Abstract
Aim
To examine whether hawthorn (Crataegus Special Extract WS 1442 {CSE}) inhibits progression in heart failure (HF) patients.
Methods
We performed a retrospective analysis of data from the HERB CHF study in which patients with mild to moderate HF were randomised to either CSE 900 mg or placebo for 6 months. The primary outcome was time to progression of HF (HF death, hospitalisation, or sustained increase in diuretics) as assessed by log-rank tests and by Cox modelling.
Results
Progression of HF occurred in 46.6% of the CSE and 43.3% of the placebo groups (OR 1.14, 95% CI = 0.56, 2.35: p = 0.86). Patients receiving CSE were 3.9 times (95% CI = 1.1 – 13.7: p = 0.035) more likely to experience HF progression at baseline. In adjusted analysis, the risk of having early HF progression in the CSE group increased to 6.4 (95% CI = 1.5, 26.5: p = 0.011). In patients with LVEF 35%, those taking CSE were at significantly greater risk (3.2, 95% CI = 1.3, 8.3: p = 0.02) than the placebo group.
Conclusions
CSE does not reduce heart failure progression in patients who have HF. CSE appears to increase the early risk of HF progression.
Keywords: Hawthorn, Heart Failure Progression, Crataegus Special Extract WS 1442, Crataegus oxycantha
Introduction
The pharmacological treatment of heart failure (HF) has seen tremendous advances in recent years. In appropriate patients, the addition of β-adrenergic and aldosterone antagonists, and the combination of hydralazine and nitrates have each substantially improved quality of life and reduced symptoms of HF, the risk of death and the rate of HF progression. Nevertheless, even with contemporary evidence based treatment regimens, progression of HF is inexorable. As the prevalence of heart failure continues to increase, there is a pressing need for new agents that could positively affect HF progression.
Inflammatory signalling and oxidative stress appear to be involved in transcriptional regulatory pathways that lead to progression of HF. (1) Elevated aldosterone levels, which have been suggested as having a significant role in left ventricular remodelling, stimulate reactive oxygen species (ROS) in the myocyte. (2) Thus, inflammatory signalling may be one of the mechanisms through which activation of the sympathetic nervous system causes left ventricular remodelling. As the neurohormonal system is triggered early in HF disease progression, drugs with anti-inflammatory effects may, in theory, reduce the risk of clinical progression.
Crataegus oxycantha, or hawthorn berries, leaves and flowers and their extracts have been used for cardiac and circulatory disorders since the first century AD. (3) C. oxycantha demonstrates numerous properties that may be beneficial in HF progression, including antioxidant activities (4–9) and anti-inflammatory effects. (10–12) A recent meta-analysis of clinical trials concluded that C. oxycantha may be a safe and effective treatment for HF. (13)
Although most previous trials of C. oxycantha have reported modest improvements in exercise capacity, quality of life (QOL) and HF-related symptoms, (13) none of these studies have examined the effect of C. oxycantha on HF progression. Consequently, we performed a secondary data analysis of a randomised, double blind, placebo controlled trial of Crataegus Special Extract WS1442 in patients with mild to moderate symptomatic HF (HERB CHF), to examine the effect of Crataegus Special Extract WS 1442 versus placebo on clinical measures of HF progression.
Methods
We retrospectively analyzed baseline, three and six month data from 120 HF patients who had completed a six month randomised, double-blind, placebo-controlled drug trial, HERB-CHF, in which treatment with Crataegus Special Extract WS 1442 was found to have a neutral effect on clinical outcomes in patients with New York Heart Association (NYHA) class II–IV symptoms. (14)
Study Population
Patients aged 18 years and older who had been diagnosed with HF (NYHA functional classes II–III) for ≥ 3 months with a left ventricular ejection fraction (LVEF) ≤ 40% (by radionuclide ventriculography, contrast left ventriculography or echocardiography, assessed during usual clinical care within the 12 months prior to randomisation) were recruited from the University of Michigan Health System cardiology clinics, and from the surrounding community by local newspaper advertisements. Patients were eligible to participate if they were receiving indicated standard therapy (if not contraindicated or intolerant) defined as a diuretic, an ACE-inhibitor or an angiotensin receptor blocker (ARB) and a β-blocker. Patients with NYHA class III symptoms were also required to receive spironolactone. Doses of these drugs had to be stable for ≥ 3 months, except for diuretics, for which ≥ 1 month of stability was required.
