintenance of ethanol self-administration, as with the validated sucrose-substitution procedure. The appetitive strength and preference of ethanol were also estimated using this new model.
Materials and methods
Animals
We used 6 cohorts (total n of 52) of ethanol naïve male Long-Evans rats (Charles River Laboratories, Wilmington, MA) for this study. Their weight at the start of the study ranged between 237–368 g (approximately 50–80 days old). We handled the rats for at least one week, and they were housed individually under a 12-hr light/dark cycle (on at 7:00 am and off at 7:00 pm). The animals had food and water ad libitum during the entire experiment except for a brief period during which they were being trained to lever-press (see below). A summary of the cohorts and the various experimental procedures to which they were subjected is shown in Table 1. All procedures complied with guidelines specified by the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin.
Table 1.
Experimental cohorts
| Cohort | na | Successful initiationb | Unsuccessful initiation | Ethanol preferencec | RRd | Extinctione | Prewaitf | Controlsg |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 6 | 2 | yes | 4 | 6 | yes | no |
| 2 | 6 | 3 | 3 | no | 4 | 3 | yes | no |
| 3 | 5 | 5 | 0 | no | 4 | 5 | yes | no |
| 4 | 12 | 5 | 1 | no | 20 | 11h | no | 6 |
| 5 | 9 | 5 | 0 | no | 20 | 9 | no | 4 |
| 6 | 12 | 8 | 4 | yes | 20 | 0 | no | no |
n = the number of rats that started the experiment
Successful initiation indicates the number of rats that met the criterion of consuming at least 0.3 g/kg of 10% ethanol in at least 4 of 5 consecutive days at the end of the initiation procedure.
Ethanol preference indicates whether that cohort underwent the pre- and post-initiation two bottle choice procedure.
RR = the final intended response requirement during the training period
Extinction = the number of rats that went through the extinction procedure at the end of the training.
Prewait indicates whether that cohort had an extra 45 min wait period in the chamber before the session began.
Controls = the number of rats that were never exposed to ethanol during the training.
Three controls were not included in the extinction analysis because they were not able to meet consistently the RR of 20.
Preference Testing
After a 7 day adaptation period to their home cage, the first (n=6) and sixth cohorts (n=8) of rats were given a two-bottle choice preference test for 5 days. Two versions of the preference test were used. For the first cohort the preference test was conducted as described in Samson (1986). Briefly, water and ethanol (5% w/v) were continuously available in the home cage from 500 ml bottles. Bottle position was alternated each day to control for side preference. The fluid consumed was recorded and the bottles were refilled at the same time each day (1:00 pm). The animals were also weighed at that time. Control bottles placed on empty cages were also monitored for spillage and evaporation. Ethanol preference was estimated by dividing the amount of ethanol consumed by the total fluid intake. For the sixth cohort a similar procedure was used except that 10% ethanol (w/v) was used during this initial preference test.
A second two-bottle choice preference test was performed immediately following the last operant drinking session in the first and last cohorts. The procedure for this second preference test was the same as described above except that 10% ethanol (w/v) was used for both cohorts. In both pre- and post-initiation preference tests, control bottles of ethanol and water were measured daily and refilled at the same time as the bottles on the rat cages.
Behavioral Training
Self-administration training took place in standard operant chambers (Med Associates Inc., St. Albans, VT). Each chamber contained a single, retractable lever on the left side (2 cm above the grid floor). After the rat reached the appropriate response requirement (RR), a retractable drinking spout entered the chamber on the right side of the same wall (5 cm above the grid floor). The metal bars that made up the grid floor were connected to the metal spout of the drinking bottle through a lickometer circuit (Med Associates). Each operant chamber was housed in a cubicle with the front doors left open during training. An interior chamber light and a sound-attenuating fan were activated with the start of each operant session. Operant chamber components and acquisition of lickometer data were controlled by PC using software from Med Associates.
After the pre-initiation preference test, rats were given 2–3 days in their home cage environment with a single bottle of water and ad libitum food. Animals were trained to lever-press for 10% (w/v) sucrose (10S). To facilitate the acquisition of lever-pressing behavior, animals were fluid deprived for 10–22 hr before each training session. Lever-pressing was established in 2–9 days. During all subsequent sessions water was once again available ad libitum for the remainder of the study.
