Abstract
Objective
The vestibular system is a major pathway to nausea and vomiting, and the vestibulo-ocular reflex (VOR) is a central component; its function can be studied using the vestibular autorotation test (VAT). We hypothesize that women with hyperemesis gravidarum (HG) may have VOR abnormalities.
Study Design
Women with HG were compared to women without HG using the VAT. Horizontal and vertical VOR gains and phases were evaluated at three frequency ranges: low (2.0–3.5 Hz), medium (>3.5 – 5.0 Hz), and high (>5.0 – 6.0 Hz) during pregnancy and postpartum.
Results
Twenty women with HG and 48 unaffected women were evaluated in early pregnancy. Women with HG had higher horizontal gains at all three frequency ranges. Horizontal phase differences were also observed at medium frequencies. No VAT differences were noted postpartum.
Conclusions
Women experiencing HG had a higher mean VOR horizontal gain and lower horizontal phase when compared to unaffected women.
Keywords: Hyperemesis gravidarum, nausea, vomiting, vestibulo-ocular reflex, vestibular, autorotation
Introduction
Hyperemesis gravidarum (HG) is the most severe form of nausea and vomiting of pregnancy (NVP). It is estimated that over 36,000 women are admitted to the hospital for HG each year in the United States, costing over $250,000,000 for hospital care alone1. To date, the etiologies of NVP and HG remain unknown, and consequently treatment avenues have been limited to the relief of symptoms and the reversal of dehydration and nutritional deficiencies. One approach to understanding the cause of HG has been to measure products of placental metabolism that could stimulate nausea and vomiting. A complimentary approach, less often employed, is to study NVP as a syndrome, focusing on the various pathways that affect susceptibility to NVP in the mother2,3.
There are several pathways by which a stimulus can evoke nausea and vomiting in humans4,5. First, toxic material within the lumen of the gut can stimulate vagal afferents that project to the dorsal brainstem. Second, absorbed toxic materials or endogenous substances in the blood can act directly on the area postrema to initiate emesis. Pathology in the intestines or other visceral organs can act via either of the pathways described above. Third, central nervous system stimuli, such as fear or trauma, can evoke the emetic reflex. Finally, a disturbance in the vestibular system, such as motion sickness or Meniere’s disease, can elicit nausea and vomiting by direct input to the vomiting center in the medulla6. Apart from this direct stimulus to nausea and vomiting from changes in the vestibular system, it is thought that the vestibular system also regulates the response of the brainstem to inputs from the other emetic pathways. The vestibular system may be thought of as controlling the sensitivity of a given individual to an emetic stimulus7.
The vestibular system has been presumed to play a role in NVP and HG. Women with NVP are more likely to report a history of motion sickness and migraine8, and avoidance of motion is one of the most commonly volunteered strategies for reducing NVP when women are surveyed9,10.
The vestibulo-ocular reflex (VOR) is responsible for coordinating head and eye movements, stabilizing gaze during changes in head position, and reducing the apparent “slip” of objects on the retina during activities that cause changes in head position. Specific VOR patterns have been described for patients with known vestibular pathology, such as Meniere’s disease or gentamicin toxicity11, but no objective testing of the VOR has previously been attempted in pregnancy. A study of VOR patterns in pregnant women, and specifically in women with HG, may help identify the elements of the reflex pathway that may be affected in HG. The purpose of this investigation was to determine if women with HG were more likely to demonstrate any abnormality of the VOR, the central component of the vestibular system, compared to women without HG.
Methods
This study was part of a rigorous protocol designed to identify abnormalities in the physiological pathways leading to or modifying nausea and vomiting among women with HG. Women with and without HG were recruited and evaluated prospectively. Women with HG were recruited as cases admitted to local hospitals for the index pregnancy, and women without HG were recruited from local clinics. Women were excluded if they had any chronic condition associated with nausea and vomiting, other than pregnancy. HG was defined as severe nausea and vomiting of pregnancy requiring treatment with IV fluids and/or parenteral nutrition. We evaluated subjects on three separate occasions: 1) the first trimester, 2) the late second trimester, and 3) postpartum. On the first visit, women completed a questionnaire for demographic, pregnancy and general health information. They also completed a specific questionnaire regarding symptoms of dizziness or imbalance, and motion sickness12.