Patients were ineligible if they had haemodynamically severe uncorrected primary valvular disease; active myocarditis; hypertrophic cardiomyopathy; restrictive cardiomyopathy; myocardial infarction, stroke, unstable angina, coronary artery bypass graft surgery, valvular surgery, cardiac resynchronization therapy or angioplasty ≤ 3 months before randomisation. In addition, patients with symptomatic or sustained ventricular tachycardia not controlled by antiarrhythmic drugs or an implantable cardioverter-defibrillator; any condition other than HF that would be expected to limit exercise (e.g., angina, peripheral vascular disease, pulmonary disease, arthritis or an orthopaedic problem severe enough to limit exercise); nursing mothers, pregnant women and those planning a pregnancy during the study period, were also excluded. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. The study was approved by the University of Michigan Medical School Institutional Review Board and was overseen by an independent Data Safety Monitoring Board. All participants provided written informed consent.
Study Procedures
Screening occurred at the end of a HF clinic visit. Interested patients were asked to undertake a 6-minute walk test. Patients who walked between 150 and 450 meters were invited to attend for a baseline visit within the following two weeks. Written informed consent was obtained at the beginning of the baseline visit. If participants were found to be clinically stable and euvolaemic at the baseline visit, they were asked to perform another 6-minute walk test. Those who walked between 150 and 450 meters were randomised and all others were deemed ineligible. Eligible patients also had their LVEFs assessed at the baseline visit by radionuclide ventriculography.
Intervention
Eligible patients were randomly assigned to receive either C. oxycantha extract, Crataegus Special Extract WS 1442 (Crataegutt forte®, Willmar Schwabe Pharmaceuticals, Karlsruhe, Germany), 450 mg twice daily, or a matching placebo. This dose was chosen based on the manufacturer’s recommendations and on doses used in previous clinical studies using this extract. Each tablet contained 450 mg dry extract of C. oxycantha leaves with flowers [4–6.6:1 extraction solvent: ethanol 45 percent] standardized to 84.3 mg of oligomeric proanthocyanidins (OPCs). The entire study was conducted using a single batch of Crataegus Special Extract WS 1442 to optimize product consistency. Content of OPCs in the study medication were independently verified using appropriate high performance liquid chromatography methods (Integrated Biomolecule; Tuscon, AZ). The OPC content at the end of the study was 84.06 mg/tablet. Placebo tablets contained lactose, and were coloured to match the placebo tablets. Participants were told to take the study medication twice per day with water and to bring all unused tablets to each study visit. Patients were seen at the study clinic three months and six months after the baseline visit.
Statistical analysis
Baseline characteristics are reported by treatment group using means and standard deviations (SD) for continuous variables, and counts and percentages for categorical variables. The primary outcome for this analysis, progression of HF, was defined as (1) death due to HF, (2) hospitalisation due to HF, or (3) sustained increase in diuretic dose for HF. An increase in diuretic dose was defined as an increase of at least 50% from baseline at either the three month or six month visit. The three parts of the primary endpoint were considered in hierarchical order, where HF death took precedence over HF hospitalisation and HF hospitalisation had precedence over increased diuretic dose. Consequently, patients with more than one endpoint were only counted once.
Our primary outcome, proportion of patients with clinical progression of HF, was analyzed using Cochran-Mantel-Haenszel techniques to calculate relative risks (RR) and 95% confidence intervals (CI). Time to progression by treatment group was determined by construction of Kaplan-Meier survival estimates and tested using the log-rank test. To test for the effect of potentially important prognostic determinants (baseline age, sex, HF aetiology, serum creatinine, NYHA functional class, serum sodium, peak oxygen consumption, LVEF, mean blood pressure, Heart Failure Survival Score (15), β-blocker and ACE inhibitor use as well as time by treatment interaction) we used Cox modelling, with backward stepwise regression to determine the most parsimonious models. An α of ≤ 0.05 was considered significant.
With a sample size of 60 per group and a 43% event rate in the placebo group, we have 84% power to detect a hazard ratio of 2.0 or greater, assuming alpha=0.05 and two-sided log rank test. For the subgroup LVEF of 35% and below, with a sample size of approximately 34 per group and a 43% event rate in the placebo group, we have 81% power to detect a hazard ratio of 2.6 or greater.