Throughout the initiation procedure, operant training sessions occurred 5–7 days per week. Each cohort of rats underwent the training in sequence, although there were differences in some of the training parameters and tests between the cohorts (summarized in Table 1). The ethanol drinking protocol began with 4 days of responding for 10S while increasing pre-wait period (waiting in the operant chamber before starting the program), wait period (before lever is available for pressing), and RR. The pre-wait period for the first three cohorts was increased incrementally to 45 min; there was no pre-wait period in the subsequent cohorts. The wait period was increased to 15 min, and the RR was increased from 1 to 4 lever-presses for the first three cohorts (n=19). The subsequent cohorts (4–6) had an RR of 20 (n=23) to match studies done using the already established sucrose-substitution procedure (Samson et al., 2001). For all sessions, the subject had 20 min to complete the RR, and completion of the RR allowed access to the bottle for 20 min. The second phase of the protocol was 3 days of access to 10S10E solution. Following this, there was no pre-wait period in the remaining sessions. Over the next 4 days, the sucrose in the solution (10S10E) was decreased to 5% sucrose/10% ethanol (5S10E) ethanol and to 2% sucrose/10% ethanol (2S10E). The rats were given 10% ethanol (w/v, 10E) for the remainder of the operant sessions. This final phase of the protocol varied in length from subject to subject. Rats continued the operant sessions until their intake reached > 0.30 g/kg for at least 4 of 5 consecutive sessions. This took 5–13 days of 10E-sessions to reach this criterion (6.8 ± 0.4 days). Fluid consumption was monitored for each operant session. The fluid intake was calculated by measuring the fluid remaining in the bottle (with a resolution of 0.1 ml) and subtracting the amount of fluid spilled (collected in small weigh boats placed under the spout).
Control groups were included in cohorts 4 and 5 in which the rats were trained to lever-press for sucrose as described above. However, ethanol was not introduced during the sucrose fade-out, and eventually the rats consumed water during the operant session. These controls were matched with rats in the ethanol group so that the total sessions were similar between the two groups, and no additional fluid restriction was performed in these controls.
Extinction Tests
After completion of the post-initiation preference test, the first cohort of animals was given an additional 4 days of operant sessions with 10E solution, and intake was reestablished to similar levels achieved before the post-initiation preference test. On day 5, a single extinction test was performed. Since they did not receive a post-initiation preference test, the remaining cohorts performed the extinction test immediately after completion of the initiation procedure. The extinction test consisted of a 15-min wait period followed by 20 min of responding with no access to the drinking bottle. A bottle of 10E was present, but no amount of responding would trigger its descent into the operant chamber. The number of responses in the 20-min period was recorded.
Blood Alcohol Concentration
The blood alcohol concentration was determined in the sixth cohort (n=8). The rats were reintroduced to the operant self-administration of 10E for 1 to 4 days to reestablish normal levels of intake of 10E after completion of the preference test. Four of the rats were allowed to drink 2 to 3 minutes before blood samples were collected. The other four rats had access to the 10E solution for the entire operant drinking session (20 min), and blood samples were collected immediately after the session ended. Blood was collected from the saphenous vein while under isoflurane anesthesia. The alcohol content in 10 µl of blood was measured using gas chromatography. The blood sample was immediately mixed with 90 µl of saturated sodium chloride solution, and the sealed sample was placed in a heated autosampler at 40 degrees C. A Varian CP 3800 gas chromatograph with a flame ionization detector and a Varian 8200 headspace autosampler was used to analyze the concentrations of ethanol in the samples. The stationary phase was an HP Innowax capillary column (30 m × 0.53 mm × 1.0 µm film thickness) and helium was the mobile phase. Resulting ethanol peaks were recorded using Varian Star Chromatography Workstation software, and calibration was achieved using external standards.
Statistics
Repeated measures ANOVAs were performed to determine if differences existed in (1) intake of 10S10E solution over time and (2) in the number of licks during the last day of 10S consumption and the first three days of consumption of 10S10E. Post hoc t-tests were used for individual comparisons using the Bonferroni correction. T-tests were used to (1) determine the effect of the pre-wait period on 10E consumption, (2) compare extinction responding in the ethanol group with that in the control group, and (3) compare the ethanol preference before and after the initiation procedure. Regression analyses were used to determine if there was a significant relationship between (1) extinction responding and ethanol intake, (2) extinction responding and water intake, and (3) total licks/session and ethanol intake in g/kg. The criterion for significance was p < 0.05.
Inspection of the lickometer data (total number of licks) during the various phases of the experiment revealed that in some cases the lickometer malfunctioned due to a lack of proper contact between the leads and the metal spout which produced low lick counts. Therefore, we carried out an analysis of the ratio between licks and ml consumed during the consumption of 10S because the volume consumed was generally high, and the measurement error would be correspondingly low. Analysis of 185 sessions with the explore command in SPSS indicated extreme outliers (< 85 and > 285 licks/ml), and therefore, licks/ml values falling within this range were further analyzed. Lickometer parameters selected for this study include latency, total licks/session, lick rate, and number of bouts. A bout is defined as a run of at least 25 licks with no more than 2 min between licks. Latency was defined as the time between the last lever-press response and the first lick. Lick rate was analyzed for the first bout (number of licks in the first bout divided by the time between the first and last licks of the first bout) and for the first half of the first bout (number of licks in the first bout divided by two, and this quantity is divided by the time between the first and median licks of the first bout).