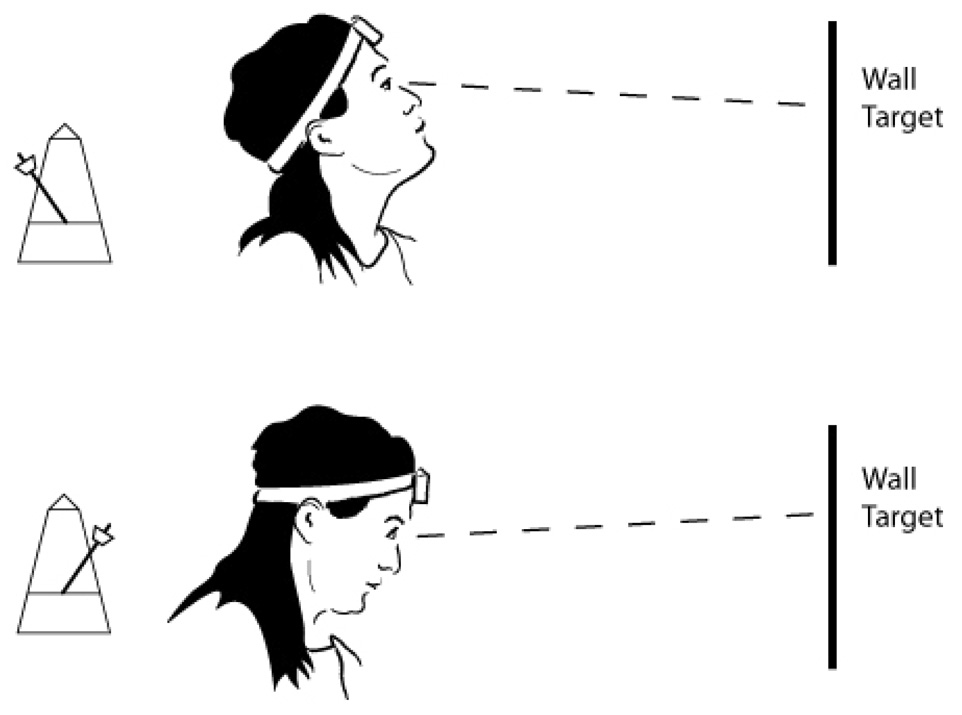
The VOR was assessed by means of the Vestibular Autorotation Test (VAT) (Western System Research, Inc. Pasadena, CA)13. The VAT is a computerized device, which consists of a head band with a built-in angular velocity sensor for measuring head movement, and electrodes for measuring eye movements. Five electrodes were placed around the eyes: two vertical electrodes, two horizontal electrodes and one reference electrode. An auditory metronome cue (tone) was generated by the computer and the subject was asked to move her head, either side to side (horizontally) or up and down (vertically), at the same frequency as the tone (Figure 1). For each trial, the cue began at a slow pace (0.5 Hz) and then became progressively faster, increasing to 6 Hz near the end of each test. While moving her head, the subject attempted to keep her eyes fixated on a wall target. Each subject performed one or more training sessions before recording of the data. The eye and head movements were recorded simultaneously by the computer. Three trials each for horizontal and vertical testing were completed, with each trial lasting18 seconds. Data from the first six seconds were used for electro-oculogram calibration, and the last 12 seconds to compute the vestibular-ocular reflex (VOR) gain and phase in both vertical and horizontal planes.
Figure 1.
Example of vertical head motion performed during the Vestibular Autorotation Test (VAT). The recording device is placed on the subject’s head, and she is instructed to keep her eyes fixated on a wall target while moving her head up and down to a metronome cue.
Gain is defined as the amplitude of eye movement divided by the amplitude of head movement. In an ideal situation, this amplitude should be −1, with the eyes moving in an equal and opposite direction to the head. The phase is the timing of head movement compared to eye movement. Ideally there should be “zero phase shift;” in other words, the eye movement should not lag behind the head movement. Horizontal and vertical VOR gains and phases were evaluated at three frequency ranges: low (2.0–3.5 Hz), medium (>3.5 – 5.0 Hz), and high (>5.0 – 6.0 Hz). For each frequency range, one of the authors (L.D-O.), who was blinded to whether the subject had HG, assigned the gain and the phase results to ordinal categories based on norms for the adult non-pregnant population: 7 = Abnormally high; 6 = Slightly high; 5 = High normal; 4 = Normal; 3 = Low normal; 2 = Slightly low; and 1 = Abnormally low. For categorical comparisons, “High” results were defined as a score > 413.
The primary analysis was a comparison of women with HG and those without HG at all three visits. For a single sub-analysis, women without HG were separated into those with and without NVP, so that VAT results for the three groups of women could be evaluated between asymptomatic women, those with NVP, and those with HG. Continuous variables are expressed as the mean ± standard deviation, median and range. Categorical data were analyzed with a Chi-square test with Yates correction, and continuous data were analyzed non-parametrically with a Kruskal-Wallis test. All calculations were performed in SAS (v. 9.1, Cary, NC). The protocol was approved by the institutional review board of the Health Sciences Campus of the University of Southern California.
