Abstract
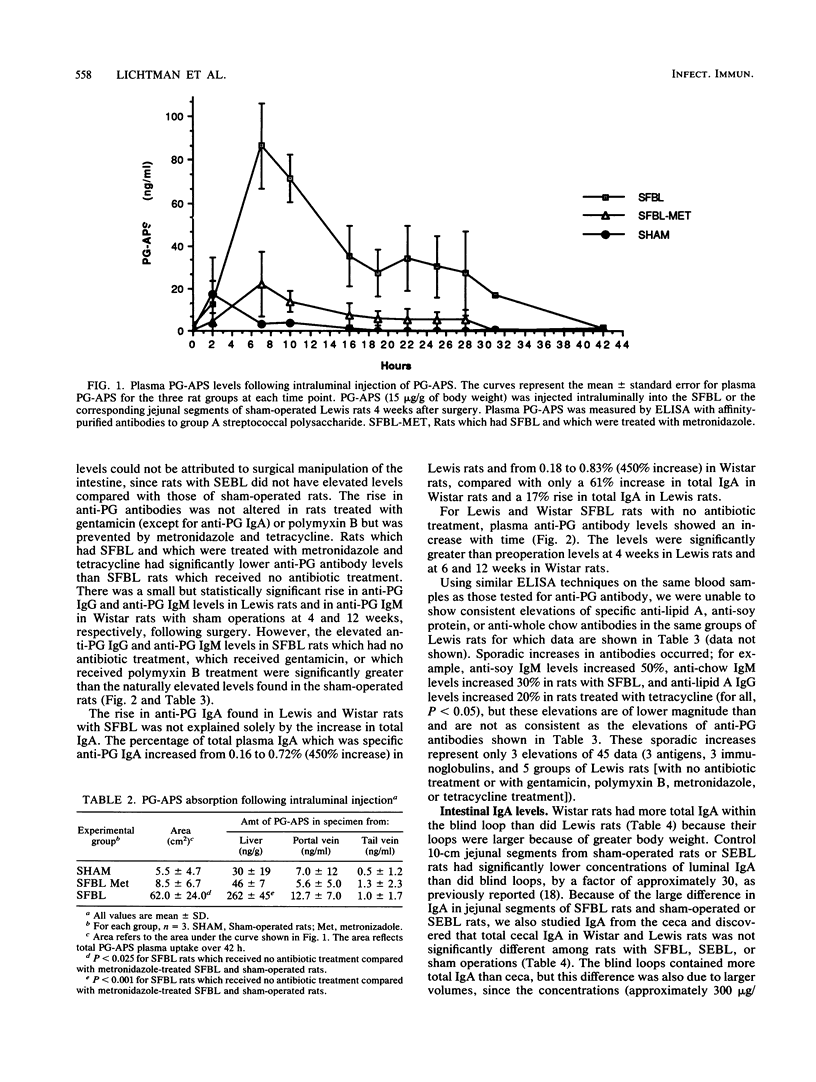
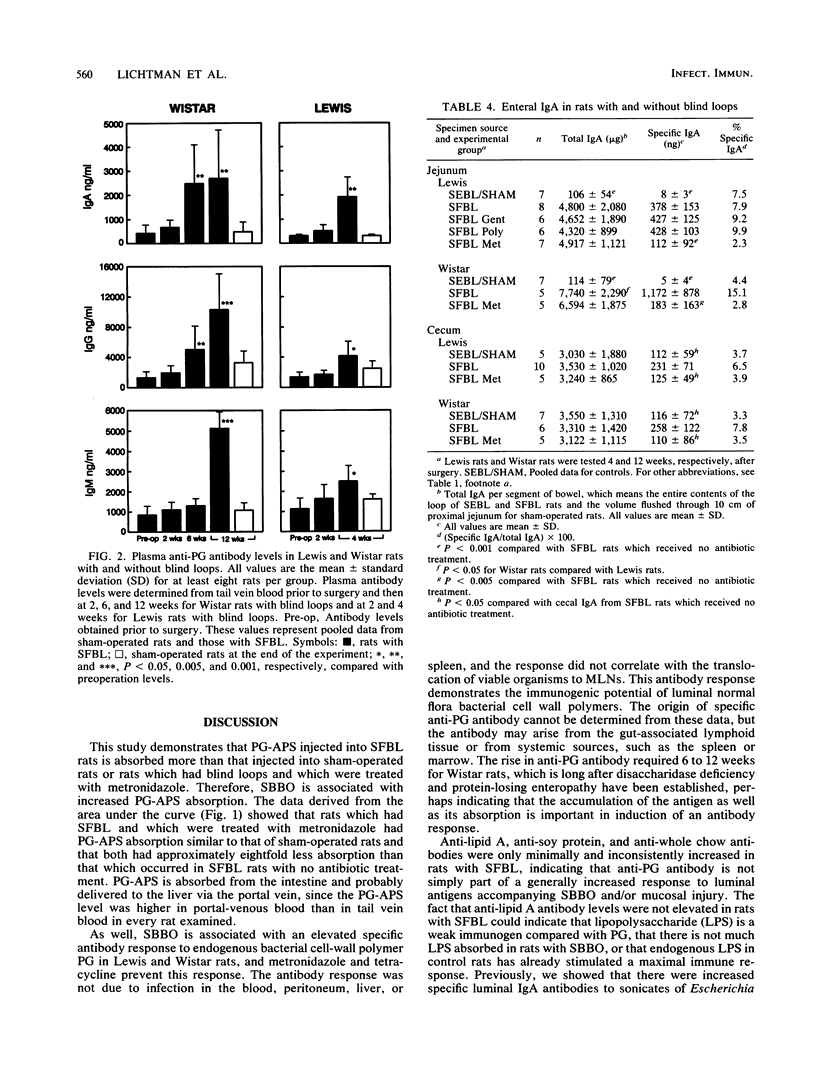
Surgical creation of jejunal self-filling blind loops (SFBL) causes small bowel bacterial overgrowth which is associated with hepatobiliary inflammation in the susceptible Lewis and Wistar rat strains. Since hepatic injury occurs when small bowel anaerobic bacterial concentrations are increased 4 to 6 log10 units per ml and hepatic bacterial cultures are negative, we postulate that the inflammation is caused by absorption of phlogistic cell wall polymers originating from bacteria within the loop. To demonstrate absorption of bacterial cell wall polymers, we measured plasma and hepatic levels of immunoreactive peptidoglycan-polysaccharide (PG-PS) following intraluminal injection as well as anti-PG antibodies as an indirect measure of absorption and/or accumulation of endogenous PG. PG-PS purified from group A streptococci was detected in plasma by enzyme-linked immunosorbent assay after intraluminal injection; rats with SFBL showed significantly more uptake into plasma and the liver than sham-operated rats or SFBL rats which were treated with metronidazole (P less than 0.025). Total plasma immunoglobulin A (IgA), IgG, and IgM levels did not differ among sham-operated rats and those with self-emptying blind loops or SFBL, but plasma anti-PG IgA (P less than 0.05), IgG, and IgM (P less than 0.01) levels were increased in rats with SFBL. Metronidazole and tetracycline prevented the elevation of anti-PG antibody, but gentamicin and polymyxin B did not. Anti-lipid A, anti-soy protein, and anti-chow antibodies in plasma were not consistently increased in rats with SFBL indicating the lack of a generalized antibody response to luminal antigens. These data suggest that PG from normal flora bacteria is absorbed from the intestinal lumen and that mucosal injury and/or increased luminal concentrations of PG, such as those induced by small bowel bacterial overgrowth, lead to enhanced absorption of potentially inflammatory bacterial polymers.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect Immun. 1981 Sep;33(3):854–861. doi: 10.1128/iai.33.3.854-861.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio G. R., Lanzavecchia A., Plebani A., Jayakar S., Ugazio A. G. Ontogeny of secretory immunity: levels of secretory IgA and natural antibodies in saliva. Pediatr Res. 1980 Oct;14(10):1111–1114. doi: 10.1203/00006450-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Ely P. H. The bowel bypass syndrome: a response to bacterial peptidoglycans. J Am Acad Dermatol. 1980 Jun;2(6):473–487. doi: 10.1016/s0190-9622(80)80148-4. [DOI] [PubMed] [Google Scholar]
