Abstract
In this study, we investigated the in vitro and in vivo biologic activity of bone morphogenetic protein 2 (BMP-2) released from four sustained delivery vehicles for bone regeneration. BMP-2 was incorporated in 1) a gelatin hydrogel, 2) poly(lactic-co-glycolic acid) (PLGA) microspheres embedded in a gelatin hydrogel, 3) microspheres embedded in a poly(propylene fumarate) (PPF) scaffold and 4) microspheres embedded in a PPF scaffold surrounded by a gelatin hydrogel. A fraction of the incorporated BMP-2 was radiolabeled with 125I to determine its in vitro and in vivo release profiles. The release and bioactivity of BMP-2 were tested weekly over a period of 12 weeks in preosteoblast W20-17 cell line culture and in a rat subcutaneous implantation model. Outcome parameters for in vitro and in vivo bioactivity of the released BMP-2 were alkaline phosphatase (AP) induction and bone formation, respectively. The four implant types showed different in vitro release profiles over the 12-week period, which changed significantly upon implantation. The AP induction by BMP-2 released from gelatin implants showed a loss in bioactivity after 6 weeks in culture, while the BMP-2 released from the other implants continued to show bioactivity over the full 12-week period. Micro-CT and histological analysis of the delivery vehicles after 6 weeks of implantation showed significantly more bone in the microsphere/PPF scaffold composites (implant 3, p < 0.02). After 12 weeks, the amount of newly formed bone in the microsphere/PPF scaffolds remained significantly higher than in the gelatin and microsphere/gelatin hydrogels (p < 0.001), however there was no statistical difference compared to the microsphere/PPF/gelatin composite. Overall, the results from this study show that BMP-2 could be incorporated into various bone tissue engineering composites for sustained release over a prolonged period of time with retention of bioactivity.
Introduction
In orthopaedic surgery, the increasing number of grafting procedures and the disadvantages of current autograft treatments (e.g. limited graft quantity and donor site morbidity) drive the quest for alternative methods to reconstruct large bone defects. In 1965, the observation of ectopic bone regeneration from its own devitalized, demineralized matrix launched the promising strategy of bone tissue regeneration using bioactive proteins.[1] Since then, many growth factors with important roles in skeletal repair have been isolated and the advances in recombinant DNA technology have enabled their large scale production for research and therapeutic purposes. Most trials have focused on members of the bone morphogenetic protein (BMP) family, due to their osteogenic capacity.[2–6] So far, these studies have resulted in two commercially available BMP-based products (BMP-2 and BMP-7) for specific orthopedic indications.[7–10]
Although BMPs can be potent osteoinductive growth factors, their administration during orthopedic applications is complicated by their short biological half-lives, localized actions and rapid local clearance.[11] To overcome these problems, effective BMP treatments of bone defects require their incorporation into a biomaterial for its local sustained delivery at the target site. The delivery vehicle should maintain local BMP concentration within the therapeutic window for a sufficient period of time to allow osteoprogenitor cells to migrate to the target site and differentiate into osteoblasts.[5, 12] Furthermore, the biomaterial should act as a biologically and biomechanically compatible framework that enhances cell migration, bone formation and mechanical stability. Although current clinical BMP delivery vehicles show promising results, optimization of the biomaterial design and site-specific pharmacological actions remain challenging. Animal studies demonstrated that the collagen powder or sponges currently used have a large initial burst release and a retention of less than 5% after 14 days of implantation.[13, 14]. In contrast to this rapid release, during normal bone repair the in situ expression profiles of BMPs known to be ectopically osteoinductive (BMP-2, BMP-4, BMP-6 and BMP-7) show an up-regulation for a longer period of time that peaks at/after 21 days.[15–17] Therefore, it is postulated that the site-specific pharmacological actions of BMPs could be improved by a more prolonged growth factor release profile that coincides with the normal rate of BMP expression and bone formation.
Sustained growth factor delivery can be established through different non-covalent retention mechanisms including physical entrapment, absorption and complexation.[18] Based upon these mechanisms, the current study employs three different polymers to design biomaterials for the sustained delivery of BMP-2. The natural polymer gelatin is a type of denatured collagen, which can be used to create hydrogels for the absorption and complexation of BMP.[19] The synthetic polymer poly(lactic-co-glycolic acid) (PLGA) is a well-characterized biodegradable material which can be used for the fabrication of microspheres for BMP encapsulation and complexation.[20–25] Poly(propylene fumarate) (PPF) is a crosslinkable linear polyester with suitable mechanical properties for bone replacement that can be used to encapsulate PLGA microspheres in order to extend their release.[26, 27]. By combining these three polymers into composite biomaterials, the release of BMP-2 could be extended for a prolonged period of time in an ectopic implantation model.[28]
Although the osteoinductive capacity of BMP-2 loaded carriers composed of these materials has been tested in vivo, little is known about the effect of prolonged growth factor retention on the stability of the protein. Therefore, the aim of this study was to assess both the in vitro and in vivo stability of released BMP-2 from the sustained delivery vehicles over time. Since the pharmacological effects of the growth factor closely depend on its structural integrity, the in vitro and in vivo biological effects of BMP-2 were used as indicator for protein stability.
Materials and Methods
Experimental design
The selection of gelatin, poly(lactic-co-glycolic acid) and poly(propylene fumarate) for the fabrication of BMP-2 delivery vehicles was based on previous work demonstrating their capability of binding BMP-2 or extending protein release.[19–22, 24–27, 29] The materials were combined into composite formulations in order to further extend BMP-2 release over a prolonged period of time. Four different implants were tested consisting of 1) BMP-2 absorbed in a gelatin hydrogel and BMP-2 loaded microspheres embedded in a 2) gelatin hydrogel, 3) PPF scaffold or 4) PPF scaffold surrounded by a gelatin hydrogel. Implants loaded with the buffer solution were used as controls. The in vitro and in vivo release profiles from the composites were determined by radiolabeling a fraction of the incorporated growth factor with 125I and correlating the measured radioactive signal to the amount of BMP-2. The in vitro biologic activity of the released BMP-2 was investigated over a period of 12 weeks in consecutive 7 day cell cultures. The in vivo BMP-2 bioactivity was determined by testing its bone forming capacity after 6 and 12 weeks of subcutaneous implantation in rats. A subcutaneous implantation site was chosen to rule out osteoconduction or periosteal bone formation as disturbing mechanisms in the BMP-2 induced bone formation.