Results
We excluded 10 of the 42 rats in the ethanol drinking groups because they did not drink greater than 0.30 g/kg of 10E for at least 4 of 5 consecutive days (Table 1). We used 31 rats for the extinction test. Fourteen rats received a pre- and post-initiation ethanol preference test. There were 10 rats used in the control groups, but 3 of these were not included in the extinction analysis because they would not consistently meet the response requirement to match the experimental group.
The first 3 cohorts were trained using a 45-min pre-wait period and had a response requirement of 4. The consumption of 10E over the last 5 days was 0.54 ± 0.04 g/kg (n = 14). We decided to delete the pre-wait period in subsequent cohorts because the modified protocol appeared to be successful, and we wanted to reduce the time needed for training. In addition, we increased the response requirement in these groups to 20. The consumption of 10E in the rats trained without the pre-wait period was 0.58 ± 0.06 g/kg (n=18). There was no significant difference in 10E consumption between the two groups (T(30) = 0.67, p > .05). In addition, we also examined selected lick parameters during the last 5 days of consumption of 10E for these two groups, and no significant differences were observed (data not shown). Therefore, we combined the two groups for subsequent analyses. The ethanol consumption of the 10S10E solution, and the last 5 days of 10E consumption are shown in Fig. 1. There is clearly a significant increase in ethanol intake after day 1 [F(2, 62) = 15.1, p < .05]. The ethanol intake increased 2.2 times from day 1 to day 2 [F(1, 62) = 26.1, p < .05]. Although the intake was slightly decreased from day 2 to day 3 of the exposure to 10S10E, this difference was not statistically significant [F(1, 62) = 0.6, p > .05]. For the 5 days of 10E solution, the average ethanol intake was fairly stable (approximately 0.6 g/kg), and there was no significant difference among the 5 days of