Results
The study population consisted of a total of 31 women with HG and 65 women without HG. Among the 65 women without HG, 49 (75.4%) had some degree of nausea and vomiting; 16 (24.6%) had no symptoms of nausea and vomiting. Because of the rigors of the study protocol, a substantial number of women from both groups either withdrew or were lost to follow up between visits (Table I). Included among these women were two women from each group who suffered a fetal loss prior to the second visit. For each study visit, Table I also describes the number of subjects who either did not complete the VAT or who had uninterpretable results, primarily due to an inability to comply with the testing because of nausea or dizziness. Women completed their first visit between June 2004 and October 2006.
Table I.
Study population denominators across three study visits by the interpretability of vestibular Autorotation Test (VAT) results.
| Number of subjects with at least one interpretable test | Number of subjects who did not have an interpretable test due to hardware problems | Number of subjects who were not available for the test | Number of subjects who did not have an interpretable test because they were too sick to comply | |
|---|---|---|---|---|
| Visit 1 | ||||
| Women without HG: N = 65 | 48 (74%) | 0 | 5 (8%) | 12 (18%) |
| Women with HG: N = 31 | 20 (65%) | 1 (3%) | 7 (23%) | 3 (10%) |
| Visit 2 | ||||
| Women without HG: N = 37 | 30 (81%) | 0 | 3 (8%) | 4 (11%) |
| Women with HG: N = 13 | 8 (62%) | 0 | 3 (23%) | 2 (15%) |
| Visit 3 | ||||
| Women without HG: N = 25 | 22 (88%) | 1 (4%) | 2 (8%) | 0 |
| Women with HG: N = 16 | 13 (81%) | 0 | 3 (19%) | 0 |
Characteristics of the participants during their first and subsequent visits are presented in Table II. The mean gestational age at Visit 1 was 13.8 ± 5.3 weeks for women with HG and 11.0 ± 1.9 weeks for women without HG (P = 0.0211), appearing somewhat greater for women with HG, due to the fact that a number of subjects with HG were too ill to come to the testing center for the battery of tests within a week of enrollment. The mean gestational age at Visit 2 was 22.7 ± 3.1 weeks for women with HG and 22.3 ± 3.0 for women without HG (P = 0.4858). Estimated gestational age at the time of delivery, for women with and without HG respectively, was 38.6 ± 1.4 weeks and 38.6 ± 2.5 weeks. Visit 3 (postpartum) was completed at 16.9 ± 10.3 weeks and 17.5 ± 10.0 weeks for women with and without HG respectively.
Table II.
Characteristics of study participants with and without hyperemesis gravidarum (HG).
| Characteristic | N (%) Women without HG | N (%) Women with HG | P Value |
|---|---|---|---|
| Age ≥ 30 | 28/65 (43.1%) | 10/31 (32.3%) | 0.4293 |
| Received more than a high school education | 28/65 (43.1%) | 8/31 (25.8%) | 0.1589 |
| Government health insurance | 49/65 (75.4%) | 24/31 (77.4%) | 1.0000 |
| Marital status | 0.7926 | ||
| Married or Living with | 46 (70.8%) | 23 (74.2%) | |
| Partner | 2 (3.1%) | 0 | |
| Divorced/Widowed/Separated | 17 (26.2%) | 8 (25.8%) | |
| Never married | |||
| Race | 0.9082 | ||
| Hispanic | 47 (72.3%) | 23 (74.2%) | |
| Black | 8 (12.3%) | 3 (9.7%) | |
| White | 7 (10.8%) | 3 (9.7%) | |
| Asian | 1 (1.5%) | 1 (3.2%) | |
| Native American | 0 | 1 (3.2%) | |
| Other/Unknown | 2 (3.1%) | 0 | |
| Reason dropped from study | 0.2456 | ||
| Fetal loss | 2 (3.1%) | 2 (6.5%) | |
| Loss to follow up | 24 (36.9%) | 11 (35.5%) | |
| Withdrew | 12 (18.5%) | 2 (6.5%) | |
| Moved | 2 (3.1%) | 0 | |
| NA | 25 (38.5%) | 16 (51.6%) | |
| Primiparous | 21/65 (32.3%) | 10/31 (32.3%) | 1.0000 |
| Women reporting nausea at time of first visit | 49/65 (75.4%) | 26/31 (83.9%) | 0.4987 |
| Ptyalism | 23/64 (35.9%) | 20/31 (64.5%) | 0.0162 |
| Weight loss prior to first visit: Yes or No (self-report) | 30/65 (46.2%) | 26/31 (83.9%) | 0.0010 |
Although not statistically significant, women with HG complained more often of dizziness that had an onset during pregnancy compared to women without HG (12/31 [38.7%] vs. 13/63 [20.6%], P = 0.106). No women with HG reported any chronic problems with dizziness. No differences in reports of motion sickness, either as a child or adult were identified.