BMP-2 radioiodination
To determine the in vitro and in vivo release profiles, a fraction of the incorporated BMP-2 (Medtronic Sofamor Danek, MN) was radiolabeled with 125I using Iodo-Gen® precoated test tubes (Pierce, Rockford, IL) according to the manufacturer’s instructions. Briefly, 100 µl of a 1.43 mg/ml BMP-2 solution, 20 µl of a 0.1 M NaOH solution and 2 mCi Na125I were added to a 1,3,4,6-tetrachloro-3α, 6α-diphenyl glycouril-coated glass test tube. The mixture was incubated for 15 min. at room temperature with gentle shaking. To separate radiolabeled protein from free radioactive iodine, the solution was dialyzed (10 kDa molecular weight cutoff (MWCO) Slide-A-Lyzer®, Pierce) for 24 hrs with 3 times media change against an aqueous BMP-2 buffer (pH 4.5) containing 5 mM glutamic acid, 2.5 wt% glycine, 0.5 wt% sucrose and 0.01 wt% Tween 80 (all from Sigma-Aldrich, St. Louis, MO). The dialysis fractions of 3 radioiodination procedures were pooled and concentrated to a final concentration of 2.6 µg/µl with a Vivaspin device (10 kDa MWCO, Sartorius AG, Germany). Trichloroacetic acid (TCA) precipitation of the final 125I-BMP-2 solution indicated 99% precipitable counts. The 125I-BMP-2 was mixed with non-labeled BMP-2 to obtain a solution with a concentration of 9.0 mg/ml and a hot:cold ratio of 1:8.
Microsphere fabrication
Poly(lactic-co-glycolic acid) (PLGA; Medisorb®, Lakeshore Biomaterials, AL) with a lactic to glycolic acid ratio of 50:50, a weight-average molecular weight (Mw) of 23,000 Dalton, and an acid number of 0.34 was used for the microsphere preparation. The microspheres were fabricated using a previously described water-in-oil-in water (W1-O-W2) double-emulsion-solvent-extraction technique.[22] The fabrication process was performed under semi-sterile conditions and all liquids were sterilized using a 0.22 µm filter. Briefly, 118 µl of the 9.0 mg/ml 125I-BMP-2/BMP-2 solution was emulsified in a solution of 500 mg of PLGA in 1.25 ml of dichloromethane using a vortex at 3050 rpm. The mixture was re-emulsified for 30 sec. in 2 ml of 1% w/v aqueous poly(vinyl alcohol) (PVA, 87–89% mole hydrolyzed, Mw=13,000–23,000, Sigma-Aldrich) solution to create the double emulsion. The content was then added to 100 ml of a 0.3 % w/v aqueous PVA solution and 100 ml of a 2% w/v aqueous isopropanol solution with stirring for 1 hr. The extraction of the dichloromethane to the external alcoholic phase resulted in precipitation of the dissolved polymers and subsequently the formation of microspheres. The microspheres were collected by centrifugation, washed twice with distilled deionized water (ddH2O), and finally vacuum-dried to a free flowing powder. Microspheres loaded with the BMP-2 buffer were used as negative controls.
Fabrication of microsphere/PPF composites
PPF with a number-average molecular weight (Mn) of 5,800 and a polydispersity index of 2.0 was synthesized via a two-step reaction process as previously described.[30] Diethyl fumarate and excess amount of 1,2-propylene glycol were polymerized together with hydroquinone (cross-linking inhibitor) and zinc chloride (catalyst) first at 100 °C for 1 h and then at 150 °C for 7 h to obtain the intermediate dimer. The intermediate dimer was polymerized to PPF via condensation under vacuum at 130 °C for another 4 h. The microsphere/PPF composites (Implant 3) were fabricated by photocrosslinking PPF with N-vinylpyrrolidinone (NVP, Acros, Pittsburgh, PA) using bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO, Ciba Specialty Chemicals, Tarrytown, NY).[27, 31] Briefly, 1.0 g of PPF and 50 µl of a 100 mg/ml BAPO/dichloromethane solution were dissolved in 0.5 ml of NVP. The PPF/NVP/BAPO paste (45 wt%) was mixed with PLGA microspheres (55 wt%) and mixed using a spatula. The mixture was transferred to a 3.0 ml syringe, forced into a glass cylindrical mold of 1.5 mm in diameter and allowed to polymerize at room temperature under UV light for 30 min. The crosslinked cylinders were removed from the glass mold, lyophilized, sectioned into 6 mm long rods, sterilized by ethanol evaporation and frozen at −20 °C until use.
Fabrication of gelatin hydrogels and composites
Gelatin (type A, 300 bloom, derived from acid-cured tissue, Sigma-Aldrich) hydrogels were fabricated as described previously.[32] The hydrogels were prepared under sterile conditions and all solution were sterilized using a 0.22 µm filter. Briefly, 1 ml of a 40°C pre-heated 10 wt% aqueous solution of gelatin was mixed with 40 µl of a 10 wt% aqueous glutaraldehyde solution for 15 seconds using a vortex at 3050 rpm. The resulting solution was cast into cylindrical glass molds with a length of 8 mm and a diameter of 3.5 mm. The gelatin was allowed to crosslink for 6 hrs at 4 °C and crosslinked gelatin hydrogels were placed in a 100 mM aqueous glycine solution for 1 hr to block the residual aldehyde groups of glutaraldehyde. Finally, the hydrogels were washed with ddH2O, frozen at −20 °C, removed from the glass mold and stored at −20 °C until use. One day before implantation, the gelatin hydrogels were loaded with 5 µL of a diluted 125I-BMP-2/BMP-2 solution. PLGA microspheres were embedded into the gelatin hydrogel by combining 72 mg microspheres per ml gelatin solution prior to the crosslinking (Implant 2). Microsphere/PPF composites were embedded in a gelatin hydrogel by placing 1 composite scaffold per mold before hydrogel crosslinking (Implant 4).
In vitro BMP-2 release and bioactivity
The in vitro biologic activity of released BMP-2 was investigated over a period of 12 weeks by determining its ability to induce an increase in alkaline phosphatase activity in a cell culture model. W20-17 cells (kindly donated by Wyeth, Madison, NJ) were cultured in the presence of BMP-2 containing microspheres or composites. The W20 clone 17 cell line is a mouse bone marrow stromal line known to respond to BMP-2 with an increase in AP activity.[33] Over the 12-week period, the composites were transferred weekly to new cell cultures in order to determine the alkaline phosphatase response over consecutive 7 day periods.
Before the start of the experiment, the W20-17 cells had been expanded, pooled to create a homogeneous mixture, and cryopreserved in multiple aliquots. The cells were cultured in Dulbecco’s Modifies Eagle’s Medium (DMEM) containing 4.5% glucose, 10% fetal bovine serum and penicillin/streptomycin. For each consecutive time point, an aliquot was thawed, expanded for 3 days and re-plated in 24-well plates at 20,000 cells/cm2 (passage 26 upon use). One day post-plating, the medium was changed and cells were exposed for a period of 3.5 or 7 days to transwells containing the BMP-2-loaded microspheres or composites. Additional cultures containing empty transwells (negative control) were used to determine the basal AP activity of W20-17 cells. To test the dose responsiveness of the stimulated alkaline phosphatase activity, cell cultures were treated with culture media containing 0.0, 0.01, 0.1, 1.0 and 5.0 µg BMP-2/ml (positive controls).
