Abstract
Sex-related differences in antinociception produced by the activation of α2-adrenoceptors (α2-ARs) have been reported, however, the precise role of gonadal steroids is still unknown. Hence, we hypothesized that estrogen and testosterone modulate antinociceptive effects of clonidine (an α2-AR agonist) on N-methyl-d-aspartate (NMDA)-and heat-induced spinal nociception. We also investigated whether estrogen-or testosterone alter the expression of α2A-adrenoceptors in the spinal cord. Sprague-Dawley (SD) rats were implanted with PE10 cannulae in the intrathecal space of the lumbosacral spinal cord and divided into male, proestrous and diestrous female, ovariectomized (OVX), estradiol-treated OVX (OVX+E), castrated male (GDX), testosterone (GDX+T) and estrogen-treated GDX (GDX+E) groups. Clonidine dose-dependently inhibited NMDA-induced scratching behavior in the male and OVX groups but to a significantly lesser extent in the OVX+E group. It also increased the tail withdrawal latency in the male, OVX, diestrous and GDX+T groups but not in the OVX+E, proestrous, GDX and GDX+E groups. Levels of α2A-AR mRNA were significantly higher in the OVX, estradiol-treated OVX, GDX and GDX+E animals. In contrast, α2A-AR protein levels were higher in estradiol-treated OVX, GDX, GDX+T and GDX+E animals as compared to the male. Indeed, no correlations were observed between changes in the mRNA or protein levels of α2A-AR and behavioral observations. These results support our hypothesis that sex-related differences in α2-AR-mediated modulation of spinal nociception are gonadal hormone-dependent: estrogen attenuates antinociceptive effects in females whereas testosterone is required for the expression of antinociception in males. In addition, results also revealed that the mechanism of action of gonadal hormones may not involve a global alternation in expression of α2A-AR in the spinal cord. Estrogen-induced attenuation of α2-AR-mediated inhibition of nociception could contribute to the higher prevalence of pain syndromes in women.
Keywords: sex differences, pain, tail flick, clonidine, analgesia, spinal cord
Sex-related differences in the perception of pain and the response to analgesics have been reported with females having lower thresholds for pain (Notermans, 1966; Neri et al., 1984; Berkley, 1997; Riley et al., 1999; Rollman et al., 2000; reviewed in Keogh et al., 2002) and a larger number of pain sites as compared to males (Fillingim and Maixner, 1995; Berkley, 1997; Fillingim et al., 1999). In addition, many pain syndromes/disorders such as fibromyalgia, rheumatoid arthritis, irritable bowel syndrome, temporomandibular joint disorder, and migraine headaches are more prevalent in women and are affected by alterations in sex hormones (Fillingim and Maixner, 1995; Marcus, 1995; Unruh, 1996; Berkley, 1997; LeResche et al., 1997; reviewed in Riley et al., 1999). Fluctuations in sex hormones during different phases of the menstrual cycle also affect pain perception and modulation in females (Tedford, 1977; Kuczmierczyk, 1986; Hapidou, 1988; Fillingim et al., 1995; reviewed in Riley et al., 1999). α2-adrenoceptors (α2-ARs) are G-protein-coupled receptors (GPCRs) that, like other GPCRs eg. opioid and GABA receptors, have been shown to elicit antinociception in humans and animals (reviewed in Yaksh, 1985; Kayser et al., 1992; Guilbaud, 1992; Eisenach, 1995; Millan, 1997, 2002). α2-AR-agonists administered systemically, intrathecally or epidurally, produce antinociception through spinal and supraspinal mechanisms (reviewed in Eisenach et al., 1996; Asano et al., 2000; Mitrovic et al., 2003) and have been utilized in treating several severe pain states, such as cancer pain and neuropathic pain (Tamsen and Grodh, 1984; Glynn et al., 1986; Glynn and O’Sullivan, 1995; Eisenach et al., 1996; Dobrydnjov et al., 2005). Gene manipulation studies have shown that among three subtypes of α2-ARs: α2A, α 2B and α2C, the α2A subtype predominantly mediates antinociceptive effects in rodents (Lakhlani et al., 1997; Stone et al., 1997; Wang et al., 2002). Sex-specific modulation of nociception by opioid receptors has been previously demonstrated by us (Zhang et al., 1998; Flores et al., 2001; Claiborne et al., 2006) and others (Cicero et al., 1996; Kepler et al., 1991; Bartok and Craft, 1997; Kest et al., 2000; reviewed in Miaskowski et al., 2000; Mogil et al., 2000; Zubieta et al., 2002; Wang et al., 2002; Stoffel et al., 2003). However, there is limited evidence regarding sex-related differences in α2-AR-induced spinal antinociception with one study showing diminished and enhanced antinociceptive effect of clonidine (an α2-AR agonist; administered intraperitoneally) after castration and ovariectomy respectively, but no change during various estrous cycle stages (Bodnar et al., 1991), and the other (Liu and Gintzler, 1999) showing involvement of α2-ARs in pregnancy-induced antinociception. Further, the precise role of gonadal hormones on sex-specific modulation of spinal nociception by α2-adrenoceptors still remains unknown. Hence, we hypothetized that the activation of α2-ARs in the spinal cord produces sex-specific, gonadal steroid (estrogen and testosterone)-dependent modulation of nociception. We also investigated whether changes in the sensitivity to α2-AR agonist could be explained by changes in spinal expression of message or protein encoding the α2A subtype of α2-ARs.
EXPERIMENTAL PROCEDURES
Animals
Adult Sprague-Dawley male, female, ovariectomized female (OVX) and castrated male (GDX) rats (225–275 g) were obtained from Harlan Sprague Dawley Inc. (Harlan, Indianapolis, IN). They were kept in the animal care facility at Meharry Medical College certified by the American Association for the Accreditation of Laboratory Animal Care (AALAC) under a 12-hour light/dark cycle (lights on: 7 AM; lights off: 7 PM) and had free access to food and water. All experimental protocols were approved by Institutional Animal Care and Use Committee of Meharry Medical College and conformed to the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pain (IASP). All efforts were made to minimize stress to the animals and the number of animals used.
Intrathecal Implantation of Cannulae
A PE-10 cannula (Intramedic, Clay Adams, Parsippany, NJ; dead volume 10 µl) was implanted intrathecally in the lumbosacral region of the spinal cord (modified from Yaksh and Rudy, 1976) in animals anesthetized with ketamine and xylazine (72 and 4 mg/kg respectively, i.p.) as detailed previously (Claiborne et al., 2006). Briefly, the animal’s head was secured in a stereotaxic frame (David Kopf) and an incision was made in the head/neck region to expose the atlanto-occipital membrane. A small opening was made in the dura with a 18-gauge needle through which the cannula was inserted in the subarachnoid space and gently pushed 8.5 cm caudally to reach the lumbosacral enlargement and was secured with dental cement. The incision was sutured, animals were kept on a heating blanket (36°C) until they recovered from anesthesia and were then returned to their home cages. Nociceptive testing began after a 5–7 days recovery period. A standard vaginal smear test was used to determine the estrous cycle stage. Females were cannulated in the diestrous phase and experiments were performed only after they underwent two regular cycles. Our laboratory has previously demonstrated that estrous cycle stages determined by vaginal smear correlate very well with the serum estradiol levels measured using radioimmunoassay (Claiborne et al., 2006). OVX and GDX rats were cannulated two weeks after the removal of gonads.