RESULTS
Socio-demographic and Clinical Characteristics
The socio-demographic and clinical characteristics of participants by treatment group are shown in Table 1. There were no differences between treatment groups for any demographic or clinical characteristics. However, despite the entry requirement of an LVEF ≤ 40% measured during a clinically-indicated study within the previous 12 months (see above), the baseline radionuclide ventriculogram performed after study randomisation detected an LVEF > 40% in 38 of the study participants. Since the baseline assessment of LVEF occurred after randomisation, we were not able to exclude these patients.
Table 1.
Socio-demographic and clinical characteristics of the study population by treatment group
| Characteristics | Placebo, (n=60) n (%) or mean (± SD) | Hawthorn, (n=60) n (%) or mean (± SD) |
|---|---|---|
| Sex | ||
| Men | 44 (73) | 46 (77) |
| Women | 16 (27) | 14 (23) |
| Age | 57.8 (± 9.0) | 54.4 (± 12.6) |
| Race | ||
| White | 50 (83) | 45 (75) |
| Blood pressure, mm Hg | ||
| Systolic | 113 (± 19) | 109 (± 15) |
| Diastolic | 66 (± 10) | 66 ± 10 |
| Heart rate, bpm | 71 ± 11 | 68 ± 11 |
| NYHA class | ||
| II | 33 (55) | 30 (50) |
| III | 27 (45) | 30 (50) |
| LVEF, % | 34.8 (±14.5) | 36.2 (±15.1) |
| LVEF ≤ 40% | 44 (73) | 38 (63) |
| Six-minute Walk Test, m | 374.0 (± 52.4) | 358.2 (± 59.2) |
| Peak Oxygen Consumption, mL\kg\min | 14.6 (± 3.8) | 14.7 (± 3.5) |
| Medications | ||
| ACE Inhibitor | 46 (77) | 48 (80) |
| ARB | 11 (18) | 9 (15) |
| ACE Inhibitor or ARB | 57 (95) | 57 (95) |
| Beta Blocker | 52 (87) | 54 (90) |
| Digoxin | 49 (82) | 47 (78) |
| Loop Diuretic | 57 (95) | 55 (92) |
| Spironolactone | 39 (65) | 39 (65) |
| Thiazide Diuretic | 10 (17) | 11 (18) |
Patient Flow
Of the 120 patients who were randomised into the HERB CHF trial, 60 were assigned to placebo and 60 to Crataegus Special Extract WS 1442. One hundred and eleven patients completed all six months of the study. In the placebo group three patients did not complete the study; one died due to HF, one had severe pneumonia and one had a United Network for Organ Sharing (UNOS) status 1 heart transplant. In the Crataegus Special Extract WS 1442 group six patients did not complete the study: three patients died, one due to aplastic anaemia and two due to HF; of the other three patients: one patient developed Grave’s disease; one developed thyomas and one had a UNOS status 1 heart transplant.
Progression of Heart Failure
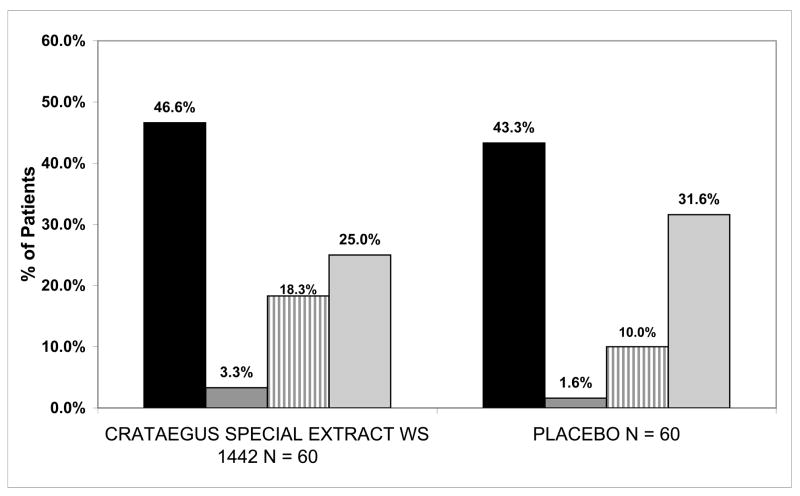
Progression of HF occurred in 46.6% (28/60) of Crataegus Special Extract WS1442 patients and 43.3% (26/60) of placebo patients. This reflects no difference in the incidence of the primary outcome between the groups (p=0.86) (Fig 1). The odds ratio of HF progression was 1.14 (95% CI = 0.56, 2.35) for Crataegus Special Extract WS1442 compared to placebo. Evaluating each component of the combined endpoint separately, Crataegus Special Extract WS1442 resulted in nominally more HF deaths (3.3%, 2/60 vs. 1.6%, 1/60) and HF hospitalisations (18.3%, 11/60 vs. 10.0%, 6/60) but less frequent need for increased diuretic (25.0%, 15/60 vs. 31.6%, 19/60) compared to placebo.