For each time point, the transwells were transferred to a new cell culture and the exposed cultures were used to determine the alkaline phosphatase activity, total protein content of the cells, and the BMP-2 release in the medium. For the first two time points, the transwells were already transferred to new cells cultures after 3.5 days. For the rest of the time points, the medium was changed after 3.5 days and the cell culture replacements were done weekly. AP activity was measured using an AP assay kit (Sigma Diagnostics, St. Louis, MO) which immediately lysed the cells. The AP activities were normalized to protein contents of the cell lysate as measured by the bicinchoninic acid assay, (BCA Protein Assay Kit, Pierce). To determine the BMP-2 release over the 12 week period, all media changes and cell lysates were assayed for 125I counts on a gamma counter. After 84 days, the implants were collected to determine the remaining activity. All 125I counts were corrected for decay and normalized to the starting amount. The in vitro release profiles and BMP-2 culture concentrations were determined by correlating the gamma-irradiation in counts/minute to the amount of incorporated growth factor in microspheres or composites.
Animals and surgical procedure
Twenty male 12-week-old Harlan Sprague Dawley rats (weight 310–335 g) were used for the experiment, according to an approved protocol by local animal care and use committee. Prior to surgery, the rats received an antibiotic prophylaxis of Tribrissen (sulfonamide, 40 mg/kg, SC) and were anesthetized with ketamine/xylazine (45/10 mg/kg, IM). After shaving and disinfecting, small skin incisions were made in the proximal part of each limb and in the lumbar area. At each site, a subcutaneous pocket was created and filled with one implant according to a randomized scheme. The four limb pockets were filled with 125I-BMP-2 containing implants and the two lumbar pockets with negative controls (implants containing buffer solution). The rats received the fluorochrome markers calcein green (10mg/kg, paravenously) and tetracycline (10mg/kg, paravenously) at 3 and 9 weeks postoperatively, respectively. Ten animals were sacrificed after 6 and 12 weeks to evaluate bone formation using micro-computed tomography (µCT) and histology.
In vivo release measurements
The in vivo BMP-2 release from the different carriers was measured by using four scintillation probes connected to digital scalers as previously described.[28] At different time points, the rats were sedated with ketamine/xylazine (15/3 mg/kg, IM) for measurements of the remaining radioactivity at the implant site over three 1-min. periods with 2 different detectors. The 125I counts were corrected for decay and normalized to the post-operative measurement at day 0.
µCT and histological analysis
After 6 and 12 weeks postoperatively, 10 rats were euthanized and the implants were excised and fixed in a 1.5% phosphate buffered glutaraldehyde solution. The implants were scanned using a µCT-system (custom built from commercially available components and described in detail previously) at 0.49° angular increments, which provided 721 views around 360°.[34] Images were recorded, digitized, and transferred to a controlling computer for reconstruction using a modified Feldkamp cone beam tomographic reconstruction algorithm. The 3-D images consisted of 20 µm cubic voxels and the radiopacity of each voxel was represented by a 16-bit grayscale value. Image analysis was performed using the Analyze software package. (Biomedical Imaging Resource, Mayo Clinic, Rochester, Mn) The volume of the subcutaneously formed bone was quantified in µCT reconstructions of the calcified tissue. The threshold for the 3D-reconstructions of the calcified tissue was determined prior to the volume quantification. All reconstructions and volume quantifications were obtained using the same standardized threshold.
After µCT analysis, the implants were dehydrated in a graded series of alcohol and embedded in methylmethacrylate for histological analysis. Sections were stained with hematoxilin/eosin, methylene blue and trichrome Goldner for evaluation of the general tissue response and bone formation. Unstained sections were used for fluorescence microscopy.
Statistical analysis
All data are given as means ± standard deviations (SD) for n = 4 (in vitro experiments) and n = 10 (in vivo experiments). Single factor analysis of variance (ANOVA) with Bonferroni’s post hoc tests for multiple comparisons was used to identify differences in mean BMP-2 implant loading and BMP-2 release at each time point. The BMP-2-induced AP activity per time-point was statistically compared to the negative and positive controls using single factor ANOVA with Dunnett’s post hoc tests and additional Bonferroni’s correction for the number of tests. The general linear model with Bonferroni-corrected post hoc tests (for composite effect) and unpaired t-tests (for time effect) were used to assess the statistical significance of the subcutaneous bone volumes. The data analyses were performed with SPSS software (version 13.0, SPSS Inc., Chicago, IL) and differences were considered significant at p<0.05.
Results
BMP-2 labeling and incorporation
The radiolabeling procedure of the protein resulted in an activity of 3.8 µCi/µg BMP-2. The addition of 125I-BMP-2 to a preosteoblast W20-17 cell line culture indicated the growth factor remained bioactive after the iodination reaction (data not shown). Based on the 125I counts before and after the microsphere fabrication procedure, the entrapment efficiency of BMP-2 was 84% or 1.4 µg per mg microspheres. The BMP-2 content of the different composite formulations is summarized in Table 1. Due to the manufacturing process, the composites consisting of PLGA microspheres in a gelatin hydrogel had a significantly lower BMP-2 loading compared to the other scaffolds.
Table 1.
Experimental groups and implant characteristics.
| Name | Implant composition | Initial activity (µCi) | BMP-2/implant (µg) |
|---|---|---|---|
| Gelatin | Gelatin hydrogel | 6.0 ± 0.5 | 9.8 ± 0.8 |
| Mps/Gelatin | Mps in a gelatin hydrogel | 3.7 ± 0.6* | 6.0 ± 1.0* |
| Mps/PPF | Mps in a solid PPF cylinder | 5.6 ± 0.5 | 9.1 ± 0.8 |
| Mps/PPF/Gelatin | Mps in a PPF cylinder surrounded by a gelatin hydrogel | 5.8 ± 0.4 | 9.5 ± 0.6 |
Mps = poly(lactic-co-glycolic acid) microparticles loaded with BMP-2
significantly lower loading relative to other implants.
In vitro BMP-2 release and bioactivity
The in vitro BMP-2 retention profiles of the PLGA microspheres and the 4 different implants were studied in a cell culture model that allowed simultaneous release and bioactivity measurements. During the first week, water absorption and implant swelling were seen in all composites. The microspheres showed a typical initial burst release which was followed by a sustained S-shaped release profile for the rest of the in vitro study (Figure 1). The released BMP-2 retained its bioactivity during the course of the study as indicated by the increased AP response over basal levels of the cells (Figure 1). The AP increase induced by the BMP-2 released from the microspheres was equal (3/11 time-points) or significantly higher (8/11 timepoints, p≤0.001) compared to comparable BMP-2 concentrations of the dose-response curve that had been directly added to the culture medium of the cells.
Figure 1.