Estrogen/Testosterone replacement
Hormone injections were performed at least two weeks after the removal of gonads. OVX rats received a single subcutaneous injection of estradiol benzoate (1 ng, 1, 10 or 100 µg/100 µl sesame oil) 48 hours prior to nociceptive testing. This method of administration has been used in neuroendocrinology (Berglund et al., 1988; Priest et al., 1995) as well as the pain fields (Ji et al., 2003, 2005). The 100 µg dose of estradiol was used in majority of experiments because it is known to reliably induce lordosis behavior in rats (Clark, 1993a). Our laboratory has previously reported a time course of dose-dependent changes in serum estradiol levels in OVX rats (Nag and Mokha, 2006) as well as in normally cycling females (Claiborne et al., 2006). Levels of estradiol were found to be within the physiological range 48 hours after estradiol injection. GDX males were given a single subcutaneous injection of testosterone propionate (250µg/100 µl sesame oil) 24 hours prior to nociceptive testing. This dose of testosterone has been shown to produce maximum copulatory behavior in castrated male rats (Clark, 1993b).
N-methyl-d-aspartic acid (NMDA)-induced nociceptive testing
NMDA (4 nmol/10 µl) (Aanonsen and Wilcox, 1987) was administered intrathecally followed by 10 µl of sterile saline. NMDA microinjection induced nociceptive scratching, biting and licking behaviors that were restricted to the hind limb region and lasted for up to 4 minutes. The number of scratches/bites, latency and duration were manually recorded. Slightly higher doses of NMDA produced frankly noxious behavior including vocalization and attempts to escape in our pilot studies and were excluded.
Drugs
Various doses (0.875, 1.75, 2.5, 3.5, and 7 µg/5 µl) of clonidine, an α2-AR agonist, were intrathecally administered in separate groups, 15 minutes prior to NMDA microinjection or after baseline readings in the tail flick experiment. Doses greater than 7 µg produced apparent sedative effects in our pilot studies and were excluded. Yohimbine (30 µg/15 µl; Solomon et al., 1989; Nag and Mokha, 2004; Nag and Mokha, 2006), a selective α2-AR antagonist, was injected intrathecally 5 minutes prior to the administration of clonidine. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in sterile saline.
Tail-flick test
The tail-flick test (D’Amour and Smith, 1941) was conducted using a tail-flick analgesiameter (Model 33T, IITC Life Science, Woodland Hills, CA). Animals were loosely restrained in a Plexiglas cylinder and habituated for 1 hr. prior to testing. The heat stimulus was applied to the dorsal surface of the tail through a radiant heat source at two separate spots located between 5–9 cm from the tip of the tail to prevent sensitization produced by heating a single spot repeatedly. A change in the tail temperature, whether induced by alteration in hormones (Hosono et al., 2001; Opas et al., 2006) or by fluctuation in room temperature and other factors, could be a confounding factor in the tail flick test (Hole and Tjolsen, 1993). To avoid this, a trigger temperature setting was used to automatically pre-warm the tail to 32°C in all groups before the intense heating started. The heat intensity was set to produce an average baseline tail-flick latency of 3.5–6.0 seconds. A cutoff latency of 15 seconds was used to prevent tissue damage. Tail-flick latencies were automatically recorded at 5 min intervals before and after drug injections.
Tissue collection, RT-PCR and Taqman real-time qPCR
Animals were deeply anesthetized with sodium pentobarbital (100 mg/kg; i.p.), the lumbar spinal cord was collected in RNAlater (0.5 ml; Ambion, Austin, TX) and stored at −80°C until further processing. RNA was isolated using the Rneasy lipid tissue mini kit (QIAGEN, Inc., Valencia, CA) and purified with Dnase I. cDNA was synthesized using the Reverse-transcriptase cDNA archive kit (Applied Biosystems, Foster City, CA). Expression of the α2A-AR subtype was measured by real-time PCR (Icycler, Biorad Laboratories, Hercules, CA) using a Taqman gene expression kit from Applied Biosystems. A two-step PCR protocol was used: 50°C, 2 min; 95°C, 10 min; 95°C for 15 seconds and 50°C for 1 min for 40 cycles. Manufacturers’ instructions were followed through each step. GAPDH gene expression was measured similarly and was used to normalize α2A-AR expression. Relative expression was calculated using cycle threshold (Ct) values by Pfaffl method (2001).
Western blotting
Lumbar spinal cord tissue was collected as described above and total tissue lysate was prepared in CLB buffer (0.5 ml/100 mg tissue; 10 mM Tris, 300 mM sucrose, 1 mM AEBSF, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM EDTA, 1 µg/µl aprotinin, 1 µg/µl iodoacetamide, 1 µg/µl leupeptin, 1 µg/µl σ-phenanthroline) by homogenization for 1 min using a dounce homogenizer. A crude plasma membrane fraction was prepared by spinning the total homogenate at 7500 RPM (5 min, 4°C) discarding the pellet, spinning again at 13000 RPM (1 hr., 4°C) and resuspending the pellet in 100 µl cold PBS as described by Galan et al. (2004). Total protein content of each sample was determined using a Lowry based DC protein assay kit (Biorad). The absorbance was read at 750 nm on a Beckman DU-360 spectrophotometer and protein concentrations were calculated against a BSA standard curve. For western blotting, equal amount of protein (15 µg/15 µl Lammeli sample buffer; Biorad) for each sample was subjected to SDS-PAGE (Biorad; 10% separating gel; 50V for 1 hr, 80V for 2 hr) after heating at 65°C in a shaking water bath. Protein bands were transferred onto nitrocellulose membrane, blocked for 1 hr and probed with β-actin (1:500; Sigma-Aldrich, St. Louis, MO) or α2A-AR (1:300; C10; Daunt et al., 1997; obtained from Dr. Brian Kobilka, Stanford University) rabbit polyclonal primary antibodies for 1 hr at room temperature. After washing, the blots were incubated with HRP-conjugated donkey anti-rabbit secondary antibody (1:3000 for α2A-AR and 1:5000 for β-actin; GE Healthcare, Pittsburgh, PA), developed using chemiluminescence (Santa Cruz Biotechnology, Santa Cruz, CA) and exposed to x-ray films. X-ray films were scanned and densitometry was performed on the bands using Labworks Image Acquisition and Analysis Software (Bioimaging Systems, Upland, CA). Immunoblotting experiments for α2A-AR were performed in the laboratories of Drs. Timothy Angelotti and Brian Kobilka after determining that the commercially available antibody for α2A-AR, used initially, did not bind specifically to the α2A-AR (based on immunoblotting in tissues from α2A-KO mice ; data not shown).
Data Analysis
All data were analyzed using SPSS (SPSS, Inc., Chicago, IL). Behavior data were submitted to analysis of variance (ANOVA) with appropriate between-(Group, Drug) and within-(Time) group factors, and dependent variables (number of scratches, latency, duration, tail withdrawal latency). Data from tail flick time course experiments was analyzed by repeated measures ANOVA followed by Fisher’s LSD post hoc test. Additionally, area under the curve (AUC) was calculated by trapezoid method using Prism (Graphpad Software, Inc., San Diego, CA) for time course plots to obtain a single measure of the overall response. The data obtained from western blotting and real-time PCR studies were analyzed by one-way ANOVA. A post-hoc test (Fisher’s LSD) was employed for intergroup comparisons where necessary and only when ANOVA yielded a significant main effect. A p-value of <0.05 was considered significant. Data were plotted as mean ± SEM.