Table III describes the VAT response ordinal categories for women with and without HG, indicating that women with HG had increased horizontal gain at all three frequency ranges and decreased horizontal phase at the medium frequency range during Visit 1. Results for Visit 2 were similar. No differences in the VAT responses were noted between women with and without HG during Visit 3. This same pattern of increased gain at all frequencies for women who had suffered from HG was noted during Visit 3 but the differences were not statistically significant. We note that for 20% – 30% of women in both HG and non-HG groups, tests of both vertical and horizontal gain and phase did not yield interpretable results, particularly at the higher frequencies. Inconsistencies between the numbers shown in Table I and Table III were due to lack of tolerance of VAT testing. VAT testing provoked nausea in some women, who subsequently could not complete the test at one or more frequency levels.
Table III.
Comparison of Vestibular Autorotation Test (VAT) response ordinal categories for women with and without hyperemesis gravidarum (HG) for Study Visits 1, 2, and 3.
| VAT response | Women without HG Mean ± s.d., Median | Women with HG Mean ± s.d., Median | P value |
|---|---|---|---|
| VISIT 1 | |||
| Horizontal gain, low Hz** | N = 44 | N=20 | 0.0066 |
| 4.07 ± 0.97 | 4.70 ± 1.13 | ||
| Horizontal gain, med Hz** | N = 45 | N = 19 | 0.0045 |
| 3.80 ± 1.25 | 4.63 ± 1.16 | ||
| Horizontal gain, high Hz* | N = 31 | N = 13 | 0.0165 |
| 4.06 ± 1.26 | 4.85 ± 1.28 | ||
| Vertical gain, low Hz | N = 46 | N = 20 | 0.6067 |
| 3.91 ± 0.72 | 3.85 ± 0.67 | ||
| Vertical gain, med Hz | N = 45 | N = 17 | 0.9199 |
| 3.87 ± 0.69 | 3.94 ± 0.24 | ||
| Vertical gain, high Hz | N = 39 | N = 12 | 0.8237 |
| 3.87 ± 0.77 | 4.00 ± 0 | ||
| Horizontal phase, low Hz | N = 44 | N = 20 | 0.3720 |
| 3.86 ± 0.95 | 3.70 ± 0.80 | ||
| Horizontal phase, med Hz* | N = 46 | N = 20 | 0.0273 |
| 4.33 ± 1.43 | 3.60 ± 1.14 | ||
| Horizontal phase, high Hz | N = 32 | N = 14 | 0.0734 |
| 4.00 ± 1.30 | 3.36 ± 1.15 | ||
| Vertical phase, low Hz | N = 46 | N = 20 | 0.2496 |
| 4.83 ± 1.22 | 5.30 ± 1.42 | ||
| Vertical phase, med Hz | N = 45 | N = 16 | 0.3755 |
| 4.49 ± 1.22 | 4.75 ± 1.13 | ||
| Vertical phase, high Hz | N = 33 | N = 10 | 0.5947 |
| 4.61 ± 1.39 | 4.80 ± 1.14 | ||
| VISIT 2 | |||
| Horizontal gain, low Hz** | N = 30 | N=8 | 0.0028 |
| 4.10 ± 0.99 | 5.25 ± 1.28 | ||
| Horizontal gain, med Hz** | N= 30 | N = 8 | 0.0073 |
| 4.00 ± 1.02 | 5.00 ± 1.31 | ||
| Horizontal gain, high Hz | N = 23 | N = 6 | 0.1774 |
| 4.00 ± 1.21 | 4.67 ± 2.25 | ||
| Vertical gain, low Hz | N = 30 | N = 8 | 0.3363 |
| 3.83 ± 0.95 | 4.13 ± 0.35 | ||
| Vertical gain, med Hz | N = 29 | N = 8 | 0.5168 |
| 3.76 ± 0.87 | 4.00 ± 0.00 | ||
| Vertical gain, high Hz | N= 23 | N = 6 | 0.8061 |
| 3.87 ± 0.76 | 4.00 ± 0.00 | ||
| Horizontal phase, low Hz* | N = 29 | N = 8 | 0.0370 |
| 4.38 ± 1.24 | 3.50 ± 1.07 | ||
| Horizontal phase, med Hz* | N = 29 | N = 8 | 0.0104 |
| 4.52 ± 1.35 | 3.38 ± 1.07 | ||
| Horizontal phase, high Hz | N = 23 | N = 6 | 0.0756 |
