Abstract
4-vinylcyclohexene diepoxide (VCD) specifically destroys small pre-antral follicles in the rodent ovary. VCD can be detoxified to an inactive tetrol by microsomal epoxide hydrolase (mEH), or by conjugation to glutathione (GSH) by glutathione S-transferase (GST). Formation of VCD-GSH adducts in the mouse ovary 4 h after VCD exposure (0.57mmol/kg/day) has been demonstrated. Because the mouse ovary expresses both mEH and GST, expression of mEH and GST pi and mu during a time-course of VCD-induced ovotoxicity was evaluated in a neonatal mouse ovarian culture system. Ovaries from postnatal day 4 (PND4) B6C3F1 mice were incubated with VCD (15μM) for 2, 4, 6, 8, 10, 12, or 15 days. Following incubation, ovaries were histologically evaluated, or assessed for mRNA or protein expression. VCD did not cause follicle loss (P>0.05) on days 2, 4, or 6 of culture. At days 8, 10, 12, and 15, VCD reduced (P<0.05) both primordial and primary follicle numbers. Increased (P<0.05) expression of mEH, GST pi and GST mu mRNA was detected after 4 days of VCD exposure. This expression was reduced on days 6 and 8, when follicle loss was underway, but increased (P<0.05) after 10 days of exposure. mEH and GST pi proteins were elevated (P<0.05) following 8 days of VCD-exposure however there was no increase in GST mu protein. These findings suggest that with continuous exposure to VCD, increased expression of detoxification enzymes may participate in retarding the onset of follicle loss, but that this loss cannot ultimately be prevented.
Keywords: 4-vinylcyclohexene diepoxide, Ovotoxicity, Microsomal epoxide hydrolase, Glutathione S-transferase
Introduction
Exposure of females to environmental or occupational chemicals can have effects on reproductive function. 4-vinylcyclohexene (VCH) is an occupational chemical formed by dimerization of 1,3-butadiene and is produced as a by-product in the pesticide, rubber, plastic and flame retardant industries (Rappaport and Fraser, 1977). A metabolite of VCH, 4-vinylcyclohexene diepoxide (VCD) is used as an industrial diluent for epoxides (IARC, 1976). VCD is ovotoxic and specifically destroys small pre-antral follicles (primordial and primary) in ovaries of rats and mice (Doerr et al., 1995; Smith et al., 1990) by enhancing the natural process of atresia (apoptosis; Springer et al., 1996a,b; Hu et al., 2001a,b). VCH is metabolized in the liver by cytochrome P450 enzyme isoforms, CYP2A and CYP2B (Fontaine et al., 2001a,b). Conversely, in the ovary the cytochrome P450 enzyme isoform CYP2E1 is thought to convert precursors to the ovotoxic form, VCD (Cannady et al., 2003; Rajapaska et al., 2007a).
Microsomal epoxide hydrolase (mEH) is an enzyme that catalyzes the addition of water to alkene epoxides and arene oxides. mEH can detoxify VCD to an inactive tetrol metabolite [4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane], and isolated rat ovarian follicles have been demonstrated to be capable of forming the tetrol metabolite (Flaws et al., 1994). Previous research in mice has shown that mEH is expressed in the ovary and mEH expression and activity are increased in small pre-antral follicles following repeated daily dosing (15d) with VCD (Cannady et al., 2002).
Glutathione S-Transferases (GST’s) comprise a large family of enzymes that catalyze the conjugation of glutathione (GSH) to a variety of xenobiotics and allow for more rapid excretion. There are a number of classes of these enzymes, alpha, mu, omega, pi, sigma, theta and zeta, and each of these in turn contains a number of isoforms. In addition to its role in glutathione conjugation, the GST family has another possible interesting indirect role in cell signaling pathways. GSTs form protein:protein interactions with JNK and ASK1 protein kinases involved in controlling cellular stress responses, apoptosis and proliferation (Adler et al., 1999; Cho et al., 2001; Davis et al., 2001; Dorion et al., 2002).
An in vitro culture system has been developed using ovaries from post-natal day 4 (PND4) mice (enriched in small pre-antral follicles) to examine in vitro effects of ovotoxicants without a metabolic influence from the liver (Devine et al., 2002, 2004). Although mEH induction in ovaries from mice dosed daily (15d) with VCD was demonstrated (Cannady et al., 2002), a correlation between VCD-induced mEH expression and follicle loss has not been determined. The mouse ovary has been shown to synthesize GSH (Luderer et al., 2001), and form VCD-GSH adducts (Rajapaska et al., 2007b), however, induction of individual GST classes in the ovary has not been examined over a period of exposure. Therefore, the present study was designed to investigate the temporal association between the effects of VCD on expression of detoxification enzymes (mEH and GST’s) as compared with follicle loss using the in vitro culture system.