Results
Estrogen-dependent sex-specific inhibition of NMDA-induced nociception by α2-ARs
Intrathecal microinjection of NMDA (4 nmol/10 µl) produced comparable nociceptive scratching behavior in the male, OVX and OVX+E groups. There were no significant differences in both measures of NMDA-induced nociception, i.e. the number of scratches (Fig. 1A) and the duration of scratching (Fig 1B) between these groups.
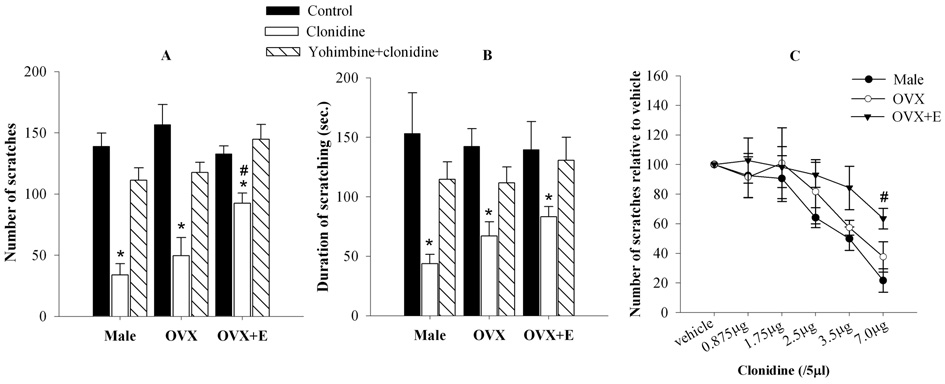
Figure 1. Activation of α2-ARs in the spinal cord produces sex-specific modulation of NMDA-induced nociception.
A, B.. Intrathecal administration of NMDA (4 nmol/10 µl) resulted in scratching behavior (A, number of scratches; B, duration of scratching) in the hindlimb region, which was comparable in all groups (males, OVX and OVX+E). Clonidine (7 µg/ 5 µl), administered intrathecally 15 minutes prior to NMDA, significantly reduced the number of scratches and duration in all three groups. However this effect of clonidine was significantly less pronounced in the OVX+E group in comparison to that in the male and OVX groups. C. Clonidine produced dose-dependent antinociception in the male, OVX and OVX+E females. Three higher doses (2.5 – 7.0 µg) of clonidine significantly reduced the number of scratches in the male and OVX groups. However, only the highest dose of clonidine (7 µg) could significantly reduce the number of scratches in the OVX+E group and this reduction was significantly less than that in clonidine-treated male or OVX groups. *p<0.05 compared to control; #p<0.05 compared to clonidine-treated male or OVX groups.
To determine whether activation of α2-ARs in the spinal cord produces sex-specific modulation of nociception, clonidine (7 µg/5 µl), an α2-AR agonist, was administered intrathecally 15 minutes prior to the administration of NMDA. Significant main effects of Group [F2,44=4.83 (scratches) p<0.05] and Drug [F2,44=47.60 (scratches); 15.45 (duration); p<0.05] as well as an interaction between the two factors (F4,44=3.14; p< 0.05; scratches only) were obtained on ANOVA. Post-hoc comparisons revealed that clonidine significantly reduced the number of scratches (Fig. 1A) and duration (Fig. 1B) in all three groups in comparison to their respective controls. However this effect of clonidine was significantly less pronounced in the OVX+E group in comparison to that in the male and OVX groups (p<0.05). The ability of yohimbine (30 µg/15 µl; i.t.; 5 minutes before clonidine), a selective α2-AR antagonist, to block the antinociceptive effects of clonidine in all groups (Fig. 1A, B) is consistent with the interpretation that the antinociception produced by clonidine is due to the activation of spinal α2-ARs. As shown in Fig. 1C, the effect of clonidine was dose-dependent in all groups. ANOVA yielded significant main effects of Group (F2,90=6.24; p<0.05) and Drug (F5,90=18.00; p<0.05) on NMDA-induced scratches. Post-hoc comparisons revealed that clonidine significantly reduced the number of scratches in the male and OVX at three higher doses (2.5 – 7.0 µg). However, only the highest dose of clonidine (7 µg) significantly reduced the number of scratches in the OVX+E group and this reduction was significantly less than that in clonidine-treated male or OVX groups (p<0.05). These findings indicate that observed differences in the ability of clonidine to suppress NMDA-induced nociceptive behavior are sex-related and not due to changes in the dose-response to clonidine.
Estrogen-dependent sex-specific modulation of heat-evoked nociception by α2-ARs
To assess whether sex-related differences in the antinociceptive properties of clonidine are specific to NMDA-induced nociception, a heat-evoked tail flick test was employed. Normally cycling females were also included in this study in order to assess whether estrogen-induced reduction in the antinociceptive effects of clonidine can be observed in normally cycling females in parallel with fluctuations in estrogen levels. Groups representing phases of higher (proestrous) and lower (diestrous) endogenous estrogen levels were included. Significant main effects of Group (F4,65=37.67; p<0.001) and Drug (F2,65=202.83; p<0.001) as well as an interaction between these two factors (F6,65=25.91; p<0.001) were obtained on ANOVA. Post hoc comparisons revealed that while baseline tail withdrawal latencies were comparable in all groups, clonidine (7 µg/ 5µl) significantly increased tail withdrawal latencies in the male, OVX and diestrous groups (with lower estrogen levels). However, clonidine had no effect in the OVX+E and the proestrous groups (with higher estrogen levels). Yohimbine fully antagonized antinociceptive effects of clonidine in the male, OVX and diestrous groups (Fig. 2A).
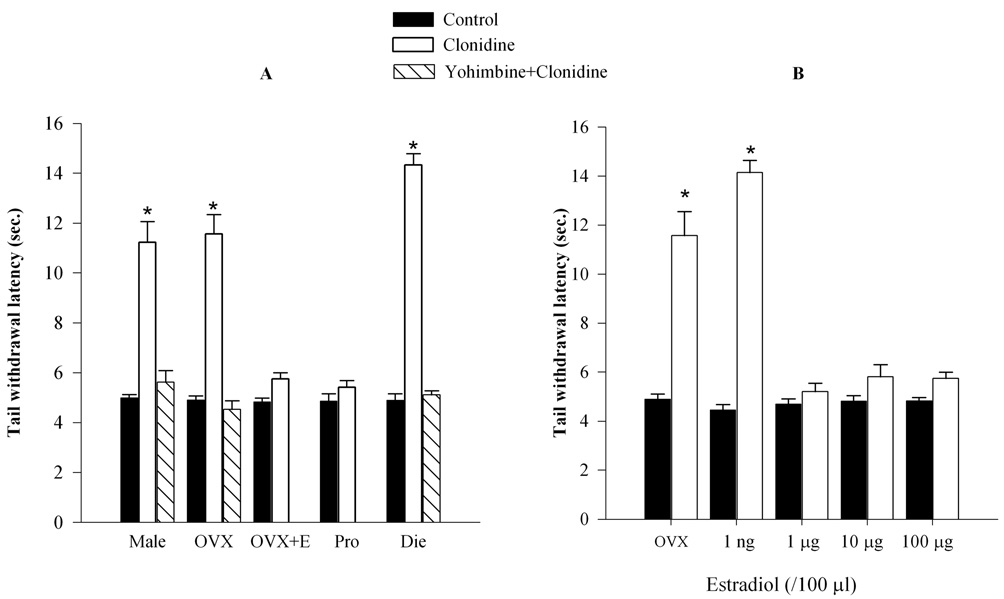
Figure 2. Clonidine increases tail withdrawal latency in male, OVX, diestrous, and in the presence of low, but not high levels of estradiol.