| 4.39 ± 1.41 | 3.33 ± 1.21 | ||
| Vertical phase, low Hz | N = 29 | N =8 | 0.1835 |
| 5.34 ± 1.37 | 4.75 ± 1.39 | ||
| Vertical phase, med Hz | N = 28 | N = 8 | 0.4098 |
| 5.14 ± 1.30 | 4.75 ± 1.16 | ||
| Vertical phase, high Hz | N = 20 | N = 5 | 0.1972 |
| 5.30 ± 1.34 | 4.60 ± 1.34 | ||
| VISIT 3 | |||
| Horizontal gain, low Hz | N = 21 | N=13 | 0.5320 |
| 4.38 ± 0.80 | 4.69 ± 1.18 | ||
| Horizontal gain, med Hz | N = 21 | N = 12 | 0.2786 |
| 4.14 ± 0.85 | 4.58 ± 1.16 | ||
| Horizontal gain, high Hz | N = 20 | N = 11 | 0.6363 |
| 3.95 ± 0.94 | 4.36 ± 1.43 | ||
| Vertical gain, low Hz | N = 20 | N = 12 | 0.8053 |
| 3.95 ± 0.51 | 4.00 ± 0.60 | ||
| Vertical gain, med Hz | N = 20 | N = 12 | 0.8864 |
| 3.95 ± 0.51 | 4.00 ± 0.85 | ||
| Vertical gain, high Hz | N = 16 | N = 10 | 0.6175 |
| 4.06 ± 0.68 | 3.90 ± 0.74 | ||
| Horizontal phase, low Hz | N = 21 | N = 13 | 0.9619 |
| 4.14 ± 0.91 | 4.00 ± 0.41 | ||
| Horizontal phase, med Hz | N = 21 | N = 12 | 0.5966 |
| 4.24 ± 1.30 | 4.00 ± 0.85 | ||
| Horizontal phase, high Hz | N = 21 | N = 10 | 0.3128 |
| 4.33 ± 0.97 | 3.90 ± 1.45 | ||
| Vertical phase, low Hz | N = 20 | N = 12 | 0.1537 |
| 5.20 ± 1.28 | 4.50 ± 0.67 | ||
| Vertical phase, med Hz | N = 20 | N = 12 | 0.2378 |
| 4.70 ± 1.13 | 4.25 ± 0.62 | ||
| Vertical phase, high Hz | N = 14 | N = 10 | 0.4839 |
| 4.21 ± 0.89 | 4.10 ± 1.10 |
Statistically significant at P < 0.05
Statistically significant at P < 0.01
Table IV describes the proportion of women with high horizontal gain results. For women with HG, high horizontal gain results were more common at Visit 1 (≥ 35%) and at Visit 2 (≥ 50%) compared to less than 10% of women without HG, whose results were similar to the non-pregnant population norms14. At the postpartum Visit 3, 25% of women who had suffered from HG still demonstrated high gain results, compared to women without HG who returned for Visit 3, of whom 19% had high results (not statistically significant).
Table IV.
Percent of women with and without HG who had High scores (> 4) vs. Normal or Low scores (≤ 4) for Horizontal Gain.
| N (%) Women without HG | N (%) Women with HG | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| VISIT 1 | ||||
| Horizontal Gain Low Hz | 4/44 (9.1%) | 7/20 (35.0%) | 5.4 (1.2–26.8) | 0.0286 |
| Horizontal Gain Med Hz | 3/45 (6.7%) | 7/19 (36.8%) | 8.2 (1.5–48.3) | 0.0078 |
| Horizontal Gain High Hz | 3/31 (9.7%) | 5/13 (38.5%) | 5.8 (0.9–41.1) | 0.0672 |
| VISIT 2 | ||||
| Horizontal Gain Low Hz | 3/30 (10.0%) | 5/8 (62.5%) | 15.0 (1.8–162.0) | 0.0060 |
| Horizontal Gain Med Hz | 2/30 (6.7%) | 4/8 (50.0%) | 14.0 (1.4–174.7) | 0.0146 |
| Horizontal Gain High Hz | 2/23 (8.7%) | 3/6 (50.0%) | 10.5 (0.9–170.8) | 0.0753 |
| VISIT 3 | ||||
| Horizontal Gain Low Hz | 5/21 (23.8%) | 4/13 (30.8%) | 1.42 (0.23–8.63) | 0.9625 |
| Horizontal Gain Med Hz | 4/21 (19.0%) | 3/12 (25.0%) | 1.42 (0.19–10.27) | 0.9679 |
| Horizontal Gain High Hz | 5/20 (25.0%) | 3/11 (27.3%) | 1.13 (0.46–7.83) | 0.7714 |
No differences in vertical gain or phase were noted either with continuous or categorical analyses.