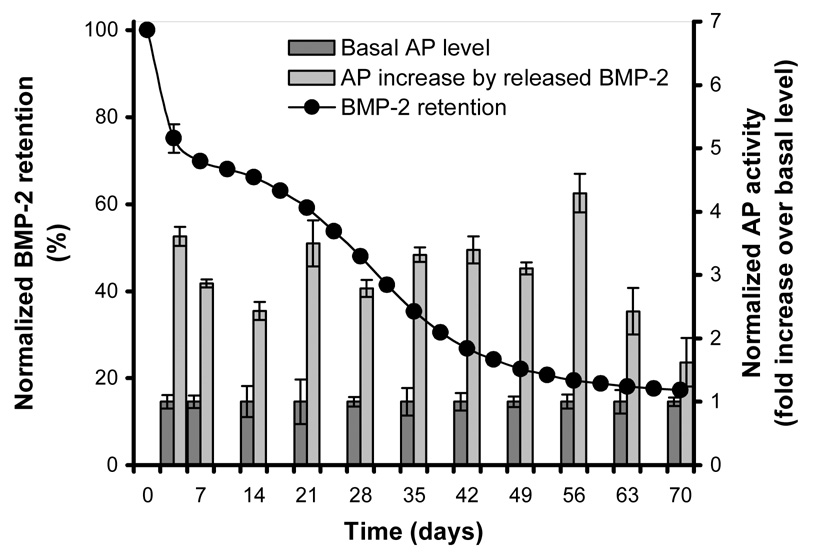
125I-BMP-2 release kinetics from PLGA microspheres and biologic activity of the released BMP-2 obtained from the same cell culture model. The left axis and open marks (○) show the normalized retention profile in the microspheres at 37 °C in culture media. The right axis and bars show the alkaline phosphatase activity of consecutive 3 or 7 day W20-17 cell cultures in the presence of BMP-2 contraining microspheres or empty transwells (control culture). The alkaline phosphatase activity at each time point was normalized to the control cultures without BMP-2.
The gelatin hydrogel-only implants showed an initial burst release of the incorporated BMP-2 of 33 (± 8) % during the first 3.5 days, which leveled off over 2 weeks to assume an almost linear sustained release (R2=0.983) for the rest of the time frame (Figure 2). The release profile of the other 3 composites showed an initial burst between 4.9 (± 0.3) % and 6.8 (± 0.2) %, which was followed by an S-shaped release profile for the microsphere/gelatin and microsphere/PPF/gelatin scaffolds. The microsphere/PPF scaffolds exhibited a linear release for the first 56 days (R2=0.998) which leveled off towards the end of the experiment (Figure 2A).
Figure 2.
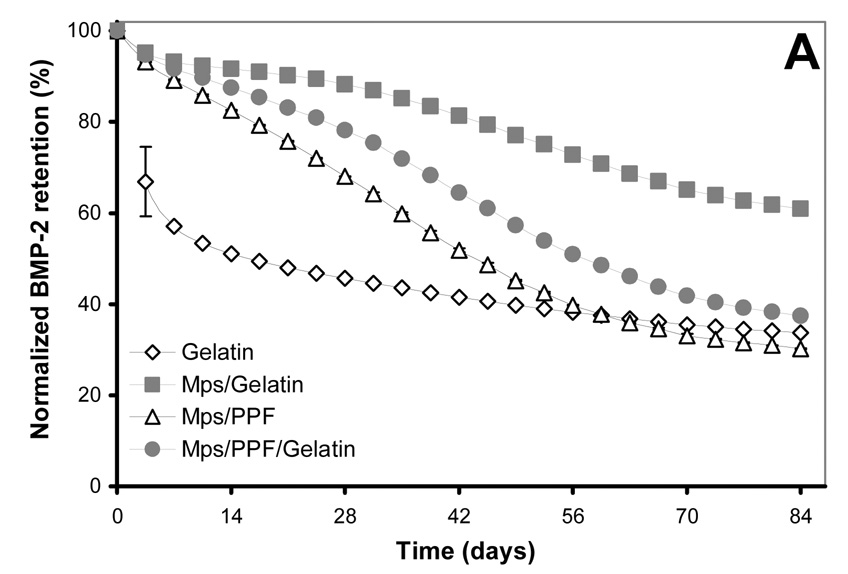
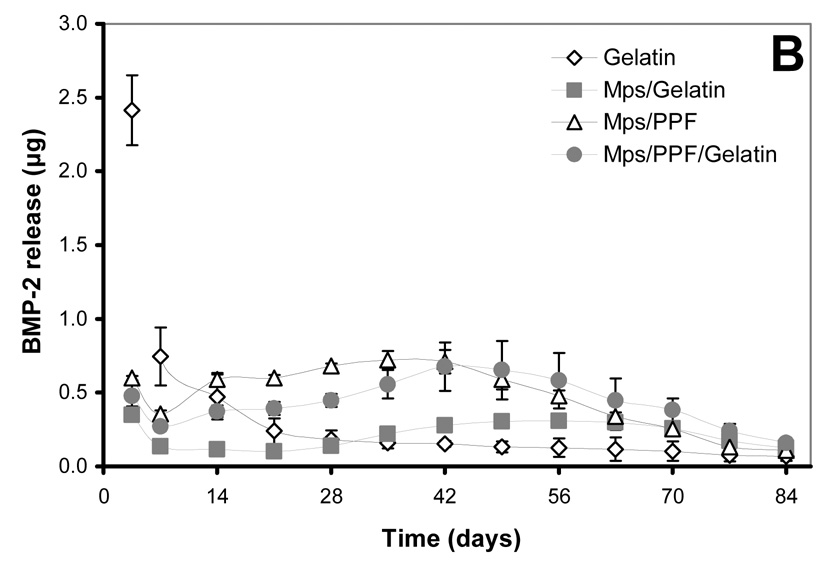
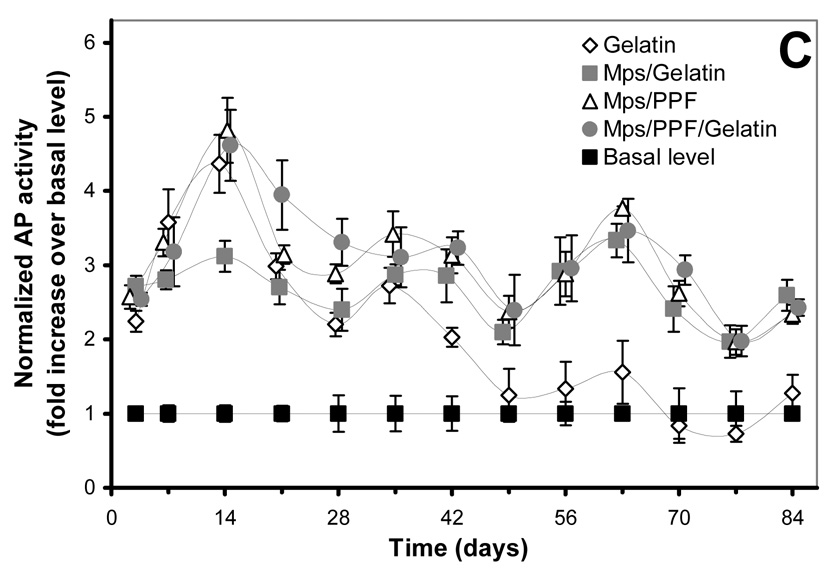
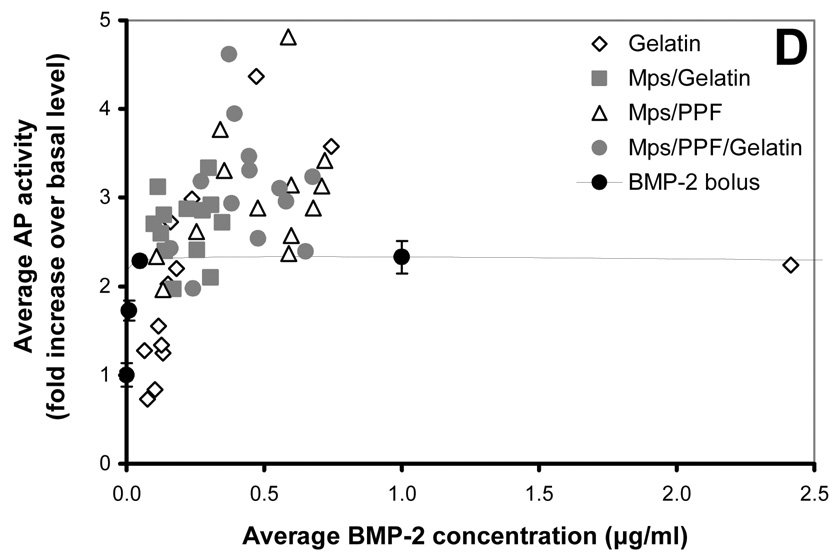
125I-BMP-2 release kinetics from the implants and its bioactivity on consecutive W20-17 cell cultures. The in vitro 125I-BMP-2 release profiles are expressed as (A) normalized release (% of the initial loading) and (B) amount of released protein (in µg) from the 4 different implants at 37 °C in the culture media. (C) AP activity of consecutive new W20-17 cell cultures exposed to empty transwells (basal level) or transwells containing the BMP-2 implants. The alkaline phosphatase activity was normalized to the cell protein contents and expressed as an increase over the basal levels for each time point. (D) Relationship between the BMP-2 concentration in the culture medium (obtained from B) and the normalized alkaline phosphatase activity (obtained from B). The line (●) shows the dose-response curve of cell cultures treated with media containing 0.0, 0.01, 0.1, 1.0 and 5.0 µg BMP-2/ml.
The bioactivity of BMP-2 released from the microspheres and composites was studied by determining the ability to induce an AP activity increase over basal W20-17 cell levels (negative controls) in consecutive 7-day cell cultures, and by comparing the cell response to a BMP-2 dose response curve directly added to the culture medium (positive control). The AP activity response and BMP-2 culture concentrations were analysed in several ways to distinguish between time-and dose-related effects (Figure 2B). Overall, the BMP-2 released from all implants was able to induce a significantly increased AP activity over basal levels during the first 42 days (p<0.001, Figure 2B). After 42 days, the alkaline phosphatase activity of cultures containing the BMP-2 releasing gelatin hydrogels did not show significantly increased AP levels above basal levels, whereas the cultures containing the microspheres or other implants continued to show significantly higher alkaline phosphatase activities for the rest of time (p<0.001). Although no AP induction over basal levels was seen after 42 days for the BMP-2 released from the gelatin hydrogels, comparable concentrations of BMP-2 containing medium in the dose response curve were still able to significantly increase the AP activity (Figure 2C). The increase of AP activity for the rest of the implants was equal or significantly higher compared to comparable BMP-2 concentrations of the dose-response curve that had been directly added to the culture medium of the cells (p<0.05)
Animals
One rat died during the release measurements as a result of an overdose of anesthetics. Another rat removed the Mps/PPF/gelatin implant from its subcutaneous pocket of the hind limb at the end of the first week. After the paravenous administration of tetracycline at the tail base, some of the rats developed a small necrotic area of the skin at the injection site. The rest of the animals showed no complications in wound healing and remained healthy during the 12 week follow up.
In vivo BMP-2 release
Compared to the in vitro profiles, the 125I-BMP-2 release of the implants was significantly different upon implantation (Figure 3). The gelatin scaffolds showed a prolonged and enlarged burst release of 92.2 (± 4.4) % within the first 14 days, which was followed by an almost linear release for the remaining amount of growth factor. The other three scaffolds also exhibited a much faster BMP-2 release upon implantation. They showed no significant burst release during the first 3 days and an S-shaped sustained release profile spanning the entire period of the 84 days.
Figure 3.
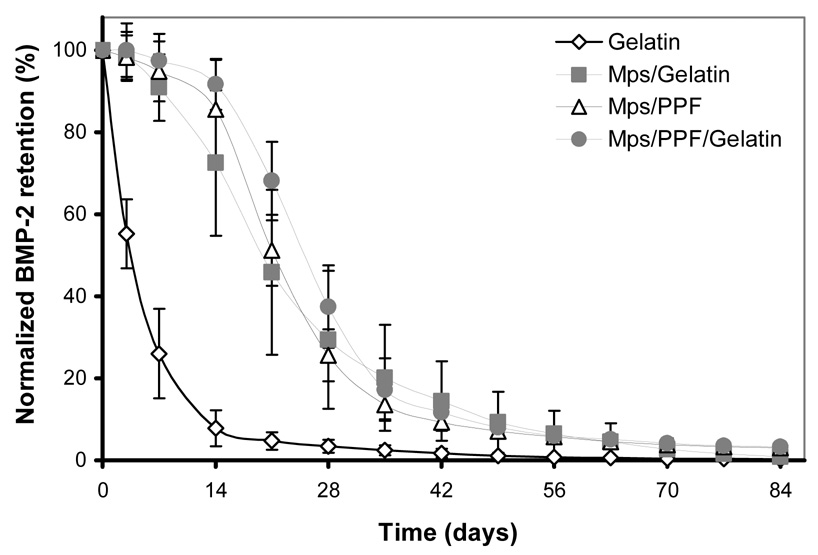
Normalized in vivo release profile of 125I-BMP-2 from the 4 different implants in a rat subcutaneous implantation model. Adapted from reference [28].
µCT analysis
After 6 and 12 weeks of implantation, all implants were easily identified and retrieved for analysis by µCT and histology. The µCT reconstructions showed calcifications in all BMP-2-loaded implants which varied in size from 5.5×10−4 to 24 mm3. The bone was formed as a solid shell on part of the surface and as a trabecular structure inside the cylindrical implants (Figure 4). No bone formation was observed in the control composites containing microspheres without BMP-2.
Figure 4.