A. Baseline tail withdrawal latencies were comparable in all groups. Clonidine (7 µg/ 5µl), administered 15 minutes prior to testing, significantly increased the tail withdrawal latencies in the male, OVX and diestrous groups (with lower estrogen levels), but not in the OVX+E and proestrous groups (with higher estrogen levels). Clonidine’s effects were blocked by yohimbine in the male, OVX and diestrous groups. B. Estrogen dose dependently attenuated the antinociceptive effect of clonidine. It completely reversed the increase in tail withdrawal latencies produced by clonidine only at higher doses (1 µg, 10 µg or 100 µg/100 µl), but not at the lowest dose (1 ng). *p<0.05 compared to control.
Estrogen attenuates antinociceptive effects of clonidine in a dose-dependent manner
To determine the “threshold” level of estradiol that diminishes the antinociceptive effects of clonidine, the effects of clonidine were tested on heat-evoked nociception in OVX animals treated with estradiol benzoate over a range of 1 ng to 100 µg. As a comparator, levels of estrogen detected in proestrous rats correlate with those measured 48 hr. after the administration of a single 100 µg dose of estradiol. AVOVA yielded significant main effects of Group (F3,40=24.44; p<0.001) and Drug (F1,40=53.41; p<0.001) as well as an interaction between these two factors (F3,40=22.41; p<0.001). Post hoc comparisons revealed that clonidine, as expected, produced a significant increase in tail-flick latency in the OVX group (Fig. 2B). Estradiol injected at a 1 ng dose did not alter this effect, whereas treatment with 1, 10 or 100 µg estradiol fully blocked the effect of clonidine (p<0.05).
Time course of the sex-specific modulation of heat-evoked nociception
To determine whether the sex-specific antinociceptive effect of clonidine on thermal nociception is present throughout the duration of the antinociceptive response, a time course study was performed. The control baseline tail withdrawal latencies were comparable in the male, OVX, OVX+E, proestrous and diestrous groups (Fig 3A). Intrathecal administration of clonidine (7 µg/ 5 µl) produced a significant increase in tail withdrawal latencies in the OVX and male groups, but not in the OVX+E group (consistent with data provided in Fig. 2A). Repeated-measures ANOVA yielded significant main effects of Time (F11,165= 8.10; p< 0.001) and Group (F4,15= 11.26; p< 0.001). Post hoc comparisons revealed that clonidine significantly increased the latencies starting at 5 min in the male and 10 min in the OVX group compared to their respective vehicle controls (all p<0.05). The antinociceptive effect of clonidine was maintained for the period of testing (90 min). In contrast, there was no effect of clonidine in the OVX+E group (Fig. 3A). Clonidine also significantly increased the tail withdrawal latencies in normally cycling females at the diestrous but not at the proestrous stage of estrous cycle (Fig. 3B). ANOVA yielded significant main effects of Time (F11, 99= 10.34; p<0.001) and Group (F2, 9= 68.22; p<0.001). The cumulative effect of clonidine over the entire time course, presented as area under the curve (Fig. 3D) was also significantly higher in the male, OVX and diestrous groups in comparison to the OVX+E, proestrous and the respective vehicle control groups (F10,43=17.11, p< 0.001). These findings are consistent with the interpretation that high levels of estrogen present naturally can also inhibit antinociceptive effects of clonidine.
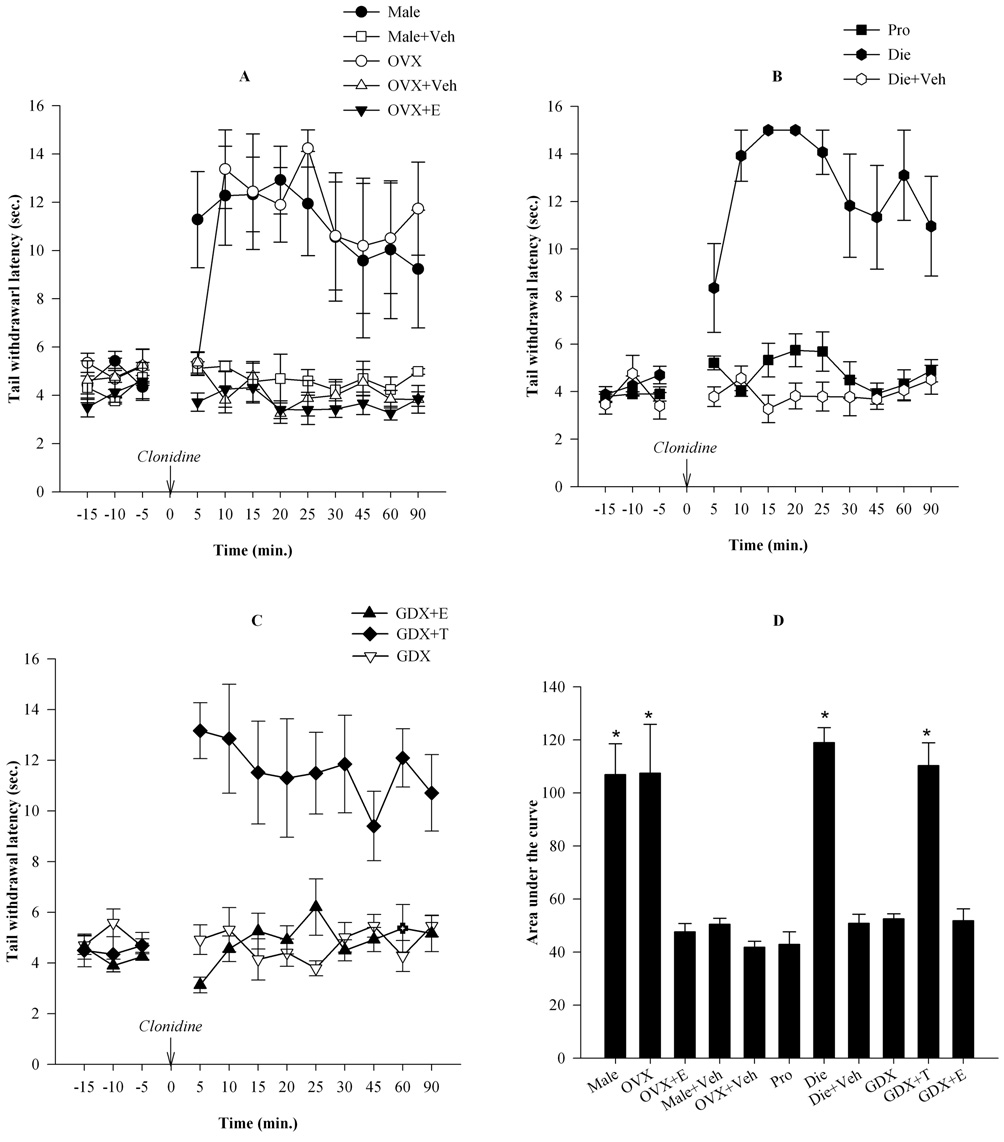
Figure 3. Estrogen attenuates whereas testosterone is required for the expression of antinociception produced by clonidine.