Materials and Methods
Reagents
VCD (mixture of isomers, >99% purity), 2-β-mercaptoethanol, 30% acrylamide/0.8% bis-acrylamide, ammonium persulfate, glycerol, N′,N′,N′,N′-Tetramethyl-ethylenediamine (TEMED), Tris base, TrisHCL, sodium chloride, Tween-20, bovine serum albumin (BSA), ascorbic acid (Vitamin C), and transferrin were purchased from Sigma-Aldrich Inc. (St Louis, MO). Dulbecco’s Modified Eagle Medium: nutrient mixture F-12 (Ham) 1X (DMEM/Ham’s F12), Albumax, penicillin/streptomycin (5000U/ml, 5000μg/ml, respectively), Hanks’ Balanced Salt Solution (without CaCl2, MgCl2, or MgSO4), Custom designed primers, and Superscript III One-Step RT-PCR System were obtained from Invitrogen Co. (Carlsbad, CA). Millicell-CM filter inserts were purchased from Millipore (Bedford, MA), and 48 well cell culture plates were obtained from Corning Inc. (Corning, NY). The mEH antibody was purchased from Detroit R and D (Detroit, MI). Rabbit polyclonal anti-GSTpi was obtained from Chemicon (Temecula, CA). Donkey anti-goat secondary antibody was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Rabbit polyclonal anti-GSTmu was purchased from Upstate Biotechnology (Lake Placid, NY). Goat anti-rabbit secondary antibody and BCA protein quantification kit were obtained from Pierce Biotechnology (Rockford, IL). Cy-5-streptavidin was obtained from Vector (Burlingame, CA). YOYO-1 was purchased from Molecular Probes (Eugene, OR). RNeasy Mini kit, QIAshredder kit, RNeasy MinElute kit, and Quantitect™ SYBR Green PCR kit were purchased from Qiagen Inc. (Valencia, CA). RNAlater was obtained from Ambion Inc. (Austin, TX). ECL plus chemiluminescence detection kit was purchased from GE Healthcare, Amersham (Buckinghamshire, UK).
Animals
Late gestation day pregnant mice (carrying B6C3F1 litters) were purchased from Harlan Laboratories (Indianapolis, IN). All animals were housed one per cage in plastic cages, and maintained in a controlled environment (22 ± 2°C; 12h light/12h dark cycles). The animals were provided a standard diet with ad libidum access to food and water, and allowed to give birth. All animal experiments were approved by the University of Arizona’s Institutional Animal Care and Use Committee.
In vitro ovarian cultures
Ovaries from PND4 female B6C3F1 mice were cultured as described by Parrott and Skinner, 1999. Briefly, PND4 female B6C3F1 mice were euthanized by CO2 inhalation followed by decapitation. Each ovary was removed, oviduct and excess tissue was trimmed, and it was placed on a piece of Millicell-CM membrane floating on 250μl of DMEM/Ham’s F12 medium containing 1mg/ml BSA, 1mg/ml Albumax, 50μg/ml ascorbic acid, 5U/ml penicillin/5μg/ml streptomycin, and 27.5μg/ml transferrin per well in a 48 well plate previously equilibrated to 37°C. Using fine forceps a drop of medium was placed to cover the top of the ovary to prevent drying. Ovaries were incubated with vehicle control or 15μM VCD. Plates containing ovaries were cultured for 2, 4, 6, 8, 10, 12 or 15 days at 37°C and 5% CO2 in air. For those cultures lasting more than 2 days, media were removed and fresh media and treatment were replaced every 2 days.
Histological evaluation of follicle numbers
Following incubation, ovaries were placed in Bouin’s fixative for 1.5h, transferred to 70% ethanol, embedded in paraffin, serially sectioned (5μm thick), and every 6th section was mounted. All ovarian sections were stained with hematoxylin and eosin. Healthy follicle populations containing oocytes were classified and counted in every 12th section. Unhealthy follicles were distinguished from healthy follicles by pyknosis of granulosa cells and intense eosinophilic staining of oocytes (Devine et al., 2002). Follicle population classification was according to the procedure of Flaws et al. (1994) which was adapted from that described by Pedersen and Peters (1968). Briefly, primordial follicles contained the oocyte surrounded by a single layer of squamous-shaped granulosa cells, primary follicles contained the oocyte surrounded by a single layer of cuboidal-shaped granulosa cells, and secondary follicles contained the oocyte surrounded by multiple layers of granulosa cells. Total follicle loss was calculated by subtracting the total number of follicles (primordial + primary) remaining following VCD treatment at each time point from total number of follicles in vehicle control treated group at that time point. These values were divided by the follicle numbers present in control ovaries, and the resulting value was multiplied by 100 to obtain a percentage; (Control-VCD)/Control × 100.
RNA isolation
Following 2, 4, 6, 8, or 10 days of in vitro culture, ovaries treated with vehicle control or VCD (15μM) were stored in RNAlater at −80°C. Total RNA was isolated (n=3; 10 ovaries per pool) using an RNeasy Mini kit. Briefly, ovaries were lysed and homogenized using a motor pestle followed by applying the mixture onto a QIAshredder column. The QIAshredder column containing ovarian tissue sample was then centrifuged at 14,000 rpm for 2 min. The resulting flow-through was applied to an RNeasy mini column, allowing RNA to bind to the filter cartridge. Following washing, RNA was eluted from the filter, and concentrated using an RNeasy MinElute kit. Briefly, isolated RNA was applied to an RNeasy MinElute spin column, and after washing, RNA was eluted using 14μL of RNase-free water. RNA concentration was determined using an ND-1000 Spectrophotometer (λ= 260/280nm; NanoDrop technologies, Inc., Wilmington, DE).
First strand cDNA synthesis and real-time polymerase chain reaction (PCR)
Total RNA (0.5μg) was reverse transcribed into cDNA utilizing the Superscript III One-Step RT-PCR System. cDNA was diluted (1:25) in RNase-free water. Two microliters of diluted cDNA were amplified on a Rotor-Gene 3000 using Quantitect™ SYBR Green PCR kit and custom designed primers as listed in Table 1. The cycling program consisted of a 15 min hold at 95°C and 45 cycles of: denaturing at 95°C for 15s, annealing at 58°C for 15s, and extension at 72°C for 20s at which point data were acquired. Product melt conditions were determined using a temperature gradient from 72°C to 99°C with a 1°C increase at each step. There was no difference in β-actin mRNA between vehicle control and VCD treated ovaries. Therefore, each sample was normalized to β-actin before quantification.