Figure 1.
Heart failure progression endpoints in the Crataegus Special Extract WS 1442 and placebo groups for the 120 patients in the HERB CHF trial.
The Kaplan-Meier curve for Crataegus Special Extract WS1442 and placebo (Fig 2.) showed a crossing pattern that could indicate changing hazards over time. We consequently performed a non-proportional Cox regression analysis using a time by treatment interaction that demonstrated the risk of HF progression between the two groups changed significantly through time (p = 0.047). The non-proportional Cox model demonstrated that patients in the Crataegus Special Extract WS1442 group were 3.9 times (95% CI = 1.1, 13.7: p = 0.035) more likely to experience a HF progression event at baseline than the placebo group. The hazard ratio (HR) for the Crataegus Special Extract WS1442 group decreased through time, so that at six months (182 days) Crataegus Special Extract WS1442 did not increase progression (HR = 0.63, 95% CI = 0.29, 1.47: p = 0.72) compared to the placebo group. In a similar fashion, when adjusted for baseline NYHA functional class, peak oxygen consumption, LVEF, Heart Failure Survival Score, and ACE inhibitor use, as well as the time by treatment interaction, the risk of having a HF progression event at baseline in the Crataegus Special Extract WS1442 group increased to HR = 6.4 (95% CI = 1.5, 26.5: p = 0.011) times greater than the placebo group. At six months (182 days), after adjustment, Crataegus Special Extract WS1442 again no longer increased progression (HR = 0.41, 95% CI = 0.14, 1.18: p = 0.64) compared to the placebo group.
Figure 2.
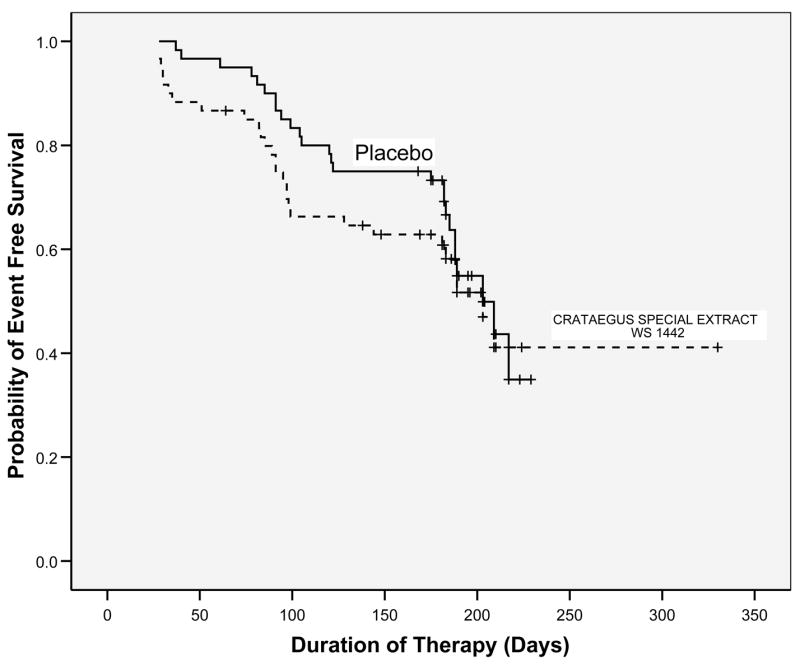
Kaplan-Meier plot of the effect of Crataegus Special Extract WS 1442 versus placebo on progression of heart failure in patients in the HERB CHF trial. Crataegus Special Extract WS 1442 had no effect on the rate of heart failure progression (hazard ratio = 1.14, (95% CI = 0.56, 2.35)
We conducted the same analyses on the subgroup of 68 participants with LVEF 35%. Results in this subgroup of patients were similar to those observed for the whole group. Progression of HF occurred in 51.5% (17/33) of Crataegus Special Extract WS1442 patients and 42.9% (15/35) of placebo patients. This difference reflects a non-significant increase in the primary endpoint of 13% (p=0.48) in the Crataegus Special Extract WS1442 group. The odds ratio of HF progression was 1.42 (95% CI = 0.55, 3.69: p = 0.48) for Crataegus Special Extract WS1442 compared to placebo.