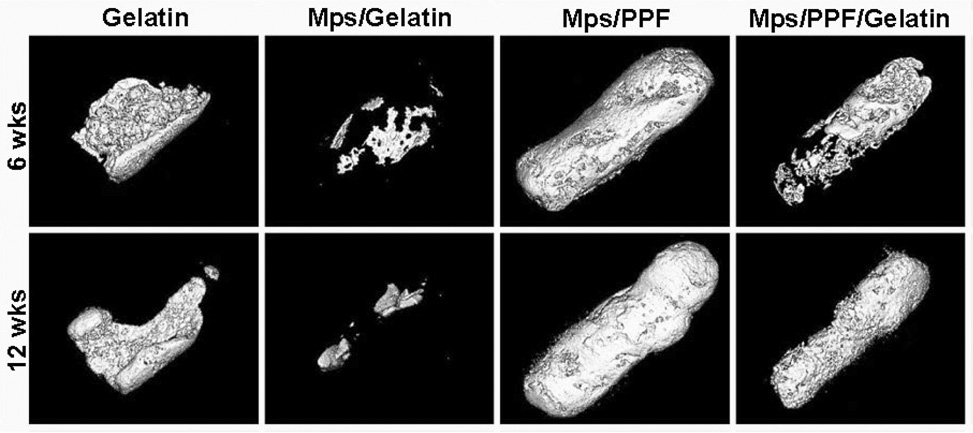
New bone formation after 6 and 12 weeks of implantation in a rat subcutaneous model. Image reconstruction was derived from µCT scans using standardized thresholds.
Quantification of the bone volume after 6 weeks of implantation showed significantly more bone in the Mps/PPF implants compared to all other implants (p≤0.012, Figure 5). The gelatin and Mps/PPF/gelatin scaffolds also contained significantly more bone compared to the Mps/gelatin composites (p≤0.036). At 12 weeks, the Mps/PPF scaffolds still contained more bone than the gelatin and Mps/gelatin scaffolds implants (p<0.001), however no statistical difference was found between the Mps/PPF and Mps/PPF/gelatin scaffolds. The bone formation in the Mps/PPF/gelatin composites remained significantly higher than the Mps/gelatin scaffolds implants (p<0.001). Only the Mps/PPF scaffold showed a significant increase in bone formation over time (p<0.001).
Figure 5.
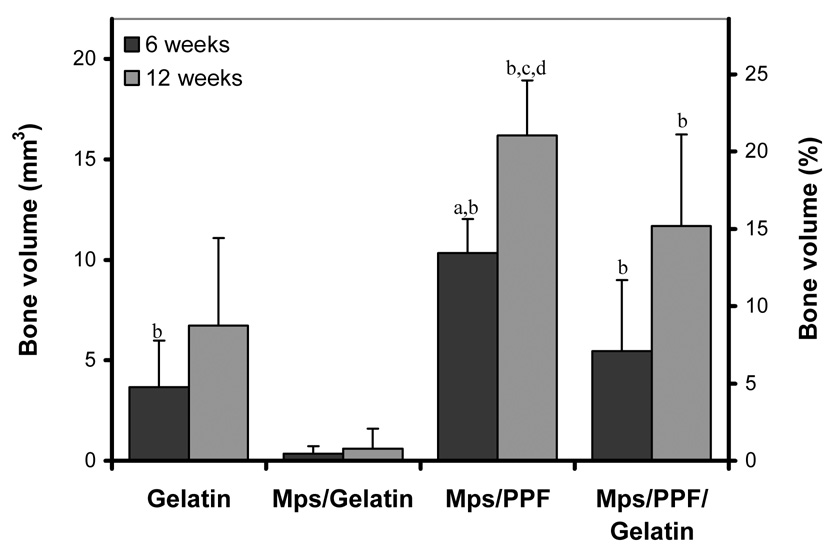
Average volumes of newly formed bone in the 4 different scaffolds after 6 and 12 weeks of subcutaneous implantation in rats (left axis), and normalized to an 8 mm long, 3.5 mm diameter cylindrical scaffold (right axis). Significant difference relative to (a) all other implants, (b) Mps/gelatin implant and (c) gelatin implant at the same time-point. (d) Significantly increase in bone formation relative to the previous time-point.
Histology
Histological evaluation confirmed the subcutaneous bone formation that was seen in the µCT scans (Figure 6). The hydrogel-containing implants showed resorption of gelatin at the surface with poor cell infiltration into the intact gelatin network (Figure 6A, 6B & 6D). As a result, the bone was mainly formed as a shell around the resorbed hydrogel. The microsphere/PPF cylinders showed a more homogeneous degradation that resulted in an interconnected porous network (Figure 6C & 6D). This network was filled with osteoid, bone and well vascularized connective tissue. Overall, the newly formed bone was found along the degrading implants and had a woven or trabecular appearance with osteoid depositions at its surface (Figure 6E). Fluorochrome analysis indicated that bone mineralization was present as early as 3 weeks after implantation (Figure 6F). The calcein green injected at 3 weeks was visible in 1/19 Mps/gelatin implants, 11/18 Mps/PPF/gelatin implants, 16/19 gelatin implants and 19/19 Mps/PPF implants. The label was mainly seen as small circles along the implant surface indicating that ossification started simultaneously at multiple sites. The 9-week label tetracycline could not be found in any of the implants. Given the µCT-measured increase in bone formation between 6 and 12 weeks and the superficial necrotic skin reactions to tetracycline injection, it was suspected that the absence of label was due to unsuccessful systemic administration.
Figure 6.
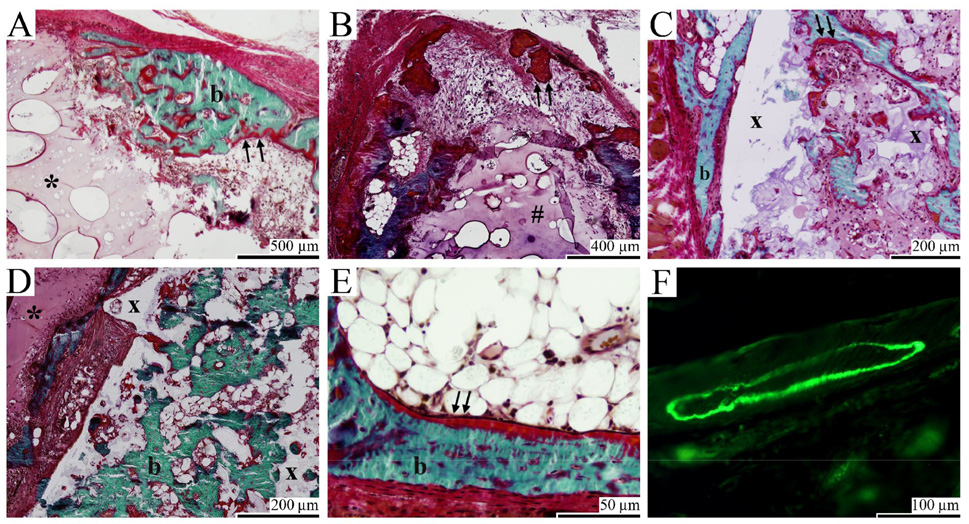
Goldner stained and unstained histological sections of gelatin (A & E), Mps/gelatin (B), Mps/PPF (C & F), Mps/PPF/gelatin (D) implants after 6 (A, C, E & F) and 12 (B & D) weeks of subcutaneous implantation in rats. (A, B & D) Surface resorption and minimal cell infiltration were seen in the gelatin and microsphere/gelatin hydrogels. (A, B, C, D & E) Bone formation (b) and osteoid deposition (arrows) were seen on the surface and inside the gelatin (*), microsphere/gelatin (#) and microsphere/PPF (x) scaffold components. (C & D) Degradation of the Mps/PPF scaffold resulted in an interconnected porous network for tissue ingrowth and bone formation inside the solid Mps/PPF cylinders. (F) Fluorochrome analysis showed calceine green deposition in newly formed bone.