intake [F(4, 124) = 1.8, p > .05]. The number of licks for the last day of 10S, the three days of 10S10E, the two days of 5S10E, the two days of 2S10E, and the last five days of 10E are shown in Fig. 2. The fluid consumption drops off dramatically on the first day of exposure to the 10S10E solution [F(1, 57) = 86.5, p < .05], but this rebounds on day 2 as described above for the intake data [F(1, 57) = 20.2, p < .05]. There was a significant correlation between the licks and ethanol intake across the sessions shown in Fig. 1 and Fig. 2 (r2 = 0.99, p < .05). This confirms that the intake measures obtained by determining the volume of fluid in the bottle before and after the session is due to consumption and is not confounded by spillage. Selected lickometer parameters for the last day of 10E self-administration were compiled to show that the data collected using this new protocol are similar to data collected using the established sucrose-substitution procedure (Doyon et al., 2003) and are shown in Table 2.
Fig. 1.
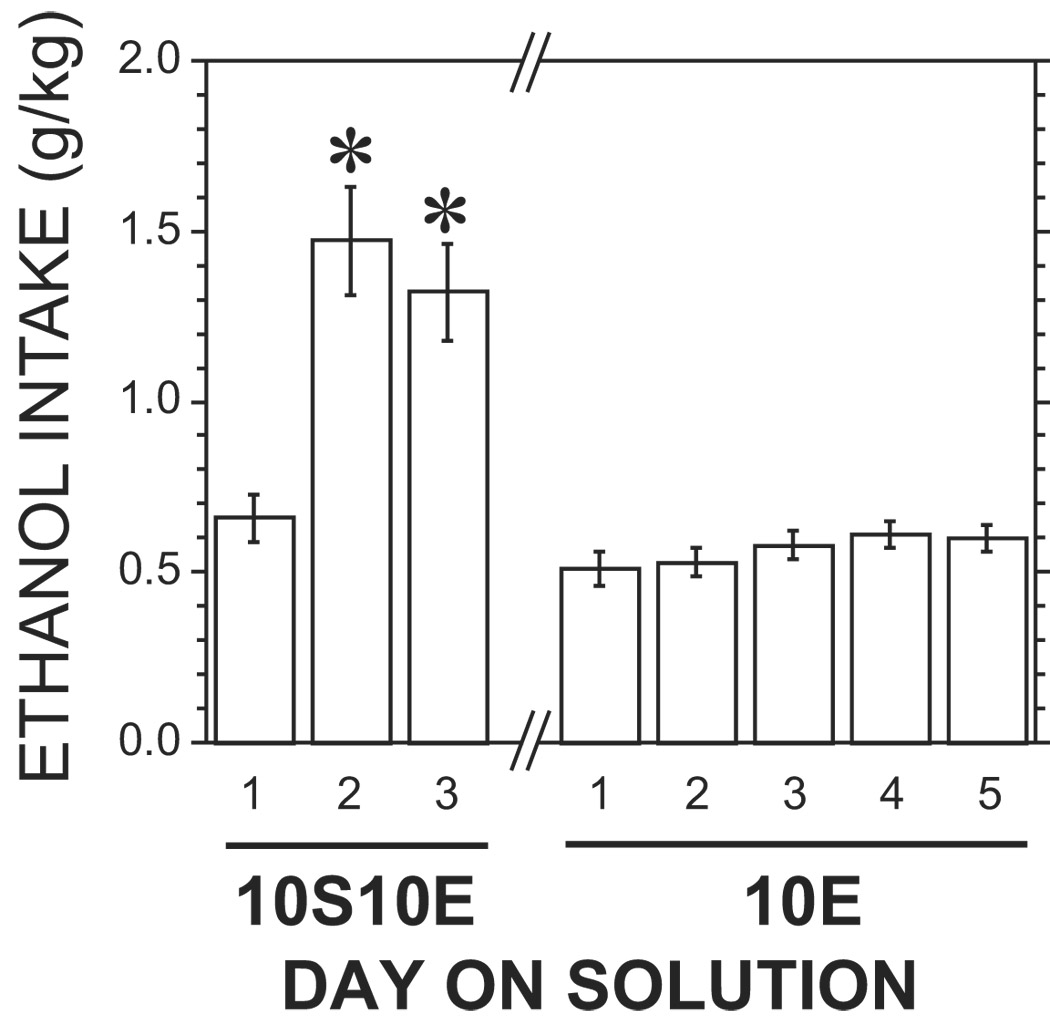
Ethanol intake during first three days of operant self-administration of 10% sucrose and 10% ethanol and last five days of 10% ethanol. Ethanol intakes (g/kg) are presented as mean ± SEM (n=32). * indicates a statistically significant difference in ethanol intake compared with day 1 (p < .05).
Fig. 2.
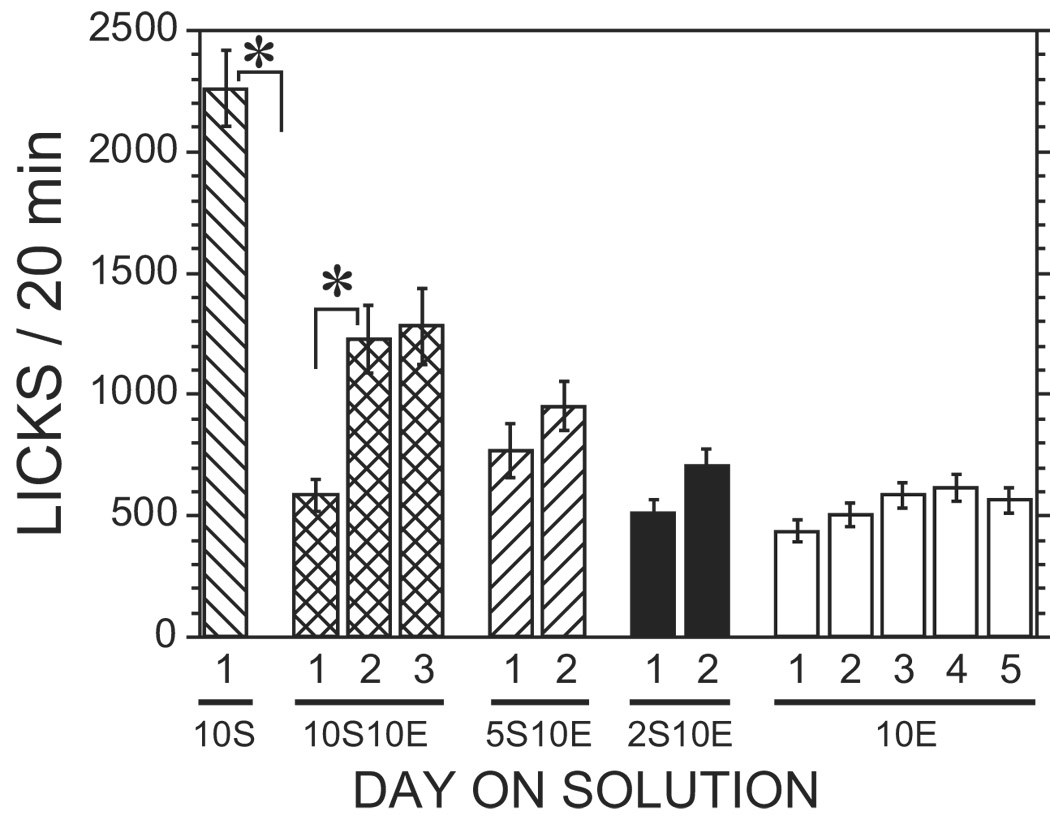
Ethanol intake (licks) during the various stages of the protocol. The licks represent the ethanol consumption during the operant self-administration of the last day of 10% sucrose, the first three days of 10% sucrose and 10% ethanol, the two days of 5% sucrose and 10% ethanol, the two days of 2% sucrose and 10% ethanol, and the last five days of 10% ethanol. Ethanol intakes (licks) are presented as mean ± SEM (n=19–22). * indicates a statistically significant difference between the indicated groups by post hoc analysis after significance was found for the overall ANOVA.