Table 1.
Primers used in real-time PCR
| Forward (5′-3′) | Reverse (3′-5′) | |
|---|---|---|
| mEH | GGGTCAAAGCCATCAGGCA | CCTCCAGAAGGACACCACTTT |
| GST alpha | CTGCAGCAGGGGTGGA | CTCTCTCCTTCATGTCCTTCC |
| GST theta | TGGAGCTGTACCTGGATCT | GTTCTTCTTGGCGAAGATATA |
| GST omega | CAGCATGAGGTTCTGC | TCATCCAGGTACTCACAAG |
| GST pi | GGCATCTGAAGCCTTTTGAG | GAGCCACATAGGCAGAGAGC |
| GST mu | TTCAAGCTGGGCCTGGAC | CAGGATGGCATTGCTCTG |
| β-actin | ACGCAGCTCAGTAACAGTCC | TCCATCCTGGCCTCACTGTC |
Protein Isolation
Pools of whole ovarian protein (10 ovaries/pool) homogenates were prepared from cultured ovaries via homogenization in tissue lysis buffer as previously described (Thompson et al., 2005). Briefly, homogenized samples were placed on ice for 30 min, followed by two rounds of centrifugation at 10,000 rpm for 15 min. Supernatant was aliquoted and stored at −80°C until further use. Protein was quantified using a standard BCA protocol on a 96-well assay plate. Emission absorbance values were detected with a λ= 540nm excitation on a Synergy™ HT Multi-Detection Microplate Reader using KC4™ software (Bio-Tek® Instruments Inc., Winooski, VT). Protein concentrations were calculated from a BSA protein standard curve.
Western Blot Analysis
SDS-PAGE (10%) was used to separate protein homogenates (10μg; n=3) and subsequently transferred onto nitrocellulose membranes as previously described (Thompson et al., 2005). Briefly, membranes were blocked for 1 h with shaking at 4°C in 5% milk in Tris-buffered saline with Tween-20 (TTBS). Membranes were incubated with primary antibody in 5% milk in TTBS overnight at 4°C. Antibody dilutions used were mEH (1:10,000), GSTpi and GST mu (1:200). Membranes were washed three times for 10 min each with TTBS. HRP-conjugated secondary antibody (1:2000 dilution) was added for 1 h at room temperature. Membranes were washed three times for 10 min each in TTBS, followed by a single wash for 10 min in Tris Buffered Saline (TBS). Western blots were detected by chemiluminescence (using ECL plus chemiluminescence detection substrate) and exposed to X-ray film. Densitometry of the appropriate bands was perfomed using LabWorks™ software from a UVP Bioimaging system (UVP Inc., Upland, CA). Individual treatment values were normalized to beta-actin and expressed as a percentage of the control mean.
Confocal microscopy
Following in vitro culture, ovaries (3/group) treated with vehicle or VCD (15μM) were fixed in 4% buffered formalin for 2 h, transferred to 70% ethanol, embedded in paraffin, serially sectioned, and every 10th section was mounted. Sections were deparaffinized (approximately 10 sections/ovary) and incubated with primary antibody directed against either mEH (1:75 dilution) or GST mu (1:25 dilution) at 4°C overnight. Specificity for these antibodies was determined by western blotting. Secondary biotinylated antibody (mEH-horse anti-goat; GST mu-goat anti-rabbit; 1:50 dilution) was applied for 1 h, followed by CY-5-streptavidin (1 h; 1:100 dilution). Sections were treated with Ribonuclease A (100μg/ml) for 1 h, followed by staining with YOYO-1 (10 min; 5nM). Slides were repeatedly rinsed with phosphate buffer saline (PBS), cover-slipped, and stored in the dark (4°C) until visualization. Primary antibody was not added to immuno-negative ovarian sections. Immunofluorescence was visualized on a Zeiss (LSM 510 NLO-Meta) confocal microscope with an argon and helium-neon laser projected through the tissue into a photomultiplier at λ = 488 and 633 nm for YOYO-1 (green) and CY-5 (red), respectively. All images were captured using a 40 X objective lens. Multiple readings were taken throughout the sections.
Statistical analysis
Comparisons were made between treatments using Statview software analysis of variance (ANOVA) and Fisher’s protected least significant difference (PLSD) multiple range test. The assigned level of significance for all tests was P < 0.05.
Results
VCD-induced follicle loss
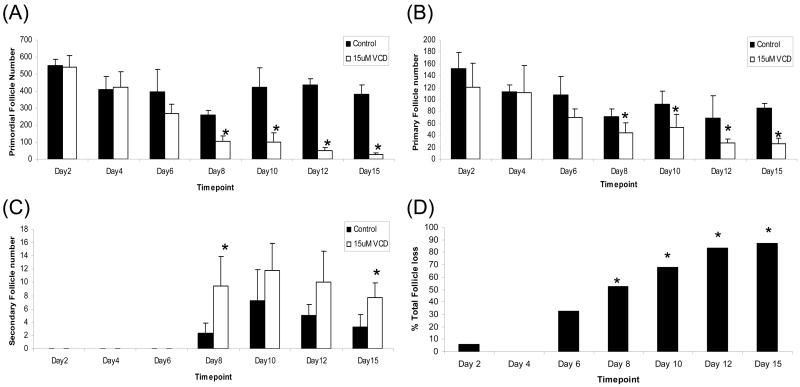
To demonstrate the ovotoxic effects of VCD, follicle loss was evaluated in ovaries of B6C3F1 mice cultured with 15μM VCD for 2, 4, 6, 8, 10, 12 or 15 days (Figure 1). Relative to control medium, incubation with 15μM VCD significantly depleted (P<0.05) primordial and primary follicles after 8 days in culture (Figure 1A, B). In the PND4 ovary, follicle populations are mainly composed of primordial and primary follicles, such that secondary follicles only appeared after 8 days in culture. There were no significant effects of VCD on secondary follicles on days 10 and 12, however on days 8 and 15, there were increased (P<0.05) numbers of secondary follicles due to VCD treatment (Figure 1C). There was a non-significant trend (P=0.06) for total follicle loss at day 6 which became significant (P<0.05) at day 8 (Figure 1D).