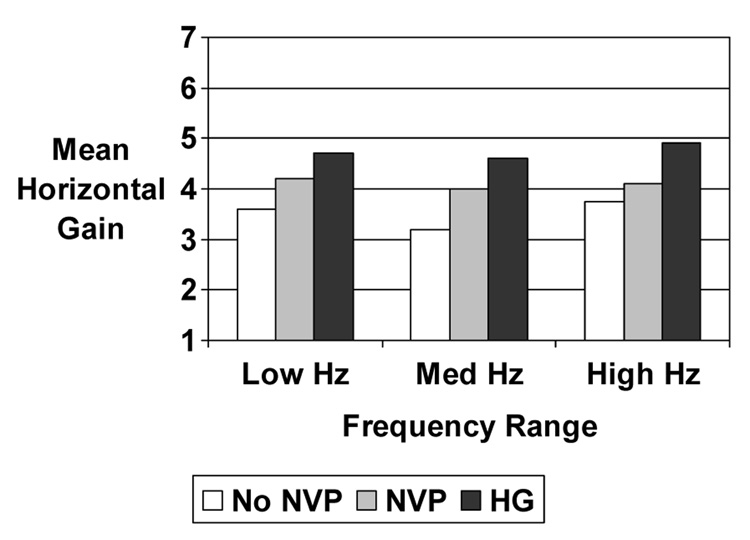
In a subanalysis, women without HG were divided into those who did and did not report nausea and vomiting during Visit 1. Three groups of women were then compared: asymptomatic women, women with ordinary NVP, and women with HG. The mean horizontal gain increased with the severity of nausea and vomiting (Figure 2).
Figure 2.
Mean horizontal gain by severity of nausea and vomiting of pregnancy during Visit 1 for all three frequency ranges. Comparison of all three groups at each frequency range by the Kruskal-Wallis test was statistically significant at P < 0.05.
Comment
This study provides the first objective evidence that women with HG demonstrate an abnormality of the vestibular system. This was demonstrated in the following ways:
Women with HG showed increased horizontal gain and decreased horizontal phase of the VOR when compared to women without HG in both the first and second trimester of pregnancy,
Approximately one third of women with HG had a high horizontal gain in the low to mid frequency ranges compared with ≤ 10% of women without HG for the first trimester visit, and 50% for the second trimester visit and
Horizontal gain of the VOR increased with the severity of NVP when comparing three groups of women: asymptomatic women, women with ordinary NVP, and women with HG during the first trimester visit.
Postpartum, 25% of women with HG appeared to have high horizontal gain results compared with 19% of unaffected women, which was not statistically significant.
Vestibular abnormalities have been classified into four separate etiological categories: peripheral (the presence of a specific vestibular lesion, e.g. Meniere’s disease); cortical (the triggering of the vestibular system, and hence the vomiting center, through cerebral cortical mechanisms such as anticipatory vomiting or acrophobia); physiological (triggering through the cerebral cortex because of motion sickness or vestibular/sensory mismatch); and central (inflammatory, humoral, or toxic processes affecting the vestibular system). These broad categories have characteristic VOR patterns when tested with the VAT. Horizontal gain and phase abnormalities are generally consistent with a central or cortical etiology, but the pattern of increased horizontal gain and decreased horizontal phase is not linked to a specific pathologic process.
How might the condition of HG be centrally affecting the vestibular system? Potential mechanisms include 1) sympathetic responses to dehydration that may cause vasospasm within the cerebral vasculature15; 2) defective ion channel processes such as those found in migraineurs16; 3) inflammatory substances that may be produced in women with HG17; and 4) estrogen or other humoral or hormonal factors that may be produced or responded to abnormally by the vestibular system18. Estrogen, for example, has been shown to trigger gastric dysrhythmias, which may themselves trigger the medullary vomiting center19. Estrogen has also been linked to cerebral vasospasm in migraine20. Changes in osmolality and vasopressin sensitivity induced by HCG may affect the electrolyte constitution of the endolymph as is seen in Meniere’s disease, resulting in vestibular dysfunction21,22.
It is possible that the changes in the VOR act in a general way to lower the threshold to nausea and vomiting by other pathways. It has been shown, for example, that motion increases the sensitivity to nicotine–induced nausea23. In addition, a history of motion-induced nausea is a well-recognized risk factor, not only for NVP and HG, but also for post operative nausea and chemotherapy induced nausea24.
Our findings of a VOR abnormality associated with HG suggest some potential treatment modalities. Some disorders of the VOR, as identified by the VAT in non-pregnant subjects, have been successfully treated by vestibular rehabilitation25. This process involves deliberately provoking the stimulus that causes nausea several times daily, until the brain accommodates to the signal and the symptoms subside. In non-pregnant adults, many of whom may be as symptomatic as women with HG, improvement is often noted after one week of therapy, with full benefit seen in 4–6 weeks26. An interesting corollary is that subjects who suffer from vestibular-mediated nausea and dizziness who limit activity, or those who are treated with medications that reduce the signal mismatch from the VOR, improve more slowly or not at all27. Since many women report that limiting motion is an important strategy for coping with NVP, there is an opportunity to explore alternative treatment strategies such as vestibular rehabilitation that take into account the vestibular component of NVP.