Discussion
This study clearly shows that biologically active BMP-2 can be released over a prolonged period of time from sustained delivery vehicles. The delivery vehicles, based on the three different polymers gelatin, PLGA, and PPF, exhibited an in vitro release of biologically active BMP-2 for at least 42 to 84 days. Compared to the in vitro retention profiles, the delivery vehicles exhibited a significantly faster in vivo BMP-2 release. Although all scaffolds showed bone induction after 42 and 84 days of subcutaneous implantation, the amount of newly formed bone varied significantly between the delivery vehicles. The carriers based on the gelatin hydrogel showed less bone formation compared to those based on PPF.
Until recently, few studies have focused on the prolonged retention of BMP-2 in delivery vehicles and its subsequent effect on the biological activity of the released protein. So far, studies performing separate pharmacokinetic and bioactivity assays of BMP-2 showed bioactivity retention of the released or retained protein up to 48 days.[29, 35] In this study, both pharmacokinetic and bioactivity assays have been combined in one experimental model to determine the dose- and time-related effects on the bioactivity. However, the W20-17 cells treated with various concentrations of BMP-2 directly added to the culture medium already reached a maximum AP response at 50 ng/ml in our experimental model, in contrast to the AP increase found previously for these cells at concentrations up to 800 ng/ml.[33] As a result, any difference in bioactivity for the released protein at concentrations over 50 ng/ml was impossible to detect. The percentage of bioactive BMP-2 in the total amount of released protein could only be reliably determined under this threshold. For values above this threshold, the bioactivity per amount of released protein could not be expressed as a percentage of the released protein.
Consecutive cell cultures in the presence of various composites showed that protein incorporation in the polymeric delivery vehicles resulted in the release of bioactive BMP-2 for at least 42, >70 and >84 days from the gelatin, PLGA microspheres only, and other implants, respectively. Since the biological activity of the protein closely depends on its structural integrity, its pharmacological effect is an indicator for the stability of the released protein. The decreased bioactivity of the gelatin hydrogels at later time points compared to the other vehicles is likely due to the difference in material properties and may indicate that gelatin is less effective in stabilizing BMP-2 in a physiologic environment at 37°C compared to materials containing PLGA microspheres.
Surprisingly, the bioactivity of the BMP-2 released by composites frequently was higher than similar concentrations of BMP-2 added directly to the control cultures. During the initial characterization of W20-17 cells, loss of growth factor bioactivity was seen at low dosages when fresh cultures were treated with BMP-2 containing media that was previously added to W20-17 cells for 24 hrs.[33] These findings could indicate that BMP-2 is susceptible to degradation or inactivation in the culture medium by molecules such as serum proteins. Therefore, the higher AP activities in the cultures exposed to the sustained delivery vehicles could be the result of the different BMP-2 administration mechanisms. The BMP-2 dose in the control cultures was immediately exposed to the culture conditions from the start of the experiment, whereas the implants had gradually released the same dose over the full 7 day culture period.
In terms of protein release, all in vitro profiles underestimated the in vivo release rate of the sustained delivery vehicles. These findings are similar to previous studies involving BMP-2 release studies with different solutions (phosphate buffered saline, fetal calf serum and/or DMEM).[11, 36] One difference between the two environments with a profound effect on the release of the entrapped and bound protein is the mechanism of implant degradation. Apart from in vitro and in vivo hydrolysis of the polymer matrix in an aqueous environment, cellular enzymatic actions accelerate implant degradation upon implantation. Furthermore, proteins such as those found in serum enhance the desorption of bound growth factors.[11, 37, 38] The accelerated degradation and faster protein desorption in the more protein-rich in vivo environment may have contributed to the underestimation of the in vitro release profiles.
The role of the sustained delivery vehicle materials during in vivo bone formation is diverse. In addition to maintaining local BMP concentrations at sufficient levels over time, implants should also provide a mechanically stable and biodegradable framework that enhances cell migration, attachment and differentiation. Although it is difficult to distinguish between release-related effects and scaffold-related effects, this study shows remarkable differences in bone formation in the various composites. Histology and µCT showed that the addition of gelatin to the scaffold construct had a negative effect on cell infiltration and efficacy of bone formation. This indicates that the biodegradable, non-porous structure of the gelatin hydrogel is a less favorable substrate for cell infiltration and bone formation compared to the biodegradable, non-porous PLGA-PPF constructs.
Another remarkable difference in bone formation was seen between the gelatin and the microsphere-gelatin hydrogels. Although part of the difference in bone formation is caused by the significant lower dose of the microsphere-gelatin hydrogels, this difference in dosage does not fully explain the decreased bone formation. Previous BMP-2 dose-response studies in a similar dose range in collagen or PLGA carriers showed a decrease of approximately 50% or less of the calcium content at a 0.2 fold lower dose in a similar range.[39, 40] In this study, a smaller difference in BMP-2 dosage (0.6 fold) resulted in a larger decrease (90%) in bone formation. Most likely, the prolongation of the retention of BMP-2 in the microsphere-gelatin hydrogel is partially responsible for the decreased osteoinductive capacity. This could indicate that BMP-2 bound to the 90% aqueous gelatin hydrogel is more susceptible to spontaneous and/or enzymatic degradation before its release compared to the protein entrapped in the dense PLGA/PPF polymer network.
In conclusion, this study demonstrates that BMP-2 can be incorporated into polymeric composites for its sustained release over a prolonged period of time. The entrapment, absorption and complexation of BMP-2 into the composites resulted in the release of biologically active protein for at least 42 days in vitro. However, the composite release profiles were significantly different upon in vivo implantation. The ectopic osteoinductive capacity of the released BMP-2 varied among the different composites. Overall, the incorporation of BMP-2 loaded PLGA microspheres in the hydrophobic, solid PPF matrix enhanced bone formation, and therefore may find use as carriers for prolonged release of bioactive proteins for bone tissue engineering.