- Gut J. P., Schmitt S., Bingen A., Anton M., Kirn A. Probable role of endogenous endotoxins in hepatocytolysis during murine hepatitis caused by frog virus 3. J Infect Dis. 1984 Apr;149(4):621–629. doi: 10.1093/infdis/149.4.621. [DOI] [PubMed] [Google Scholar]
- King C. E., Toskes P. P. Protein-losing enteropathy in the human and experimental rat blind-loop syndrome. Gastroenterology. 1981 Mar;80(3):504–509. [PubMed] [Google Scholar]
- King C. E., Toskes P. P. Small intestine bacterial overgrowth. Gastroenterology. 1979 May;76(5 Pt 1):1035–1055. [PubMed] [Google Scholar]
- Kutteh W. H., Prince S. J., Phillips J. O., Spenney J. G., Mestecky J. Properties of immunoglobulin A in serum of individuals with liver diseases and in hepatic bile. Gastroenterology. 1982 Feb;82(2):184–193. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemaitre-Coelho I., Jackson G. D., Vaerman J. P. Relevance of biliary IgA antibodies in rat intestinal immunity. Scand J Immunol. 1978;8(5):459–463. doi: 10.1111/j.1365-3083.1978.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Lichtman S. N., Sartor R. B., Keku J., Schwab J. H. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990 Feb;98(2):414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- Lichtman S., Sherman P., Forstner G. Production of secretory immunoglobulin A in rat self-filling blind loops. Local secretory immunoglobulin A immune response to luminal bacterial flora. Gastroenterology. 1986 Dec;91(6):1495–1502. doi: 10.1016/0016-5085(86)90207-6. [DOI] [PubMed] [Google Scholar]
- Mellander L., Carlsson B., Hanson L. A. Appearance of secretory IgM and IgA antibodies to Escherichia coli in saliva during early infancy and childhood. J Pediatr. 1984 Apr;104(4):564–568. doi: 10.1016/s0022-3476(84)80548-x. [DOI] [PubMed] [Google Scholar]
- Menge H., Köhn R., Dietermann K. H., Lorenz-Meyer H., Riecken E. O., Robinson J. W. Structural and functional alterations in the mucosa of self-filling intestinal blind loops in rats. Clin Sci (Lond) 1979 Feb;56(2):121–131. doi: 10.1042/cs0560121. [DOI] [PubMed] [Google Scholar]
- Nolan J. P., Leibowitz A. I. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978 Sep;75(3):445–449. [PubMed] [Google Scholar]