Figure 1. Time-course of VCD-induced ovotoxicity in B6C3F1 PND4 mouse ovaries.
Ovaries from PND4 B6C3F1 mice were cultured with control medium or 15μM VCD for 2, 4, 6, 8, 10, 12 or 15d. Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. (A) Primordial, (B) Primary, and (C) Secondary follicles were classified and counted. (D) Total follicle loss in VCD treated ovaries as a percentage of control treatment. Values are mean ± SE total follicles counted/ovary, n=5; * Different (p<0.05) from control. NOTE: Secondary follicles do not form until day 8 in culture.
Expression of GST class mRNA in B6C3F1 mouse ovaries
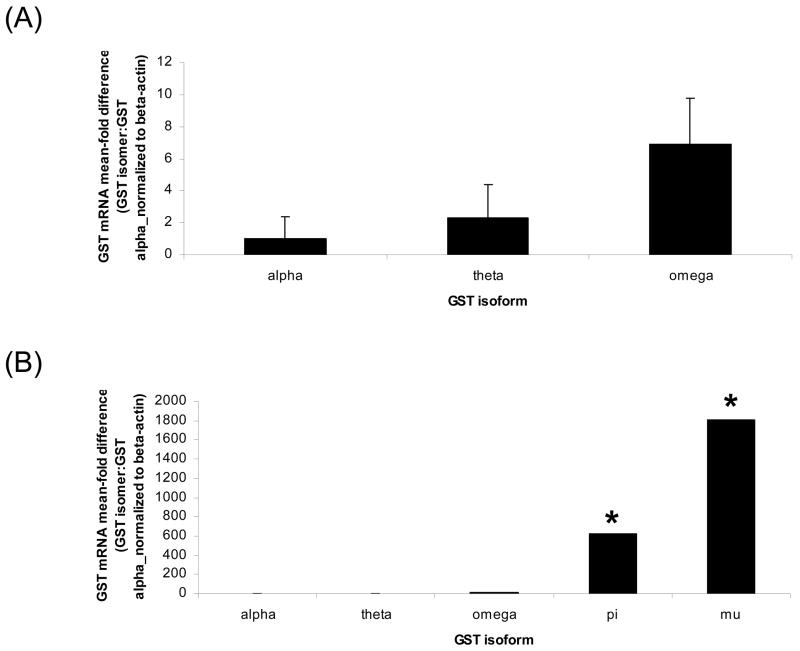
The relative distribution of GST classes in PND4 mouse ovaries was evaluated using primers designed to conserved regions of each GST class. GST alpha mRNA was at the lowest relative abundance, therefore, mRNA encoding the other classes were compared to GST alpha as a relative fold difference as shown in Figure 2A and B. The relative order of GST class mRNA abundance in the murine ovary were mu>pi>omega>theta>alpha. GST pi and mu mRNA were substantially greater relative to GST alpha, Theta and Omega.
Figure 2. GST isoform distribution in unstimulated B6C3F1 PND4 mouse ovaries.
Total RNA was isolated from PND4 B6C3F1 mice as described in materials and methods. One microgram of total RNA was reverse transcribed to cDNA and analyzed for GST isoform and β-actin mRNA by RT-PCR. Values are mean fold level, ± SE; n=3 (8–10 ovaries per pool), relative to GST alpha. Values are mean ± S.E.; n=3.
VCD-induced effects on mEH, GST pi and GST mu mRNA expression
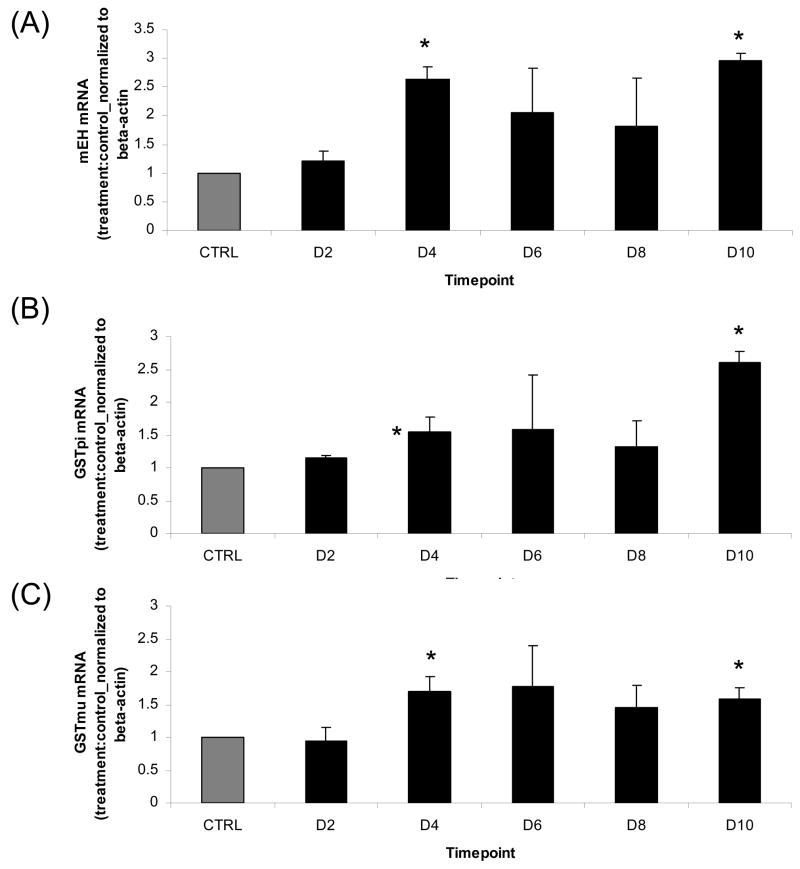
The effects of VCD exposure on induction of mEH, GST pi and GST mu mRNA expression in cultured neonatal mouse whole ovaries were evaluated. Ovaries from B6C3F1 PND4 mice were cultured with 15μM VCD for 2, 4, 6, 8 or 10 days (Figure 3). VCD-induced mRNA expression was determined by normalization to beta-actin mRNA with fold-changes being expressed as a ratio to control treatment.