A. Intrathecal administration of clonidine (7 µg/ 5 µl) produced a significant increase in tail withdrawal latencies in OVX and male groups, but not in OVX+E groups. Clonidine significantly increased the latencies in the male and OVX groups, and maintained its effects for the period of testing (90 min). However, there was no effect of clonidine in the OVX+E group. B. In normally cycling females, clonidine significantly increased the tail withdrawal latencies at the diestrous stage, but not at the proestrous stage. C. Clonidine was completely ineffective in castrated males. Estradiol treatment did not alter this absence of effect, however, testosterone replacement in GDX males restored the antinociceptive effects of clonidine. D. The cumulative effect of clonidine over time was determined using area under the curve analysis. AUC was significantly higher in the male, OVX, diestrous and GDX+T groups in comparison to the OVX+E, proestrous, GDX, GDX+E and vehicle control groups. *p<0.05
Testosterone is required for the expression of the antinociceptive effect of clonidine in the male
To determine if testosterone plays a role in mediating antinociceptive effects of clonidine in the male, GDX males were administered testosterone (250 µg/ 100µl sesame oil, s.c.) 24 hours prior to, or estradiol (100 µg/100 µl sesame oil) 48 hours prior to nociceptive testing. The antinociceptive effect of clonidine was abolished in GDX males (Fig. 3C). However, clonidine produced a significant increase in the tail withdrawal latency in GDX males treated with testosterone propionate (GDX+T). In contrast, estrogen did not alter the lack of effect of clonidine in the GDX group. ANOVA revealed significant main effects of Time (F11, 99= 5.60; p<0.001) and Group (F2, 9= 12.85; p<0.001). Post-hoc comparisons revealed that clonidine significantly increased tail withdrawal latencies in the GDX+T group at all time points (p<0.05), but not in the GDX and GDX+E groups. The baseline tail withdrawal latencies were comparable in all groups. As shown in Fig. 3D, AUC was significantly higher in the GDX+T group in comparison to the GDX and GDX+E groups (F10,43=17.11, p< 0.001). These findings indicate that testosterone is required for the expression of the antinociceptive effects of clonidine in the male, whereas, as shown in Fig. 1–3, estrogen suppresses the effects of clonidine in the female.
Expression of the α2A-AR subtype mRNA or protein in the spinal cord does not correlate with sex-specific modulation of pain
As mentioned earlier, there are three α2-AR subtypes (Bylund et al., 1992; Bylund et al., 1994; reviewed in Ruffolo, 1994; Hein and Kobilka, 1998; Saunders and Limbird, 1999), and it is the α2A subtype, which principally mediates the antinociceptive effects of clonidine in the spinal cord (Lakhlani et al., 1997; Stone et al., 1997; Wang et al., 2002). To determine if changes in expression of the α2A-AR could explain the effect of estrogen to suppress the antinociceptive effects of clonidine, the expression of the α2A-AR gene relative to that of GADPH was determined in the lumbosacral region of the spinal cord in the male, OVX, OVX+E (1 ng -100 µg), proestrous, diestrous, GDX, GDX+T and GDX+E rats using real-time PCR. The male group was used as the control against which all other groups where compared. ANOVA revealed a significant main effect of Group (F7, 32=14.65; p<0.001). Post hoc comparisons showed that α2A-AR mRNA levels were significantly higher in OVX, 10 µg estradiol-treated OVX, GDX and GDX+E groups in comparison to males (Fig. 4A). However, there was no significant difference between the male and OVX groups treated with 1 ng, 1 µg and 100 µg estradiol, proestrous, diestrous and GDX+T groups. At 1 µg estrogen, no changes in receptor mRNA expression occurred, even though, at this dose, behavioral effects, in terms of tail withdrawal latencies, are maximal (Fig.2B and 4A). These data emphasize that there is no correlation between estrogen-or testosterone-induced changes in the level of α2A-AR mRNA (Fig. 4A) and the behavioral suppression of α2A-AR induced antinociception (Fig. 1, Fig 2 and Fig 3).
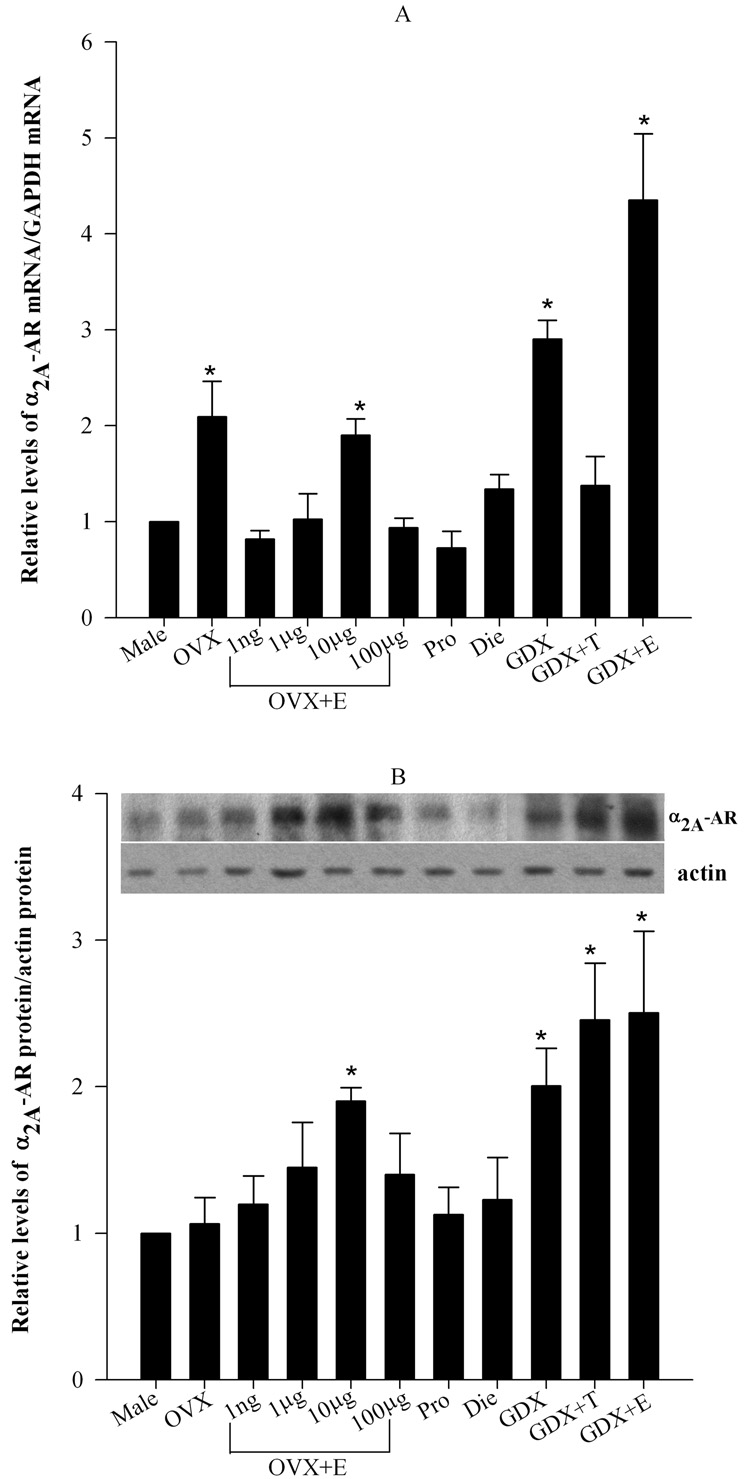
Figure 4. There is no correlation between the expression of α2-AR and antinociception.