In 20% – 30% of women in both HG and non-HG groups, tests of both vertical and horizontal gain and phase did not yield interpretable results at Visits 1 and 2, particularly for higher frequencies. Such a drop off in test performance at higher frequencies is not uncommon in ill subjects. At Visit 3, the proportion of tests without interpretable data at higher frequencies was close to the expected range of 5% for otherwise healthy, younger subjects. Based on observations at the time of testing, it seemed that a significant proportion of women could not comply with the instructions for rapid head movement because the testing provoked increased nausea in both women with and without HG.
The finding that 25% of women with HG still had high results at the post partum examination is consistent with the hypothesis that some women have subclinical abnormalities of the VOR that may make them susceptible to NVP and HG. A similar phenomenon has been identified in testing for suitability jet fighter pilot service and some aspects of naval service. Findings on the VAT have been used to screen apparently healthy subjects to identify those who will not be able to tolerate certain stresses on the vestibular system posed by motion, weightlessness and high G-forces28,29. If this is the case, it might be possible to test non-pregnant women for abnormalities in the VAT in order to identify those at risk for HG. Vestibular rehabilitation prior to pregnancy could be a preventive strategy if such a subset of women were identified.
We emphasize that these results are preliminary. First, generalizability of our findings may be limited because of the preponderance of Hispanic women studied. Second, the severity of illness of women with HG does not easily allow for the rigorous testing required for such investigations. In this particular study, each visit required several hours of testing, including both psychological evaluations and some physical tests, such as the VAT and the electrogastrogram, both of which can provoke vomiting. Although vestibular abnormalities were noted, many women could not complete all three visits, and there was insufficient power to study both horizontal and vertical gain and phase for all patients across all three frequency ranges. Further studies can be expected to produce a more complete picture of the VOR abnormalities in these patients.
Finally, we wish to emphasize that we are presenting evidence of an association between VOR abnormalities and the presence of HG. These abnormalities are not known to be a cause or a result of HG. It remains for future studies to identify potential hypotheses regarding the reasons why HG appears to be associated with such abnormalities.
In summary, this is the first demonstration that a subset of women with hyperemesis gravidarum has abnormalities in the VOR pathway. These abnormalities appear consistent with a central physiological mechanism. Further exploration of the role of the vestibular system in both NVP and HG is warranted, and may eventually lead to viable treatment or prevention strategies.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Lorena Gonzalez for her assistance with patient assessment.
This research was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, contract number N01-HD-2-3342.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Some preliminary results from this manuscript were presented orally at the Society of Maternal Fetal Medicine 28th Annual Meeting, Dallas, Texas; February 1, 2008.
Condensation
Compared to unaffected women, women with hyperemesis gravidarum had a higher mean horizontal gain and lower horizontal phase of the vestibulo-ocular reflex.
References
- 1.Jiang HG, Elixhauser A, Nicholas J, Steiner C, Reyes C, Brierman AS. Care of women in U.S. Hospitals, 2000. Rockville, MD: Agency for Healthcare Research and Quality; 2000. HCUP Fact Book No. 3. AHRQ Pub. No. 02-0044. [Google Scholar]
- 2.Goodwin TM. Nausea and vomiting of pregnancy: An obstetric syndrome. Am J Obstet Gynecol. 2002;186(5 Suppl 2):S184–S189. doi: 10.1067/mob.2002.122592. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin TM, Niebyl J, Catz C, Romero R. Understanding and treating nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186(5 Suppl 2):S181. doi: 10.1067/mob.2002.122595. [DOI] [PubMed] [Google Scholar]
- 4.Miller AD. Central mechanisms of vomiting. Digestive Diseases and Sciences. 1999;44:39S–43S. [PubMed] [Google Scholar]
- 5.Andrews PLR. Physiology of nausea and vomiting. British Journal of Anesthesiology. 1992;69:2S–19S. doi: 10.1093/bja/69.supplement_1.2s. [DOI] [PubMed] [Google Scholar]