- Sartor R. B., Bond T. M., Compton K. Y., Cleland D. R. Intestinal absorption of bacterial cell wall polymers in rats. Adv Exp Med Biol. 1987;216A:835–839. doi: 10.1007/978-1-4684-5344-7_97. [DOI] [PubMed] [Google Scholar]
- Sartor R. B., Bond T. M., Schwab J. H. Systemic uptake and intestinal inflammatory effects of luminal bacterial cell wall polymers in rats with acute colonic injury. Infect Immun. 1988 Aug;56(8):2101–2108. doi: 10.1128/iai.56.8.2101-2108.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B., Cromartie W. J., Powell D. W., Schwab J. H. Granulomatous enterocolitis induced in rats by purified bacterial cell wall fragments. Gastroenterology. 1985 Sep;89(3):587–595. doi: 10.1016/0016-5085(85)90455-x. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Importance of intestinal mucosal immunity and luminal bacterial cell wall polymers in the aetiology of inflammatory joint diseases. Baillieres Clin Rheumatol. 1989 Aug;3(2):223–245. doi: 10.1016/s0950-3579(89)80019-6. [DOI] [PubMed] [Google Scholar]
- Severijnen A. J., Hazenberg M. P., van de Merwe J. P. Induction of chronic arthritis in rats by cell wall fragments of anaerobic coccoid rods isolated from the faecal flora of patients with Crohn's disease. Digestion. 1988;39(2):118–125. doi: 10.1159/000199614. [DOI] [PubMed] [Google Scholar]
- Sherman P., Lichtman S. Small bowel bacterial overgrowth syndrome. Dig Dis. 1987;5(3):157–171. doi: 10.1159/000171170. [DOI] [PubMed] [Google Scholar]
- Sherman P., Wesley A., Forstner G. Sequential disaccharidase loss in rat intestinal blind loops: impact of malnutrition. Am J Physiol. 1985 Jun;248(6 Pt 1):G626–G632. doi: 10.1152/ajpgi.1985.248.6.G626. [DOI] [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toskes P. P., Giannella R. A., Jervis H. R., Rout W. R., Takeuchi A. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975 May;68(5 Pt 1):1193–1203. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Allen J. B., Wilder R. L., Paglia L., Hand A. R. Bacterial cell wall-induced hepatic granulomas. An in vivo model of T cell-dependent fibrosis. J Exp Med. 1986 Apr 1;163(4):884–902. doi: 10.1084/jem.163.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L., Toskes P. P., Baer H. Importance of anaerobic bacteria in the cobalamin malabsorption of the experimental rat blind loop syndrome. Gastroenterology. 1981 Feb;80(2):313–320. [PubMed] [Google Scholar]