A. Levels of α2A-AR mRNA were quantified in the lumbar spinal cord using real-time PCR and normalized by levels of GADPH mRNA. α2A-AR mRNA levels were significantly higher in OVX, 10 µg estradiol-treated OVX, GDX and GDX+E groups in comparison to males. However, there was no significant difference between the male and OVX groups treated with 1 ng, 1 µg and 100 µg estradiol, proestrous, diestrous and GDX+T groups. B. Levels of α2A-AR protein in crude plasma membrane fractions were analyzed in the lumbar spinal cord using optical density and were normalized with β-actin. The top panel shows representative α2A-AR and β-actin immunoblots. The bottom panel represents the densitometric quantification of α2A-AR normalized to β-actin and compared to the male group. Significant increase in α2A-AR protein levels was noted in the OVX+E (10 µg), GDX, GDX+T and GDX+E groups. However, there were no significant differences in the OVX, OVX+E (1 ng, 1 µg and 100 µg), proestrous and diestrous groups in comparison to the male. *p<0.05
Protein level of α2A-AR, relative to that of β-actin, was determined in the lumbosacral region of the spinal cord by western blotting using a polyclonal antibody against the α2A-AR subtype developed in the laboratory of Dr. Brian Kobilka (Fig. 4B). ANOVA revealed a significant main effect of Group (F10,32= 6.64, p<0.05). Post hoc comparisons showed that α2A-AR protein levels were elevated in the OVX+E (10 µg),GDX, GDX+T and GDX+E groups. However, there were no significant differences in the OVX, OVX+E (1 ng, 1 µg and 100 µg), proestrous and diestrous groups in comparison to the male. These changes do not correlate with either the mRNA expression in the spinal cord (Fig. 4A) or the observed antinociceptive effects in behavioral studies (Fig 1–3).
Discussion
This is the first comprehensive study to demonstrate that activation of α2-ARs in the spinal cord produces gonadal hormone-dependent, sex-specific antinociception. Estrogen attenuates antinociception in the female whereas testosterone is required for the expression of antinociception in the male. Attenuation of α2-AR-mediated antinociception by estrogen was observed in response to exogenous estrogen in ovariectomized animals as well as in normally cycling females in parallel with fluctuations in endogenous estrogen levels. Further, it was the presence of testosterone, and not the low levels or the absence of estrogen in the male, that is necessary for the expression of antinociception.
In the present study, activation of α2-AR in the spinal cord produced sex-specific modulation of the NMDA-induced nociceptive scratching behavior. The NMDA-induced scratching behavior is related to nociception and not itch for several reasons: 1) the sensations of itch and pain have been shown to be distinct and antagonistic. Itch is mediated by itch selective C primary afferent fibers and a subset of itch selective spinothalamic neurons that do not respond to noxious mechanical or thermal stimuli (Schmelz et al., 1997 and Andrew and Craig, 2001); 2) slightly higher concentrations of NMDA produced frankly noxious behavior which was evident from vocalization responses and attempts to escape; 3) similar concentrations of NMDA that produce the scratching behavior also produce a hyperalgesic response to thermal stimuli, which is blocked by mu opioid agonists (Aanonsen and Wilcox, 1987; Wilcox, 1988).
The present study also revealed that α2-AR-mediated sex-related differences occur not only in response to NMDA-induced nociceptive behavior but also in response to thermal nociception. Clonidine produced a significant antinociceptive effect in the male, OVX, and diestrous animals, but not in the proestrous or the OVX+E animals. While exogenous estrogen dose-dependently attenuated antinociceptive effects of clonidine in OVX group, high levels of endogenous estrogen attenuated this effect in proestrous females compared to diestrous females (lower estrogen levels). Such bidirectional effects of estrogen in modulating activity of opioid receptors have been reported in animals (Claiborne et al., 2006) and human studies (Smith et al., 2006). Sex-related differences in antinociception produced by systemic injection of clonidine (Bodnar et al., 1991, Mitrovic et al., 2003) have been previously reported. Our results advance these previous findings in important ways. First, we tested the effects of clonidine applied locally (i.t.) in the spinal cord. Previous studies used systemic clonidine injection that may affect multiple spinal as well as supraspinal mechanisms. Blockade of endogenous α2-AR antinociception by intrathecal yohimbine in hormone-induced or normal pregnancy has been shown (Liu and Gintzler, 1999) but that may be affected by an altered hormonal milieu (sustained high levels of estrogen and progesterone) in addition to other changes present during a complex condition like pregnancy. Second, we demonstrated that it is estrogen that decreases the antinociceptive effect of α2-AR activation in the female. Third, we demonstrated that testosterone is required for the expression of antinociception in the male. Bodnar et al. (1991) concluded their study with the results derived from castration and did not substantiate their findings by hormonal replacement. In addition, they briefly mentioned (without providing any data) that estrous cycle did not alter clonidine’s effect. On the other hand Mitrovic et al. (2003) reported sex differences in G protein-coupled, inwardly rectifying K+ channel 2 (GIRK2) knock out and wild type mice without investigating the role of sex steroids. Fourth, we revealed for the first time that the modulation of α2-AR antinociception is not paralleled by changes in levels of mRNA or protein of the α2A-AR subtype in the spinal cord of the rat.
The action of estrogen in the present study i.e. attenuatation of α2-AR-mediated analgesia, was similar to those from our previous study with another GPCR – ORL1 (Claiborne et al., 2006). However, estrogen’s actions may not be generalizable and may depend upon GPCR type, route of drug administration and therefore the site of action (spinal versus supraspinal), genotype and source of the experimental animals. Indeed, supraspinal morphine (a mu-opioid receptor agonist) injection produced most potent antinociception in the proestrous phase (high estrogen) relative to the met-diestrus phases (low estrogen) in SD rats from Charles River Laboratories (Kepler et al., 1989; Shane et. al., 2007), whereas Bernal et al. (2007) reported the most potent morphine antinociception in the diestrous phase relative to the estrous phase in SD rats from Taconic Farms.