Figure 3. Time-course of VCD effect on mEH, GST pi and GST mu mRNA.
Ovaries from PND4 B6C3F1 mice were cultured with control medium or 15μM VCD for 2, 4, 6, 8, or 10d. Following incubation, total RNA was isolated as described in materials and methods. One microgram total RNA was reverse transcribed to cDNA and analyzed for (A) mEH, (B) GST pi, (C) GST mu and β-actin mRNA by RT-PCR. Values are mean fold change ± SE; n=3 (8–10 ovaries per pool). Values are mean ± S.E.; n=3. * Different (p<0.05) from control.
There was no difference in mEH, GST pi or GST mu expression between control and VCD-incubated ovaries on day 2. After 4 and 10 days of VCD exposure, abundance of mRNA encoding mEH, GST pi and GST mu were increased (P<0.05) by 164%, 55% and 70%, respectively, on day 4 and by 197%, 160% and 159%, respectively, on day 10 (Figure 3 A, B, C). At the time-points when VCD-induced ovotoxicity (follicle loss) is being initiated (days 6 and 8), there was no significant difference in mEH, GST pi or GST mu expression due to VCD exposure when compared to control treatment.
VCD-induced effects on mEH, GST pi and GST mu protein expression
The effect of VCD exposure on mEH, GST pi and GST mu protein levels was evaluated by western blotting. Ovaries were collected from B6C3F1 PND4 mice and cultured with 15μM VCD for 4 and 8 days. After 4 days of VCD-exposure there was no difference in mEH, GST pi or GST mu protein expression between VCD and control treatment ovaries (data not shown). After 8 days of VCD exposure, mEH protein expression was increased by 113% compared to control treated ovaries (P<0.05; Figure 4A). Similarly, GST pi protein expression was increased by 47% with VCD exposure by day 8 (P<0.05; Figure 4B). There was no change in GST mu protein expression at either day 4 or day 8 due to VCD exposure (P>0.1; Figure 4C).
Figure 4. Effect of VCD on mEH, GSTpi and GSTmu protein expression on day 8.
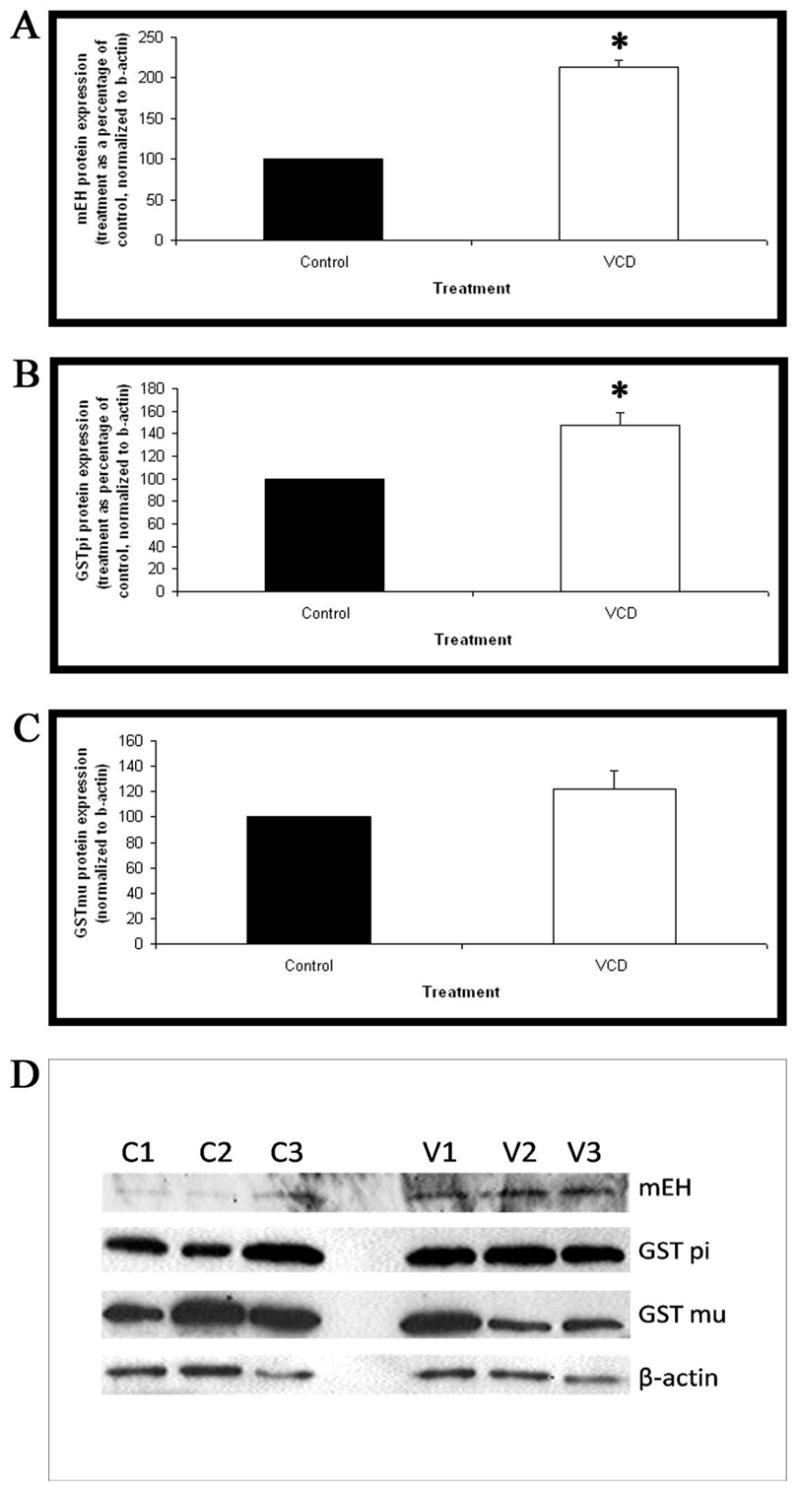