- 6.Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: Is the vestibular system involved? Am J Obstet Gynecol. 2002;186(5 Suppl 2):S204–S209. doi: 10.1067/mob.2002.122602. [DOI] [PubMed] [Google Scholar]
- 7.Sanger GJ, Andrews PLR. Auton Neurosci. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Heinrichs L. Linking olfaction with nausea and vomiting of pregnancy, hyperemesis gravidarum and migraine headache. Am J Obstet Gynecol. 2002;186(5 Suppl 2):S215–S219. doi: 10.1067/mob.2002.123053. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien B, Naber S. Nausea and vomiting during pregnancy: Effects on the quality of women’s lives. Birth. 1992;19(3):138–143. doi: 10.1111/j.1523-536x.1992.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 10.Dilorio C. First trimester nausea in pregnant teenagers: incidence, characteristics and intervention. Nursing Research. 1985;34(5):372–374. [PubMed] [Google Scholar]
- 11.O’Leary DP, Davis LL, Li S. Predictive monitoring of high-frequency vestibulo-ocular reflex rehabilitation following gentamicin ototoxicity. ACTA Otolaringol (Stockh) 1995 Suppl 520:202–204. doi: 10.3109/00016489509125228. [DOI] [PubMed] [Google Scholar]
- 12.Golding JF. Brain Research Bulletin. 1998;47(5):507–516. doi: 10.1016/s0361-9230(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary DP, Davis LL. High-frequency autorotational testing of the vestibulo-ocular reflex. Neurologic Clinics. 1990;8(2):297–312. [PubMed] [Google Scholar]
- 14.Hirvonen TP, Aalto H, Piikko I, Juhola M. Comparison of two head autorotation tests. J Vestibular Research. 1999;9:119–125. [PubMed] [Google Scholar]
- 15.Kanayama N, Khatum S, Belayet HM, Yamashita M, Yonezawa M, Kobayashi T, Terao T. Vasospasms of cerebral arteries in hyperemesis gravidarum. Gyn Obstet Invest. 1998;46:139–141. doi: 10.1159/000010018. [DOI] [PubMed] [Google Scholar]
- 16.Baloh RW. Neurotology of migraine. Headache. 1997;37:615–621. doi: 10.1046/j.1526-4610.1997.3710615.x. [DOI] [PubMed] [Google Scholar]
- 17.Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Research Bulletin. 1998;47(5):395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 18.Martin VT, Behbehani M. Ovarian hormones and migraine headache: Understanding mechanisms and pathogenesis – Part 1. Headache. 2006;46:3–23. doi: 10.1111/j.1526-4610.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- 19.Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186:S198–S203. doi: 10.1067/mob.2002.122598. [DOI] [PubMed] [Google Scholar]
- 20.Martin VT, Behbehani M. Ovarian hormones and migraine headache: Understanding mechanisms and pathogenesis – Part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 21.Davison JM, Gilmore EA, Durr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol. 1984;15:F105–F109. doi: 10.1152/ajprenal.1984.246.1.F105. [DOI] [PubMed] [Google Scholar]
- 22.Davison JM, Shiells EA, Phillips PR, Lindheimer MD. Serial evaluation of vasopressin release and thirst in human pregnancy. Role of hCG in the osmoregulatory changes of gestation. J Clin Invest. 1988;81:798–806. doi: 10.1172/JCI113386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zingler VC, Denecke K, Jahn K, von Meyer L, Krafczyk S, Krams M, Elfont R, Brandt T, Strupp M, Glasauer S. The effect of nicotine on perceptual, ocular motor, postural, and vegetative functions at rest and in motion. J Neurol. 2007;254(12):1689–1697. doi: 10.1007/s00415-007-0621-9. [DOI] [PubMed] [Google Scholar]
- 24.Baker PD, Morzorati SL, Ellett ML. The pathophysiology of chemotherapy-induced nausea and vomiting. Gastroenterology Nursing. 2005;28(6):469–480. doi: 10.1097/00001610-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Herdman SJ. Vestibular Rehabilitation. 2nd ed. Philadelphia: F.A. Davis; 2000. [Google Scholar]
- 26.Whitney SL, Furman JM. Vestibular Rehabilitation. Chapter 25. In: Jacobson GP, Shepard N, editors. Balance Function Assessment and Management. San Diego, California: Plural Publishing; 2008. pp. 543–583. [Google Scholar]
- 27.Horak FB, Jones-Rycewicz C, Black FO, Shumway-Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngology-Head and Neck Surgery. 1992;106(2):175–180. [PubMed] [Google Scholar]
- 28.Klokker M, Brock-Nannestad L, Mikines P, et al. Vestibular autoratation test in primary selection of military student pilots using neural networks. Aviation, Space and Environmental Medicine. 1999;70:959–961. [PubMed] [Google Scholar]
- 29.Nachum Z, Gordon CR, Shahal B, Spitzer O, Shupak A. Active high-frequency vestibule-ocular reflex and seasickness susceptibility. Laryngoscope. 2002;112(1):179–182. doi: 10.1097/00005537-200201000-00031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.