Estrogen receptors (ERα and ERβ) are known to be present in the dorsal horn of the spinal cord (Amanduson et al., 1995; Shughrue et al., 1997; Amanduson et al., 1999; 1998; VanderHorst, et al., 2005). Therefore, estrogen can directly attenuate the α2-AR-mediated antinociception by a number of possible mechanisms including, a) decreased expression of the α2A gene, and b) decreased coupling of α2A receptors to G proteins (Gi/Go). We investigated whether estrogen down regulates the levels of α2-AR mRNA or protein. Results, however, indicate that there was no clear correlation between estrogen-induced molecular changes and behavioral findings. Therefore, one has to look for mechanisms beyond the estrogen-induced down-regulation of the α2A-AR gene/protein. Although estrogen has been shown to decrease the α2A-AR mRNA levels and α2A-AR binding density and elevate G protein coupled receptor kinase (GRK2) in the cerebral cortex (Karkanias et al., 1997), it has also been shown to not alter the α2A-AR gene expression or protein levels in the hypothalamus. It rather stabilizes α2A-AR phosphorylation, uncoupling the receptor from G proteins (Ansonoff and Etgen, 2001). It is, therefore, possible that estrogen may uncouple α2A receptor from G proteins by promoting phosphorylation of the receptor in the spinal cord. Nevertheless, changes in mRNA and protein levels of α2A-adrenoceptors observed in the present study are relative due to technical limitation and need to be further explored by additional studies with detailed measures e.g. time course, separation of spinal tissue (dorsal versus ventral and even within the dorsal horn) etc. to study these changes in diverse populations of neurons. The use of entire lumbar spinal cord tissue in the present study could mask estrogen-induced changes in gene expression that are neuron-specific, e.g. estrogen might differentially regulate gene expression depending on the neuron type i.e. down-regulates the α2A-AR expression in projection neurons and/or up-regulates the expression in GABA containing interneurons. Testosterone induced up-regulation of the α2A gene in the dorsal horn could contribute to the antinociception in the male since testosterone has been shown to elevate the levels of α2A-AR mRNA in the cerebral cortex (Dygalo et al., 2002).
In conclusion, the results of this study supported our hypothesis that the activation of α2-ARs in the spinal cord produces sex-specific, gonadal steroid-dependent modulation of nociception. Our finding that estrogen suppresses α2A-AR-induced antinociception in the spinal cord, which may contribute to the higher prevalence of pain syndromes in women, will aid in the design and administration of analgesic agents in women during the reproductive years and after menopause. Furthermore, our finding that testosterone is required for α2-AR-mediated antinociception in the male also suggest a strategy for enhancing antinociception in aging men.
Acknowledgements
This study was supported by NIH grants GM081096 (SSM), NS050654 (TA), U54 NS41071, RR03032 and MH 65782. We are thankful to Dr. Lee Limbird for her input and comments on the manuscript and Drs. Limbird and Brian Kobilka for facilitating the collaboration with Dr. Angelotti at Stanford University. We would also like to thank Dr. R.G. Wiley and Robert Kline IV for showing us the intrathecal cannulation procedure and Dr. Danny Winder for sharing tissues from α2A-KO and wild-type mice.
ABBREVIATIONS
- α2-AR
α2-adrenoceptors
- ANOVA
Analysis of variance
- AUC
Area under the curve
- Ct
Cycle threshold
- DiE
Diestrous
- GDX
Castrated male
- GDX+E
Estradiol-treated castrated male
- GDX+T
Testosterone-treated castrated male
- GIRK2
G protein-coupled, inwardly rectifying K+ channel 2
- GPCR
G-protein coupled receptor
- I.P.
Intraperitoneal
- I.T.
Intrathecal
- KF
Kölliker-Fuse
- NMDA
N-methyl-d-aspartic acid
- OVX
Ovariectomized
- OVX+E
Estradiol-treated ovariectomized
- ProE
Proestrous
- RIA
Radioimmunoassay
- S.C.
Subcutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanonsen LM, Wilcox GL. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J Pharmacol Exp Ther. 1987;243:9–19. [PubMed] [Google Scholar]
- Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett. 1995;196:25–28. doi: 10.1016/0304-3940(95)11828-k. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen AM. Receptor phosphorylation mediates estradiol reduction of alpha2- adrenoceptor coupling to G protein in the hypothalamus of female rats. Endocrine. 2001;14:165–174. doi: 10.1385/ENDO:14:2:165. [DOI] [PubMed] [Google Scholar]
- Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000;90:400–407. doi: 10.1097/00000539-200002000-00030. [DOI] [PubMed] [Google Scholar]
- Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- Berglund LA, Derendorf H, Simpkins JW. Desensitization of brain opiate receptor mechanisms by gonadal steroid treatments that stimulate luteinizing hormone secretion. Endocrinology. 1988;122:2718–2726. doi: 10.1210/endo-122-6-2718. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav Brain Res. 2007;177:126–133. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB, Blaxall HS, Iversen LJ, Caron MG, Lefkowitz RJ, Lomasney JW. Pharmacological characteristics of alpha 2-adrenergic receptors: comparison of pharmacologically defined subtypes with subtypes identified by molecular cloning. Mol Pharmacol. 1992;42:1–5. [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr., Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J Neurosci. 2006;26:13048–13053. doi: 10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT. Analysis of female sexual behavior: proceptivity, receptivity and rejection. Methods Neurosci. 1993a;14:54–75. [Google Scholar]
- Clark JT. Component analysis of male sexual behavior. Methods Neurosci. 1993b;14:32–53. [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol. 1997;51:711–720. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- Dobrydnjov I, Axelsson K, Gupta A, Lundin A, Holmstrom B, Granath B. Improved analgesia with clonidine when added to local anesthetic during combined spinal-epidural anesthesia for hip arthroplasty: a double-blind, randomized and placebo-controlled study. Acta Anaesthesiol Scand. 2005;49:538–545. doi: 10.1111/j.1399-6576.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- Dygalo NN, Kalinina TS, Sournina NY, Shishkina GT. Effects of testosterone on alpha2A-adrenergic receptor expression in the rat brain. Psychoneuroendocrinology. 2002;27:585–592. doi: 10.1016/s0306-4530(01)00094-4. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–221. [Google Scholar]
- Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS, Mason GA. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med. 1997;59:512–520. doi: 10.1097/00006842-199709000-00008. [DOI] [PubMed] [Google Scholar]
- Flores CA, Wang XM, Zhang KM, Mokha SS. Orphanin FQ produces gender-specific modulation of trigeminal nociception: behavioral and electrophysiological observations. Neuroscience. 2001;105:489–498. doi: 10.1016/s0306-4522(01)00179-8. [DOI] [PubMed] [Google Scholar]
- Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–323. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Glynn C, O'Sullivan K. A double-blind randomised comparison of the effects of epidural clonidine, lignocaine and the combination of clonidine and lignocaine in patients with chronic pain. Pain. 1996;64:337–343. doi: 10.1016/0304-3959(95)00119-0. [DOI] [PubMed] [Google Scholar]