Ovaries from PND4 B6C3F1 mice were cultured with control medium or medium containing 15μM VCD for 8 days. Following incubation, total protein was isolated and Western blotting carried out for (A) mEH, (B) GST pi and (C) GST mu. (D) Representative western blots for mEH, GST pi, GST mu and beta-actin (Control=C1,C2,C3; VCD=V1,V2,V3). Values are normalized to beta-actin protein and expressed as treatment as a percentage of control mean ± S.E.; n=3. * Different (p<0.05) from control.
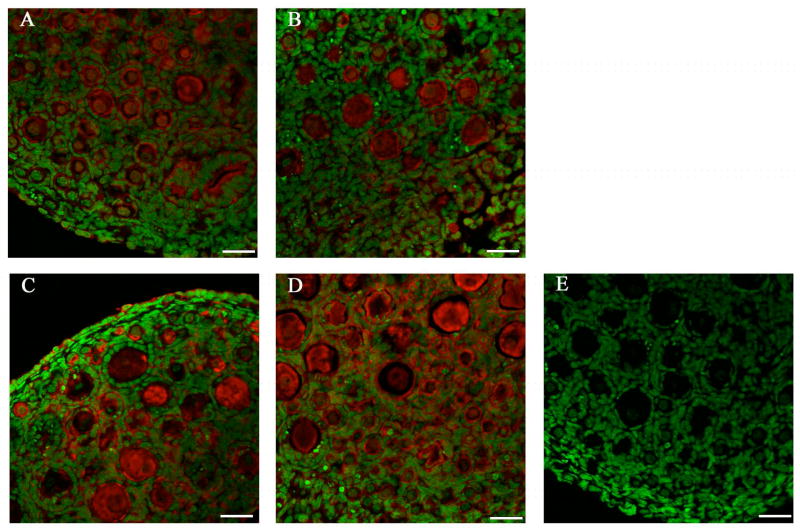
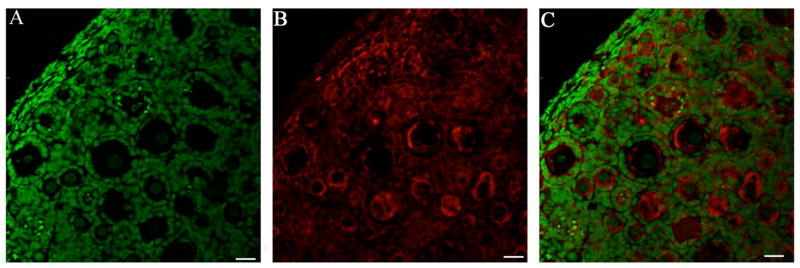
The effects of VCD exposure on mEH protein level was evaluated by immunofluorescence and analyzed by confocal microscopy. Ovaries were collected from B6C3F1 PND4 mice and cultured with 15μM VCD for 4 or 8 days. Staining for mEH protein was observed in the oocyte nucleus and cytoplasm, granulosa cells and interstitial compartments of the mouse ovary. After 4 days of VCD exposure, there was no difference in mEH protein staining relative to control (Figure 5 A,B). After 8 days of VCD exposure, staining for mEH protein was more intense in VCD-exposed mouse ovaries relative to controls (Figure 5 C,D). GST mu protein staining was also performed and staining was observed in oocyte nucleus and cytoplasm, granulosa cells and interstitial compartments of the mouse ovary (Figure 6), however, no change in GST mu protein staining was observed due to VCD exposure (data not shown). GST pi protein staining was not performed due to the appearance of more than one band on the western blot.
Figure 5. Effect of VCD on mEH protein expression.
B6C3F1 ovaries were cultured with vehicle control (A,C) or 15μM VCD (B,D) for 4 (A,B) and 8 (C,D) days and processed for confocal microscopy as described in materials and methods. mEH (Cy-5 red stain) and genomic DNA (green YOYO1 stain) overlay at 40X magnification. (E) Immuno-negative staining (no primary antibody). Scale-bar equal to 25μM.
Figure 6. GST mu protein expression in PND4 cultured ovary.