Kaplan-Meier analysis also demonstrated the possibility that the risk of having HF events appeared to change through time between the two groups. A non-proportional Cox regression analysis indicated that time by treatment interaction was not significant (p = 0.13). When adjusted for baseline peak oxygen consumption, cause of HF, serum sodium, and ACE inhibitor use, the risk of having a HF progression event in the Crataegus Special Extract WS1442 group was HR = 3.2 (95% CI = 1.3, 8.3: p = 0.02) times higher than in the placebo group.
Discussion
C. oxycantha in the formulation and dose administered in this study when added to optimum evidence based therapy did not decrease the risk of our primary outcome, clinical HF progression compared to placebo. There were, however, significantly more HF progression events at baseline in the Crataegus Special Extract WS1442 group versus the placebo group. Subgroup analysis of data from patients with LVEF ≤ 35 % demonstrated similar results, although more ill patients who received Crataegus Special Extract WS1442 were at a significantly increased risk of having a HF progression event throughout the 6 months of the study and not just at baseline.
It is unclear why patients who receive Crataegus Special Extract WS1442 are at greater risk at baseline or if more ill throughout the entire 6 months of the study for having a HF related hospitalisation, death or increased diuretic use. Our results could well be due to chance, as this study was a secondary data analysis and had limited power due to the small sample size. The analysis in sicker patients, with an even smaller sample size, was even more limited and vulnerable to chance outcomes. Moreover, as other clinical trials examining C. oxycantha for HF have not examined the occurrence of clinical HF events through time, we are unable to determine if this is a unique conclusion of our study or a common pattern with C. oxycantha intake in this population. The Survival and Prognosis: Investigation of Crataegus Extract WS 1442 in Congestive Heart Failure (SPICE) trial has recently been completed. The SPICE trial was a randomised clinical trial in nearly 3,000 HF patients with LVEF ≤ 35%, the primary endpoint was cardiac morbidity, non-fatal myocardial infarction and hospitalisation due to HF progression over 24 months. (16) The SPICE trial used Crataegus Special Extract WS1442 at the same dose as in our study and enrolled patients with similar baseline demographic and clinical characteristics. (17) However, since the SPICE trial has not yet been published, only data presented orally and in the associated abstract are currently available, allowing only limited comparisons with our study data. The SPICE trial reported similar results to our study, in that there was no significant difference in HF progression between the active and placebo groups (27.9% vs. 28.9%: p = NS), although clearly our trial had a higher percentage of HF progression events (46.6% vs. 43.3%). (16) Kaplan-Meier curves have not yet been presented for the SPICE trial, consequently it is unclear if the relative risk between the active and placebo group changed through time, and if there was an increased risk in the Crataegus Special Extract WS1442 group at baseline or in sicker patients.
One possible explanation for the increased risk for progression of HF at baseline in the Crataegus Special Extract WS1442 group and for patients with LVEF ≤ 35% (in the adjusted analysis) is the possibility of herb-drug interactions. As the majority of patients with HF are on several different drugs, many interactions are theoretically possible. In particular, the flavonoids in C. oxycantha are chemically similar to other flavonoids that have demonstrated P-glycoprotein activity, which may lead to various drug-flavonoid interactions. (18–20) Only one study has examined possible interactions between C. oxycantha and a commonly administered HF medication, digoxin. This study found that Crataegus Special Extract WS 1442 (450 mg twice daily) did not significantly alter the pharmacokinetic parameters of digoxin. (21) Further, several flavonoids in C. oxycantha such as the vitexin rhamnosides have been demonstrated to have low oral absorption or undergo extensive conjugation before reaching the systemic circulation. (22–23) Both the negative study examining potential interactions with digoxin and the low level absorption of the main flavonoids present in C. oxycantha diminish the potential of possible C. oxycantha-drug interactions. It is possible that constituents other than flavonoids in C. oxycantha may cause herb-drug interactions, but currently there is not enough information to determine whether C. oxycantha-drug interaction led to the increased early risk of HF progression.