Table 2.
Lickometer parameters on last day of 10E self-administration
| Parametera | Value (n=26b) |
|---|---|
| Latency to begin drinking (min) | 0.076 ± 0.033 |
| Number of bouts | 1.8 ± 0.2 |
| Total licks | 558 ± 48 |
| Initial bout response rate (licks/min) | 181 ± 19 |
| Response rate for half of first bout (licks/min) | 269 ± 18 |
See Methods for definition of each parameter and how it was calculated.
Six rats were not included due to malfunction of the lickometer or because the licks/volume measurements were determined to be outliers (see Methods). Values are shown as mean ± SEM. All values are representative of the last 5 days of 10E self-administration except for the latency. The mean latency is almost double that observed for the previous days of 10E self-administration because one rat had a latency of 0.89 min on the last day. Without this value the latency is 0.043 ± 0.005 (n=25).
The effect of the initiation procedure on ethanol preference was measured using a two-bottle choice procedure in two ways. In the first method the concentration of ethanol during the pre-initiation preference test was 5%, and this was changed to 10% for the post-initiation preference test. The preference for 5% ethanol during the last 3 days of the test was 0.13 ± 0.05 (n=6) before the initiation procedure, and the preference for 10% ethanol was 0.29 ± 0.03 (n=6) after initiation. This suggests that the initiation procedure increased ethanol preference, but we did not do a statistical analysis because the two-bottle choice solutions were different for the pre- and post-initiation tests. In order to more directly compare ethanol preference before and after the initiation procedure, we replicated the study using 10% ethanol for both preference tests (Table 3). The ethanol preference during the last 3 days of the post-initiation test was approximately 3-fold higher than that in ethanol naïve rats (T(7) = 3.9, p < 0.05). Although the loss of ethanol solution from the control bottles (due to spillage and evaporation) is high relative to the overall change in volume of ethanol bottles during the pre-initiation test, the spillage values are smaller than those from the drinking bottles indicating that small amounts of ethanol were consumed. The low values obtained during the pre-initiation test period likely reflect the strong aversion for 10% ethanol in naïve Long-Evans rats. Therefore, the results of both methods of assessing ethanol preference confirm that the initiation procedure produced an increase in ethanol preference.
Table 3.
Ethanol preference testing before and after initiation of ethanol self-administration
| Before initiation | After initiation | |||||
|---|---|---|---|---|---|---|
| Day 3 | Day 4 | Day 5 | Day 3 | Day 4 | Day 5 | |
| Ethanol (ml)a | 2.2 ± 0.4 | 2.3 ± 0.4 | 2.4 ± 0.4 | 7.0 ± 0.9 | 5.9 ± 0.9 | 7.2 ± 1.4 |
| Water (ml) b | 37.0 ± 1.5 | 35.8 ± 1.7 | 36.2 ± 1.7 | 35.1 ± 2.0 | 33.8 ± 2.2 | 29.8 ± 2.1 |
| Preference c | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.17 ± 0.02 | 0.15 ± 0.03 | 0.19 ± 0.03 |
| Dose (g/kg/d) | 0.50 ± 0.09 | 0.53 ± 0.09 | 0.53 ± 0.08 | 1.15 ± 0.16 | 0.98 ± 0.17 | 1.20 ± 0.26 |
Values shown are mean ± SEM (n=8) for ethanol and water consumption and preference.