B6C3F1 ovaries were cultured with control media for 8 days and processed for confocal microscopy as described in materials and methods. (A) Genomic DNA (green YOYO1 stain), (B) GST mu (Cy-5 red stain) and (C) Combined overlay of GST mu (Cy-5 red stain) and Genomic DNA (green YOYO1 stain) at 40X magnification. Scale-bar equal to 25μM.
Discussion
VCD, the ovotoxic metabolite of the occupational chemical VCH, specifically targets small pre-antral follicles in the ovaries of rats and mice. Extensive exposure results in depletion of the follicular pool and premature ovarian failure (Mayer et al., 2002; Devine and Hoyer, 2005). Therefore, chemicals that damage ovarian small pre-antral follicles could pose a risk for early menopause in exposed women. Because the liver is the major organ for metabolism of xenobiotic chemicals, research has focused on how the liver metabolizes VCH and VCD. However, extra-hepatic organs also express detoxification enzymes (Krishna and Klotz, 1994) thus, it is possible that hepatic metabolism can potentially produce active metabolites for delivery via the circulation to other organs, in which detoxification can occur. Several studies have recently shown the potential of the ovary to directly metabolize these compounds (Cannady et al., 2002; 2003; Rajapaska et al., 2007a,c).
Two main systems in the ovary are available for detoxification of VCD; mEH and the GST family. Previous studies in mice have shown that ovarian mEH expression is induced following exposure to VCD (Cannady et al., 2002), and that rat ovarian follicles in vitro can convert VCD to the inactive tetrol metabolite (Flaws et al., 1994). Furthermore, the GST family catalyzes the conjugation of VCD with GSH. VCD-GSH conjugates can be further processed and excreted from the body. The formation of VCD mono- and di-GSH adducts was demonstrated in both the liver and ovary from mice dosed with VCD for 15 days (Rajapaska et al., 2007b). Therefore, the mouse ovary has the potential to detoxify VCD by VCD-GSH conjugate formation. Thus, an in vitro whole ovarian culture system was used in the present study without the metabolic influence of the liver to determine the time-course of ovarian expression of detoxification enzymes (Devine et al., 2002; 2004).
As previously shown, VCD exposure induced ovotoxicity in the PND4 ovary and was specific for primordial and primary follicles. On 2, 4, and 6 days of culture, there was no effect (P>0.05) of VCD (15μM) on follicle numbers. However, relative to control incubations, VCD caused primordial and primary follicle loss (P<0.05) beginning on day 8. By day 15, approximately 90% of healthy follicles had been lost. Secondary follicles first developed in culture on day 8. There were increased numbers of secondary follicles due to VCD exposure on days 8 and 15, but not on days 10 and 12. The reason for this small effect on follicle numbers is unclear, however, this could be due to increased recruitment of remaining healthy primordial and small primary follicles into the secondary pool in response to VCD.
Induction of mRNA encoding mEH by VCD in the mouse ovary was seen on day 4 of culture. In control ovaries, GST pi and mu mRNA levels were much greater than those for GST alpha, theta and omega. Additionally, as with mEH, exposure to VCD increased (P<0.05) these levels by day 4 of culture. Thus, after 4 days of VCD exposure, mRNA levels were increased by 164%, 55% and 70%, for mEH, GST pi and GST mu, respectively, relative to control incubations. On days 6 and 8, the trend for increased expression of mEH, GST pi and GST mu was not significant. This reflects a highly variable response between individual ovaries during the time of impending follicle loss. Interestingly, by 10 days of VCD exposure, mRNA expression of mEH, GST pi and GST mu were again consistently increased (P<0.05) compared to control treated ovaries. Because this latter increase was seen at a time of >70% loss of follicles, it suggests that non-target ovarian cells may be expressing increased levels of these detoxification enzymes. Such cells may be located in the interstitium, and supports a capacity for the interstitial ovarian compartment to express high levels of xenobiotic detoxification enzymes. This suggests a potential role of the interstitium to provide protection of the germ cell population (ovarian follicles) against xenobiotic exposure (Cannady et al., 2003, Rajapaska et al., 2007a,c).
While mRNA levels were increased for all three genes studied at day 4 of exposure, increased protein expression was not seen until day 8 of culture. Interestingly, only mEH and GST pi protein levels were increased over the timecourse. No increase in GST mu protein was seen. Staining for both mEH and GST mu proteins was performed in cultured ovaries. Although immunofluorescent staining for both mEH and GST mu proteins were extensive throughout the ovary, only mEH protein staining was apparently increased by VCD. This observation confirmed the western blot results. Thus, it appears that the more robust response to VCD exposure in expression of detoxification enzymes resides with mEH. However, it cannot be ruled out that GST pi and mu also contribute.
In addition to the catalysis of glutathione conjugation, GST pi and mu have been shown to play roles in hepatic cell signaling pathways via protein:protein interactions. In particular, GST pi protein has been shown to bind to the Jun N-terminal kinase (JNK)-c-jun protein complex, thus inhibiting phosphorylation of c-jun and activation of JNK-c-jun mediated signaling pathways, such as induction of apoptosis (Adler et al., 1999; Davis et al., 2001). Previous research has shown that VCD exposure causes increased JNK activity and c-jun phosphorylation in the ovary (Hu et al., 2002). Therefore, the potential for the involvement of GST pi in inhibiting pro-apoptotic pathways in the ovary is plausible.