This study had several limitations. As with all secondary analyses this study was not designed a priori to detect HF progression events. As such, we did not have a data collection method in place to monitor changes in HF medications continuously over the six month study period. Instead we collected information about medications taken, their dose and frequency at baseline, three and six months. At three and six months, we noted if the patient’s diuretic dose was increased from their last visit. As a consequence we could have included patients who did not truly have an increased sustained diuretic dose and missed including patients with sustained increased diuretic dose. These potential misclassifications, however, should have been non-differentially spread across both groups, thus keeping the risk relationships between the two groups the same.
Another major limitation of this study is its small sample size. With 54 events in 120 patients we are able to detect relatively moderate changes in hazard ratios (2 times or greater) between the groups but not small differences. This is even more pronounced in the subgroup analyses of patients with LVEF of ≤ 35% where we have 32 events in 68 patients.
We suggest that healthcare providers use caution when considering prescribing C. oxycantha to patients with HF. Patients already taking C. oxycantha should also be observed closely for any adverse events that could be temporally related to C. oxycantha ingestion. We make these recommendations because Crataegus Special Extract WS1442, at a commonly recommended dose, significantly increased the risk for progression of HF at baseline and in the more clinically compromised patients with LVEF ≤ 35% in this study. Moreover, Crataegus Special Extract WS1442 increased risk consistently in more compromised patients with LVEF ≤ 35%.
In summary, Crataegus Special Extract WS 1442 does not reduce heart failure progression in patients who have mild to moderate HF. Crataegus Special Extract WS 1442 appears to increase the early risk of heart failure progression.
Acknowledgments
We are grateful for the work of the members of the Data Safety Monitoring Board (Drs. Gary Chase, Jonathan Sackner Bernstein, Allen Sedman, Cynthia Long and Shan Wong), and for the contributions of our study team: Drs. Robert Cody, Todd Koelling, David Bradley Dyke, Audrey Wu, Ragaven Baliga (UM Heart Failure and Transplant Management Program); Brian Nordin (exercise physiology); Dr. Roberta Tankanow (research pharmacy); E. Mitchell Seymour (laboratory sciences); Dr. James Corbett (nuclear medicine); Margaret Ann Murphy (data management); Alexis Zirpoli and Amie Litzinger (manuscript preparation); Katherine Rice, Patricia Stimac, Robert Adwere-Boamah, Amy Blume and Fayeannette Pierce (clinical research assistants); Drs. Sara Warber and Steven Bolling (U-M Complementary and Alternative Medicine Research Center). We would also like to thank Dr. Willmar Schwabe Pharmaceuticals and Dr. Günter Meng for generously providing the Crataegus Special Extract WS 1442 and matching placebo.