average loss for control ethanol bottle was 2.1 ± 0.4 ml before initiation and 1.5 ± 0.1 ml after initiation
average loss for control water bottle was 1.6 ± 0.3 ml before initiation and 1.5 ± 0.1 ml after initiation
Ethanol preference was significantly higher after initiation compared with that before initiation (paired t-test, p < 0.05)
In the first extinction test carried out on rats trained to drink 10E with an RR of 4, the mean responses during the single extinction session were 64 ± 14 lever-presses in 20 min (n=14), a value clearly higher than the 4 responses required during training. As shown in Fig. 3, the relationship between extinction responding and intake was not significant [F(1,12) = 2.1, p > .05, R2 = 0.15]. However, since the RR was relatively low, we repeated the extinction test with another group of animals using a RR of 20. In addition, we compared the ethanol group with a control group which was trained to lever-press for sucrose, but had the sucrose faded out to water during the same period as the ethanol group. Three of the ten rats in the control group did not maintain the response requirement of 20, and so they were not included in the subsequent extinction analysis. However, the control rats did consistently consume water if the response requirement was met (11.0 ± 2.6 ml, n=7). The correlation between the extinction responding and intake was not significant for the ethanol group (r2 = 0.01, p > 0.05), but it was significant for the control group (r2 = 0.75, p < 0.05). The relatively high level of drinking in the control group and the significant correlation were due to 4 out of the 7 rats that had extremely high intakes that were clustered near the top of the range. The number of lever-presses during the extinction trial under these conditions was 112 ± 22 (n=10) for the ethanol drinking rats and 140 ± 48 (n=7) for the control rats [T(9) = 0.55, p > .05].
Fig. 3.
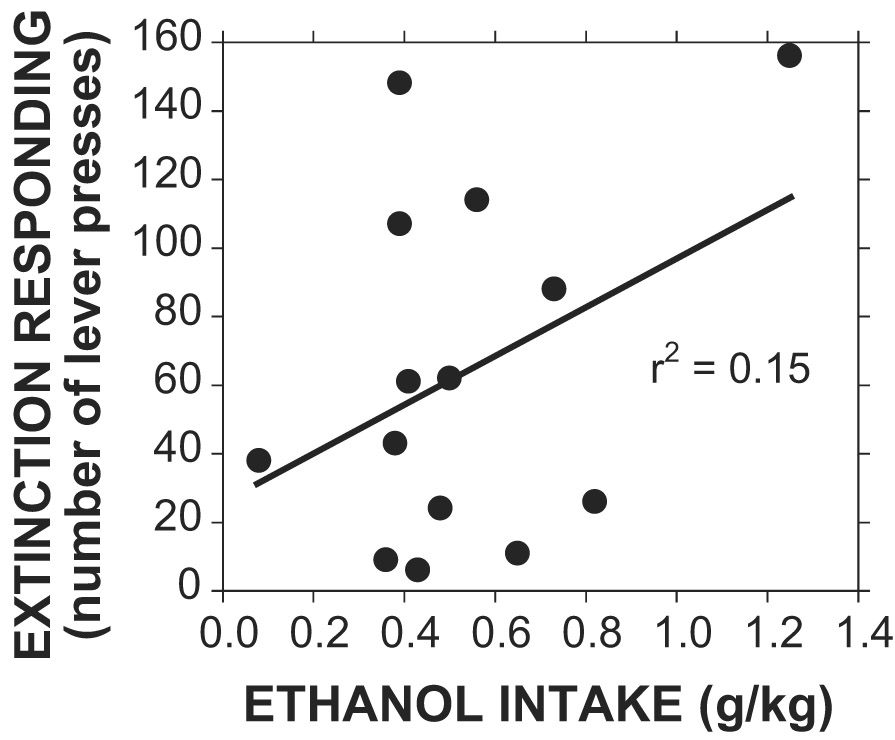
Extinction responding versus prior day ethanol intake. Ethanol intake is shown in g/kg for the last day of access to 10% ethanol for rats that had been trained using an RR of 4 (n=14). The line indicates the linear regression which was not statistically significant.
We also determined blood alcohol concentration after ethanol intake. The sixth cohort used in the study (Table 1) was trained to self-administer ethanol after performing a response requirement of 20. Two groups were used which differed in the time between the beginning of consumption and the time at which blood samples were taken from the saphenous vein (13.0 ± 2.0 and 27 ± 0.3 min), and gas chromatography was used to determine the blood alcohol concentration. The group with the short delay consumed 0.42 ± 0.05 g/kg (n=4) and had a blood alcohol concentration of 0.77 ± 0.43 mM (3.5 mg/dl). The group with the longer delay consumed 0.71 ± 0.09 g/kg (n=4) and had a blood alcohol concentration of 4.5 mM (21 ± 3 mg/dl). The blood alcohol concentration was almost 6-fold higher in the group with the longer delay compared with that in the short delay group, although the higher consumption of alcohol likely contributed to this difference also.