VCD-induced ovotoxicity requires repeated daily dosing in vivo, or continuous exposure in vitro. In fact, a single dose of VCD was seen to protect against follicle loss (Borman et al., 1999). Results of the present study show that the requirement for repeated dosing with or continuous exposure to VCD may relate, in part, to its early induction of ovarian detoxification enzymes. However, this is not sufficient to prevent the ultimate onset of ovotoxicity once the ovary becomes overwhelmed by the repeated chemical assault. This finding raises concerns about low-dose repeated exposure of women to ovotoxic environmental or occupational chemicals which may pose a risk equal to or greater than a single exposure to a higher dose (Borman et al., 2000).
In summary, the increase in expression of mRNA encoding three enzymes, mEH, GST pi and GST mu, involved in detoxification of VCD prior to follicle loss may retard the onset of VCD-induced ovotoxicity due to increased detoxification of VCD. The increase in GST pi and GST mu expression to detoxify VCD may indirectly lead to increased follicle loss from apoptosis by relieving the inhibitory interactions of GSTs with pro-apoptotic proteins. At day 10 when VCD-induced follicle loss is substantial, the ovary contains residual ovarian tissue which expresses mEH, GST pi and GST mu protein. Therefore, the increase in expression of these three enzymes measured on day 10 is likely due to their relative enrichment in that tissue.
Acknowledgments
This work was supported by National Institutes of Health (grant number ES09246) and Center Grant 06694. Authors wish to thank Andrea Grantham for histological processing of ovarian tissue and Patricia Christian for assistance with immunohistochemistry and confocal analysis.
Footnotes
This work was supported by National Institutes of Health grant ES09246 and Center Grant 06694.
Summary sentence
Ovotoxicity in mice follows initial induction of detoxification enzymes which become overwhelmed by repeated exposure to VCD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler V, Yin Z, Fuch SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB. A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl Pharmacol. 1999;158:244–252. doi: 10.1006/taap.1999.8702. [DOI] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries. Toxicol Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of cytochrome P450 2E1, 2A, and 2B in the mouse ovary: The effect of 4-vinylcyclohexene and its diepoxide metabolite. Toxicol Sci. 2003;73:423–430. doi: 10.1093/toxsci/kfg077. [DOI] [PubMed] [Google Scholar]
- Cho SG, Lee YH, Park H, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, Shim J, Kim Y, Dong MS, Lee MJ, Kim SG, Ichijo H, Choi EJ. Glutathione S-Transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- Davis W, Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- Devine PJ, Rajapaska KS, Hoyer PB. In vitro ovarian tissue and organ culture: a review. Frontiers in Bioscience. 2002;7:1979–1989. doi: 10.2741/devine. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Hoyer PB. Initiation of delayed ovotoxicity by in vitro and in vivo exposure of rat ovaries to 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2004;19:71–77. doi: 10.1016/j.reprotox.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Hoyer PB. Ovotoxic environmental chemicals: indirect endocrine disruptors. In: Nog R, editor. Endocrine disruptors: effects on male and female reproductive system. Boca Raton, Fl: CRC Press; 2005. pp. 67–100. [Google Scholar]
- Doerr JK, Hooser SB, Smith BJ, Sipes IG. Ovarian toxicity of 4-vinylcyclohexene and related olefins in B6C3F1 mice:role of diepoxides. Chem Res Toxicol. 1995;8:963–969. doi: 10.1021/tx00049a010. [DOI] [PubMed] [Google Scholar]
- Dorion S, Lambert H, Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of gluathione S-transferase Mu from ASK1. J Biol Chem. 2002;277:30792–30797. doi: 10.1074/jbc.M203642200. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Fontaine SM, Hoyer PB, Halpert JR, Sipes IG. Role of induction of specific hepatic cytochrome P450 isoforms in epoxidation of 4-vinylcyclohexene. Drug Metab Dispos. 2001a;29:1236–1242. [PubMed] [Google Scholar]
- Fontaine SM, Hoyer PB, Sipes IG. Evaluation of hepatic cytochrome P4502E1 in the species-dependent bioactivation of 4-vinylcyclohexene. Life Sci. 2001b;69:923–934. doi: 10.1016/s0024-3205(01)01170-5. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001a;65:87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001b;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monograph on the evaluation of carcinogenic risk of chemicals to humans. Vol. 11. International Agency for Research on Cancer; Lyon, France: 1976. Cadmium, nickel, some epoxides, miscellaneous industrial chemicals and general considerations on volatile anaesthetics; pp. 141–145. [PubMed] [Google Scholar]
- Krishna DR, Koltz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet. 1994;26:144–160. doi: 10.2165/00003088-199426020-00007. [DOI] [PubMed] [Google Scholar]
- Luderer U, Kavanagh TJ, White CC, Faustman EM. Gonadotropin regulation of glutathione synthesis in the rat ovary. Reprod Toxicol. 2001;15:495–504. doi: 10.1016/s0890-6238(01)00157-5. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinol. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Rajapaska KS, Cannady EA, Sipes IG, Hoyer PB. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol Appl Pharmacol. 2007a;221:215–221. doi: 10.1016/j.taap.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaska KS. Thesis. Tucson, U.S.A: The University of Arizona; 2007b. The role of ovarian metabolism in 4-vinylcyclohexene metabolites and 7, 12-dimethylbenz[a]anthracene-induced ovotoxicity in mice. [Google Scholar]
- Rajapaska KS, Sipes IG, Hoyer PB. Involvement of microsomal epoxide hydrolase in ovotoxicity caused by 7,12-dimethylbenz[a]anthracene. Toxicol Sci. 2007c;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, Fraser DA. Air sampling and analysis in a rubber vulcanization area. Am Ind Hyd Assoc J. 1977;38:205–210. doi: 10.1080/0002889778507601. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996a;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer LA, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996b;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]