This research was supported by a grant to the University of Michigan Complementary and Alternative Medicine Research Center from the National Center for Complementary and Alternative Medicine (P50 HL 061202 01). Research resources were also provided by the General Clinical Research Center of the University of Michigan (M01-RR00042). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
The authors report no conflicts of interest
This trial is registered in ClinicalTrials.gov ID: NCT00343902
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular Mechanisms in Heart Failure. Journal of the American College of Cardiology. 2006;48:A56–A66. [Google Scholar]
- 2.Kuster GM, Kotlyar E, Rude MK, et al. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005;111:420–427. doi: 10.1161/01.CIR.0000153800.09920.40. [DOI] [PubMed] [Google Scholar]
- 3.Weihmayr T, Ernst E. Therapeutic effectiveness of Crataegus. Fortschr Med. 1996;114:27–29. [PubMed] [Google Scholar]
- 4.Veveris M, Koch E, Chatterjee SS. Crataegus Special Extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sciences. 2004;74:1945–55. doi: 10.1016/j.lfs.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 5.Krzeminski T, Chatterjee SS. Ischemia and early reperfusion induced arrhythmias: beneficial effects of an extract of Crataegus oxyacantha L. Pharm Pharmacol Lett. 1993;3:45–8. [Google Scholar]
- 6.Periera da Silva A, Rocha R, Silva CM, Mira L, Duarte MF, Florencio MH. Antioxidants in medicinal plant extracts. A research study of the antioxidant capacity of Crataegus, Hamamelis and Hydrastis. Phytotherapy Research. 2000;14:612–6. doi: 10.1002/1099-1573(200012)14:8<612::aid-ptr677>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Bahorun T, Aumjaud E, Ramphul H, et al. Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Nahrung. 2003;47:191–8. doi: 10.1002/food.200390045. [DOI] [PubMed] [Google Scholar]
- 8.Kirakosyan A, Seymour E, Kaufman PB, Warber S, Bolling S, Chang SC. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J Agric Food Chem. 2003;51:3973–3976. doi: 10.1021/jf030096r. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Chang Q, Zhu M, Huang Y, Ho WKK, Chen ZY. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12:144–152. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee SS, Koch E, Jaggy H, Krzeminski T. In vitro and in vivo studies on the cardioprotective action of oligomeric procyanidins in a Crataegus extract of leaves and blooms. Arzneimittel-Forschung. 1997;47:821–5. [PubMed] [Google Scholar]
- 11.Schwitters B. OPC in Practice. Rome: Alfa Omega Edetrice; 1993. [Google Scholar]
- 12.Masquelier J. Pycnogenols: Recent advances in the therapeutical activity of procyanidins. In: Beal JL, Reinhard E, editors. Natural products as medicinal agents: plenary lectures of the International Research Congress on Medicinal Plant Research, Strasbourg, July 1980. Stuttgart: Hippokrates Verlag; 1981. [Google Scholar]
- 13.Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med. 2003;114:665–74. doi: 10.1016/s0002-9343(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson KD HERB-CHF (Hawthorn Extract Randomized Blinded Chronic HF Study) Late-Breaking and Recent Clinical Trials. J Card Fail; 8th Annual Scientific Meeting of the Heart Failure Society of America; Toronto, Ontario, Canada. 2004. Supp (Abstract 2832) [Google Scholar]
- 15.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;12:2660–7. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 16.Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. Survival and prognosis: investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)--rationale, study design and study protocol. Eur J Heart Fail. 2000;2:431–437. doi: 10.1016/s1388-9842(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 17.Holubarsch CJ, WS C, Meinertz T, et al. Late breaking clinical trials-3, Sessions 414-5. New Orleans, LA: American College of Cardiology; 2007. Crataegus extract WS1442 postpones cardiac death in patients with congestive heart failure class NYHA II-III: A randomized placebo-controlled, double-blind trials in 2681 patients. (Abstract) [Google Scholar]
- 18.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66:338–345. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 19.Yoo HH, Lee M, Chung HJ, Lee SK, Kim DH. Effects of diosmin, a flavonoid glycoside in citrus fruits, on P-glycoprotein-mediated drug efflux in human intestinal Caco-2 cells. J Agric Food Chem. 2007;55:7620–7625. doi: 10.1021/jf070893f. [DOI] [PubMed] [Google Scholar]
- 20.Lohner K, Schnabele K, Daniel H, et al. Flavonoids alter P-gp expression in intestinal epithelial cells in vitro and in vivo. Mol Nutr Food Res. 2007;51:293–300. doi: 10.1002/mnfr.200600225. [DOI] [PubMed] [Google Scholar]
- 21.Tankanow R, Tamer HR, Streetman DS, et al. Interaction study between digoxin and a preparation of hawthorn (Crataegus oxyacantha) J Clin Pharmacol. 2003;43:637–642. [PubMed] [Google Scholar]
- 22.Liang M, Xu W, Zhang W, et al. Quantitative LC/MS/MS method and in vivo pharmacokinetic studies of vitexin rhamnoside, a bioactive constituent on cardiovascular system from hawthorn. Biomed Chromatogr. 2007;21:422–429. doi: 10.1002/bmc.777. [DOI] [PubMed] [Google Scholar]
- 23.Zuo Z, Zhang L, Zhou L, Chang Q, Chow M. Intestinal absorption of hawthorn flavonoids--in vitro, in situ and in vivo correlations. Life Sci. 2006;79:2455–2462. doi: 10.1016/j.lfs.2006.08.014. [DOI] [PubMed] [Google Scholar]