Discussion
During the standard sucrose-fading method for initiation of ethanol self-administration, the concentration of ethanol is slowly increased over days from 2–10%. Because it is not clear how the changing ethanol concentrations contribute to behavioral changes, this procedure limits the interpretation of experiments that may be designed to investigate mechanisms that promote the initiation of ethanol self-administration behavior. In the present study we modified the initial phase of the standard sucrose-fading procedure by exposing rats to a constant concentration of 10S10E. Our main findings were that we can get a stable intake and an increase in ethanol preference by using an ethanol initiation procedure with a fixed concentration of ethanol and sucrose for 3 days, followed by a sucrose fade-out. Moreover, the concentration of ethanol was kept constant in this protocol, and this may allow a more detailed analysis of the factors that contribute to the development of ethanol self-administration behavior, especially during the first few days of exposure to ethanol.
In previous studies of ethanol self-administration using the sucrose-fading procedure, preference for ethanol was clearly increased after the sucrose-fading procedure as measured by an unlimited access two bottle choice method (Samson, 1986; Tolliver et al., 1988; Samson et al., 1989; Files et al., 1996). For example, in the studies cited above the preference before the sucrose-fading procedure was in the range of 5–24%, and this increased to approximately 42–70%. The modified method we used also increased ethanol preference several fold, similar to the previous work. Two issues should be considered here. First, in the initial preference test the concentration of ethanol used for the post-initiation test was higher (10%) than the pre-initiation test (5%), and we did not do a statistical analysis of the change in preference because of this. However, rats have a lower preference for 10E compared with 5% ethanol (Shoaib and Almeida, 1996; Almeida et al., 1998). Our data indicated that in naïve rats the preference for 5% ethanol was 0.11–0.15. After the initiation procedure the preference for 10E was 0.27–0.32. This is clearly higher than the original pre-initiation preference for 5% ethanol. However, we also replicated the preference test using 10% ethanol for both the pre- and post-initiation tests, and in this case we also observed a statistically significant 3.2-fold increase in ethanol preference (Table 3). It should be noted that there are several methodological differences between the previous studies and the present one. However, it is unlikely that these differences influence the preference measurement since the results are similar. Overall, the results of the preference test support the idea that the modification we introduced produced a similar behavioral change compared with the traditional sucrose-fading method.
This modified protocol successfully initiated ethanol self-administration in 76% of the rats. The overall level of ethanol intake and the pattern of ethanol consumption during the 20-min access period (measured by a lickometer) were similar to previous data using the well-established sucrose-fading protocol (Table 2) (Doyon et al., 2003; Czachowski et al., 2001). Moreover, we did not observe a significant relationship between ethanol intake in the session prior to the extinction test and the number of lever-presses during the extinction trial (Fig. 3), which is also similar to previous studies (Samson et al., 2001; Samson et al., 2003). Thus, the change in the procedure that we introduced early in the training period did not significantly alter the eventual establishment of stable intake patterns of 10E solutions or appetitive responding for ethanol.
It has been suggested by Samson et al. (2003) that measures of ethanol intake alone may not accurately describe the appetitive strength of ethanol. To further examine this issue, extinction tests were performed in two groups of rats that were trained using two different RRs: 4 and 20. In both cases the extinction responding was much higher than the original RR (15-fold increase and 5.6-fold increase for the RRs of 4 and 20, respectively), results consistent with the idea that reliable ethanol self-administration had been established using our modified protocol. In addition, we also examined a control group of rats which were trained to drink 10S with an RR of 20 and never had access to ethanol. The extinction responses were higher (although not significantly) in the control group that was trained to lever-press for sucrose, but then had the sucrose faded out similar to the ethanol group. This level of extinction responding in the control group has been previously reported for rats trained with the standard sucrose-fading protocol (Samson et al., 2003). These findings suggest that the exposure to sucrose during training has a long lasting influence on appetitive responding. However, the results of this control experiment also raise the possibility that the extinction responding in the ethanol group is influenced by the previous exposure to sucrose, and this potential confound makes it difficult, if not impossible, to relate the level of responding in the ethanol group to the level of ethanol reinforcement that had been established. In any case, these findings emphasize the importance of using “alternate fluid” control groups and the continued investigation of differences between fluid- and ethanol-reinforced responding (e.g., Doyon et al., 2003, 2004, 2005).