Acknowledgements
The authors gratefully acknowledge the National Institutes of Health (R01 AR45871 and R01 EB03060) and The Netherlands Organization for Health Research and The Netherlands Organization for Health Research and Development ZonMW (Agiko 920-03-325) for financial support. The authors thank Dr. Andras Heijink, Dr. Shanfeng Wang, Mr. James Greutzmacher, Mr. James Herrick, and Mrs. Julie Burgess from the Tissue Engineering and Biomaterials laboratory at Mayo Clinic for their contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Reddi AH, Cunningham NS. Initiation and promotion of bone differentiation by bone morphogenetic proteins. J Bone Miner Res. 1993;8:S499–S502. doi: 10.1002/jbmr.5650081313. [DOI] [PubMed] [Google Scholar]
- 3.Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM, Pang RH, Corbett C, Oskaynak E, Oppermann H, Rueger DC. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–20362. [PubMed] [Google Scholar]
- 4.Wang EA, Rosen V, D'Alessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, LaPan P, Luxenberg DP, McQuaid D, Moutsatsos IK, Nove J, Wozney JM. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wozney JM. Overview of bone morphogenetic proteins. Spine. 2002;27:S2–S8. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 6.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 7.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 8.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 9.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Vaccaro AR, Anderson DG, Patel T, Fischgrund J, Truumees E, Herkowitz HN, Phillips F, Hilibrand A, Albert TJ, Wetzel T, McCulloch JA. Comparison of OP-1 Putty (rhBMP-7) to iliac crest autograft for posterolateral lumbar arthrodesis: a minimum 2-year follow-up pilot study. Spine. 2005;30:2709–2716. doi: 10.1097/01.brs.0000190812.08447.ba. [DOI] [PubMed] [Google Scholar]
- 11.Ruhe PQ, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J Mater Sci Mater Med. 2006;17:919–927. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 12.Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27:S16–S23. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 13.Committee for Medicinal Products for Human Use EMA. Scientific discussion OP-1. 2004 [Google Scholar]
- 14.Uludag H, D'Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 16.Groeneveld EH, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 17.Niikura T, Hak DJ, Reddi AH. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463–1471. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- 18.Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004:197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 20.Boyan BD, Lohmann CH, Somers A, Niederauer GG, Wozney JM, Dean DD, Carnes DL, Schwartz Z. Potential of porous poly-D,L-lactide-co-glycolide particles as a carrier for recombinant human bone morphogenetic protein-2 during osteoinduction in vivo. J Biomed Mater Res. 1999;46:51–59. doi: 10.1002/(sici)1097-4636(199907)46:1<51::aid-jbm6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Duggirala SS, Mehta RC, DeLuca PP. Interaction of recombinant human bone morphogenetic protein-2 with poly(d,l lactide-co-glycolide) microspheres. Pharm Dev Technol. 1996;1:11–19. doi: 10.3109/10837459609031413. [DOI] [PubMed] [Google Scholar]
- 22.Oldham JB, Lu L, Zhu X, Porter BD, Hefferan TE, Larson DR, Currier BL, Mikos AG, Yaszemski MJ. Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng. 2000;122:289–292. doi: 10.1115/1.429662. [DOI] [PubMed] [Google Scholar]
- 23.Ruhe PQ, Boerman OC, Russel FG, Spauwen PH, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Schrier JA, DeLuca PP. Recombinant human bone morphogenetic protein-2 binding and incorporation in PLGA microsphere delivery systems. Pharm Dev Technol. 1999;4:611–621. doi: 10.1081/pdt-100101400. [DOI] [PubMed] [Google Scholar]
- 25.Woo BH, Fink BF, Page R, Schrier JA, Jo YW, Jiang G, DeLuca M, Vasconez HC, DeLuca PP. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm Res. 2001;18:1747–1753. doi: 10.1023/a:1013382832091. [DOI] [PubMed] [Google Scholar]
- 26.Kempen DH, Lu L, Kim C, Zhu X, Dhert WJ, Currier BL, Yaszemski MJ. Controlled drug release from a novel injectable biodegradable microsphere/scaffold composite based on poly(propylene fumarate) J Biomed Mater Res. 2006;77:103–111. doi: 10.1002/jbm.a.30336. [DOI] [PubMed] [Google Scholar]
- 27.Kempen DHR, Kim CW, Lu L, Dhert WJA, Currier BL, Yaszemski MJ. Controlled release from poly(lactic-co-glycolic acid) microspheres embedded in an injectable, biodegradable scaffold for bone tissue engineering. Materials Science Forum. 2003;426–432:3151–3156. [Google Scholar]
- 28.Kempen DHR, Lu L, Classic KL, Hefferan TE, Creemers LB, Maran A, Dhert WJ, Yaszemski MJ. A non-invasive screenings model for simultaneous evaluation of multiple in vivo growth factor release profiles. doi: 10.1016/j.jconrel.2008.05.004. Accepted for publication by the Journal of Controled Release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–3748. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Lu L, Yaszemski MJ. Bone-tissue-engineering material poly(propylene fumarate): correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules. 2006;7:1976–1982. doi: 10.1021/bm060096a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kempen DHR, Kruyt MC, Lu L, Wilson CE, Florschutz AV, Creemers LB, Yaszemski MJ, Dhert WJ. The effect of autologous BMSCs seeding and BMP-2 delivery on the ectopic bone formation in a microspheres/poly(propylene fumarate) composite. doi: 10.1089/ten.tea.2007.0376. Submitted to Tissue Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375–4383. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 33.Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318–1324. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen SM, Demirkaya O, Ritman EL. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with X-ray micro-CT. Am J Physiol. 1998;275:H1103–H1114. doi: 10.1152/ajpheart.1998.275.3.H1103. [DOI] [PubMed] [Google Scholar]
- 35.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59:573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 36.Li RH, Bouxsein ML, Blake CA, D'Augusta D, Kim H, Li XJ, Wozney JM, Seeherman HJ. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J Orthop Res. 2003;21:997–1004. doi: 10.1016/S0736-0266(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 37.Laffargue P, Fialdes P, Frayssinet P, Rtaimate M, Hildebrand HF, Marchandise X. Adsorption and release of insulin-like growth factor-I on porous tricalcium phosphate implant. J Biomed Mater Res. 2000;49:415–421. doi: 10.1002/(sici)1097-4636(20000305)49:3<415::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Lind M, Overgaard S, Soballe K, Nguyen T, Ongpipattanakul B, Bunger C. Transforming growth factor-beta 1 enhances bone healing to unloaded tricalcium phosphate coated implants: an experimental study in dogs. J Orthop Res. 1996;14:343–350. doi: 10.1002/jor.1100140303. [DOI] [PubMed] [Google Scholar]
- 39.Bessho K, Carnes DL, Cavin R, Ong JL. Experimental studies on bone induction using low-molecular-weight poly (DL-lactide-co-glycolide) as a carrier for recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 2002;61:61–65. doi: 10.1002/jbm.10169. [DOI] [PubMed] [Google Scholar]
- 40.Fujimura K, Bessho K, Kusumoto K, Konishi Y, Ogawa Y, Iizuka T. Experimental osteoinduction by recombinant human bone morphogeneticprotein 2 in tissue with low blood flow: a study in rats. Br J Oral Maxillofac Surg. 2001;39:294–300. doi: 10.1054/bjom.2001.0647. [DOI] [PubMed] [Google Scholar]