- Glynn CJ, Jamous MA, Teddy PJ, Moore RA, Lloyd JW. Role of spinal noradrenergic system in transmission of pain in patients with spinal cord injury. Lancet. 1986;2:1249–1250. doi: 10.1016/s0140-6736(86)92678-4. [DOI] [PubMed] [Google Scholar]
- Hapidou EG, De Catanzaro D. Sensitivity to cold pressor pain in dysmenorrheic and non-dysmenorrheic women as a function of menstrual cycle phase. Pain. 1988;34:277–283. doi: 10.1016/0304-3959(88)90123-6. [DOI] [PubMed] [Google Scholar]
- Hein L, Kobilka BK. Clinical and molecular pharmacology of adrenergic receptors. Naunyn-Schmeidebergs Arch Pharmacol. 1998;358:R575. [Google Scholar]
- Hole K, Tjølsen A. The tail-flick and formalin tests in rodents: changes in skin temperature as a confounding factor. Pain. 1993;53:247–254. doi: 10.1016/0304-3959(93)90220-J. [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen XM, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K. Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1341–R1347. doi: 10.1152/ajpregu.2001.280.5.R1341. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Karkanias GB, Li CS, Etgen AM. Estradiol reduction of alpha 2-adrenoceptor binding in female rat cortex is correlated with decreases in alpha 2A/D-adrenoceptor messenger RNA. Neuroscience. 1997;81:593–597. doi: 10.1016/s0306-4522(97)00359-x. [DOI] [PubMed] [Google Scholar]
- Kayser V, Besson JM, Guilbaud G. Evidence for a noradrenergic component in the antinociceptive effect of the analgesic agent tramadol in an animal model of clinical pain, the arthritic rat. Eur J Pharmacol. 1992;224:83–88. doi: 10.1016/0014-2999(92)94822-d. [DOI] [PubMed] [Google Scholar]
- Keogh E, Herdenfeldt M. Gender, coping and the perception of pain. Pain. 2002;97:195–201. doi: 10.1016/S0304-3959(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- Kiefel JM, Bodnar RJ. Roles of gender and gonadectomy in pilocarpine and clonidine analgesia in rats. Pharmacol Biochem Behav. 1992;41:153–158. doi: 10.1016/0091-3057(92)90075-q. [DOI] [PubMed] [Google Scholar]
- Kuczmierczyk AR, Adams HE. Autonomic arousal and pain sensitivity in women with premenstrual syndrome at different phases of the menstrual cycle. J Psychosom Res. 1986;30:421–428. doi: 10.1016/0022-3999(86)90081-4. [DOI] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via "hit and run" gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69:153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- Liu NJ, Gintzler AR. Gestational and ovarian sex steroid antinociception: relevance of uterine afferent and spinal alpha(2)-noradrenergic activity. Pain. 1999;83:359–368. doi: 10.1016/s0304-3959(99)00120-7. [DOI] [PubMed] [Google Scholar]
- Marcus DA. Interrelationships of neurochemicals, estrogen, and recurring headache. Pain. 1995;62:129–139. doi: 10.1016/0304-3959(95)00052-T. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Gear RW, Levine JD. Sex-related differences in analgesic responses. Sex, gender and pain. In: Fillingim RB, editor. Sex, gender and pain. Seattle: IASP Press; 2000. pp. 209–230. [Google Scholar]
- Millan MJ. The role of descending noradrenergic and serotinergic pathways in the modulation of nociception: focus on receptor multiplicity. In: Dickenson AH, Besson JM, editors. The Pharmacology of Pain. Springer: 1997. pp. 385–446. [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci U S A. 2003;100:271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Nag S, Mokha SS. Activation of alpha2-adrenoceptors in the trigeminal region produces sex-specific modulation of nociception in the rat. Neuroscience. 2006;142:1255–1262. doi: 10.1016/j.neuroscience.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Nag S, Mokha SS. Estrogen attenuates antinociception produced by stimulation of Kolliker-Fuse nucleus in the rat. Eur J Neurosci. 2004;20:3203–3207. doi: 10.1111/j.1460-9568.2004.03775.x. [DOI] [PubMed] [Google Scholar]
- Neri M, Agazzani E. Aging and right-left asymmetry in experimental pain measurement. Pain. 1984;19:43–48. doi: 10.1016/0304-3959(84)90063-0. [DOI] [PubMed] [Google Scholar]
- Notermans SL. Measurement of the pain threshold determined by electrical stimulation and its clinical application. I. Method and factors possibly influencing the pain threshold. Neurology. 1966;16:1071–1086. doi: 10.1212/wnl.16.11.1071. [DOI] [PubMed] [Google Scholar]
- Opas EE, Gentile MA, Kimmel DB, Rodan GA, Schmidt A. Estrogenic control of thermoregulation in ERalphaKO and ERbetaKO mice. Maturitas. 2006;53:210–216. doi: 10.1016/j.maturitas.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleeger M, Straneva PA, Fillingim RB, Maixner W, Girdler SS. Menstrual cycle, blood pressure and ischemic pain sensitivity in women: a preliminary investigation. Int J Psychophysiol. 1997;27:161–166. doi: 10.1016/s0167-8760(97)00058-5. [DOI] [PubMed] [Google Scholar]
- Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res Mol Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- Riley JL, III, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Lautenbacher S, Johanes KS. Sex and gender differences in responses to experimentally induced pain in humans. In: Fillingim RB, editor. Sex, gender, and pain, Progess in pain research and management. Seattle: IASP Press; 2000. pp. 165–190. [Google Scholar]
- Ruffolo RR., Jr. alpha-Adrenoceptors. Monogr Neural Sci. 1984;10:224–252. [PubMed] [Google Scholar]
- Saunders C, Limbird LE. Localization and trafficking of alpha2-adrenergic receptor subtypes in cells and tissues. Pharmacol Ther. 1999;84:193–205. doi: 10.1016/s0163-7258(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane R, Bernal SY, Rozengurtel S, Bodnar RJ. Estrus phase differences in female rats in morphine antinociception elicited from the ventrolateral periaqueductal gray. Int J Neurosci. 2007;117:811–822. doi: 10.1080/00207450600910259. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Winzer-Serhan U, Leslie F, Hokfelt T. Distribution of alpha2-adrenoceptor mRNAs in the rat lumbar spinal cord in normal and axotomized rats. Neuroreport. 1999;10:2835–2839. doi: 10.1097/00001756-199909090-00025. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RE, Gebhart GF. Intrathecal morphine and clonidine: antinociceptive tolerance and cross-tolerance and effects on blood pressure. J Pharmacol Exp Ther. 1988;245:444–454. [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamsen A, Gordh T. Epidural clonidine produces analgesia. Lancet. 1984;2:231–232. doi: 10.1016/s0140-6736(84)90523-3. [DOI] [PubMed] [Google Scholar]
- Tedford WH, Jr., Warren DE, Flynn WE. Alteration of shock aversion thresholds during the menstrual cycle. Percept Psychophysiol. 1977;21:193–196. [Google Scholar]
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Wang XM, Zhang ZJ, Bains R, Mokha SS. Effect of antisense knock-down of alpha(2a)- and alpha(2c)-adrenoceptors on the antinociceptive action of clonidine on trigeminal nociception in the rat. Pain. 2002;98:27–35. doi: 10.1016/s0304-3959(01)00464-x. [DOI] [PubMed] [Google Scholar]
- Wilcox GL. Pharmacological studies of grooming and scratching behavior elicited by spinal substance P and excitatory amino acids. Ann N Y Acad Sci. 1988;525:228–236. doi: 10.1111/j.1749-6632.1988.tb38608.x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zhang KM, Wang XM, Peterson AM, Chen WY, Mokha SS. alpha2-adrenoceptors modulate NMDA-evoked responses of neurons in superficial and deeper dorsal horn of the medulla. J Neurophysiol. 1998;80:2210–2214. doi: 10.1152/jn.1998.80.4.2210. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]