It is interesting to examine the first few days of consumption of the ethanol-containing solution. The consumption of fluid dramatically declines during the initial exposure to 10S10E compared with the previous day consumption of 10S. However, this rebounds on the second and third day of exposure to the ethanol-containing solution (Fig. 1 and Fig. 2). The reasons for these changes in fluid consumption behavior are not clear. The drop in consumption during the first day exposure to ethanol may be due to factors such as the novelty of the solution and natural aversion to the relatively high concentration of ethanol. Comparison of the lick rates for the first 30 seconds of fluid consumption revealed that the rate was significantly lower during the first day of 10S10E (127 ± 12, n = 20) than that of the previous day (196 ± 5) (T(19) = 5.5, p < 0.05, paired t-test). This suggests that palatability of the solution affected the consummatory behavior (Davis and Smith, 1988). The rebound on days 2 and 3 may be due to factors such as habituation to the aversive flavor, the natural preference for the calories provided by both the sucrose and ethanol, and the reinforcing properties of the sucrose and ethanol. Further studies are required to determine the potential involvement of these factors.
In summary, we tested a modification of a well-established method for initiating ethanol self-administration in which rats are initially exposed to a relatively high and constant concentration of ethanol (10%) along with 10S for three days. This modified protocol produced similar patterns of intake and ethanol-seeking behavior and successfully initiated measurable ethanol self-administration in a large majority of rats. The consumption of 10E solutions produced reasonable blood alcohol concentrations. The findings from this study provide the necessary foundation for future studies of the processes that regulate the initiation of ethanol drinking that will not be confounded by changing ethanol concentrations.
Acknowledgments
This work was supported by a grant from NIH/NIAAA (AA11852). We thank Sheneil Anders and Jason Lo for excellent technical assistance. JC was supported by a training grant from NIAAA (AA07471), and ECH was supported by training grants from NIDA (DA018926) and NIAAA (AA07471).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J. Clin. Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol. Clin. Exp. Res. 2001;25:344–350. [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of lick rate measures the positive and negative feedback effects of carbohydrates on eating. Appetite. 1988;11:229–238. doi: 10.1016/s0195-6663(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J. Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Ramachandra V, Samson HH, Czachowski CL, Gonzales RA. Accumbal dopamine concentration during operant self-administration of a sucrose or a novel sucrose with ethanol solution. Alcohol. 2004;34:261–271. doi: 10.1016/j.alcohol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol. Clin. Exp. Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Brice GT, Deitrich RA, Draski LJ. Initiation of ethanol self-administration by the sucrose-substitution method with HAS and LAS rats. Alcohol. Clin. Exp. Res. 1996;20:677–681. doi: 10.1111/j.1530-0277.1996.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol. Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Rogowski A, Rokicki D, Kostowski W, Bienkowski P. Relationship between dopamine D2 receptor-associated responses and operant ethanol self-administration in the rat: A factor analysis. Alcohol Alcohol. 2003;38:305–309. doi: 10.1093/alcalc/agg081. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol. Clin. Exp. Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A. Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol. 2001;24:205–209. doi: 10.1016/s0741-8329(01)00157-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int. Rev. Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Chappell A, Legg B. Measuring the appetitive strength of ethanol: use of an extinction trial procedure. Alcohol. 2003;31:77–86. doi: 10.1016/j.alcohol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. I. Ethanol initiation and limited access operant self-administration. Alcohol. Clin. Exp. Res. 1998;22:2133–2146. [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 1999;147:274–279. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Lumeng L, Li TK. Ethanol reinforcement in the alcohol nonpreferring rat: initiation using behavioral techniques without food restriction. Alcohol. Clin. Exp. Res. 1989;13:378–385. doi: 10.1111/j.1530-0277.1989.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Schwarz-Stevens K, Samson HH, Tolliver L, Lumeng L, Li TK. The effects of ethanol initiation procedures on ethanol reinforced behavior in the alcohol-preferring rat. Alcohol. Clin. Exp. Res. 1991;15:277–285. doi: 10.1111/j.1530-0277.1991.tb01869.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Almeida OFX. Absence of tolerance to the aversive stimulus properties of ethanol following oral ethanol self-administration. Alcohol. 1996;13:175–180. doi: 10.1016/0741-8329(95)02039-x. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH. Ethanol preference following the sucrose-fading initiation procedure. Alcohol. 1988;5:9–13. doi: 10.1016/0741-8329(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Vacca G, Serra S, Brunetti G, Carai M, Samson HH, Gessa GL, Colombo G. Operant self-administration of ethanol in Sardinian alcohol-preferring rats. Alcohol. Clin. Exp. Res. 2002;26:1678–1685. doi: 10.1097/01.ALC.0000036285.62071.DA. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J. Pharmacol. Exp. Ther. 1993;267:250–258. [PubMed] [Google Scholar]


