Abstract
Alcoholism is a common, heritable, chronic relapsing disorder. GABAA receptors undergo allosteric modulation by ethanol, anesthetics, benzodiazepines and neurosteroids and have been implicated in the acute as well as the chronic effects of ethanol including tolerance, dependence and withdrawal. Medications targeting GABAA receptors ameliorate the symptoms of acute withdrawal. Ethanol induces plasticity in GABAA receptors: tolerance is associated with generally decreased GABAA receptor activation and differentially altered subunit expression. The dopamine (DA) mesolimbic reward pathway originating in the ventral tegmental area (VTA), and interacting stress circuitry play an important role in the development of addiction. VTA GABAergic interneurons are the primary inhibitory regulators of DA neurons and a subset of VTA GABAA receptors may be implicated in the switch from heavy drinking to dependence. GABAA receptors modulate anxiety and response to stress; important elements of sustained drinking and relapse. The GABAA receptor subunit genes clustered on chromosome 4 are highly expressed in the reward pathway. Several recent studies have provided strong evidence that one of these genes, GABRA2, is implicated in alcoholism in humans. The influence of the interaction between ethanol and GABAA receptors in the reward pathway on the development of alcoholism together with genetic and epigenetic vulnerabilities will be explored in this review.
Keywords: GABA, ethanol, neurosteroids, benzodiazepines, tolerance, withdrawal, reward, VTA, stress, anxiety genes, GABRA2
Introduction
Although alcohol consumption is a social pleasure for many, a significant number of individuals are unable to keep within safe limits and cross over the divide between social drinking and addiction. Alcoholism is common; the 12 month prevalence for alcohol use disorders (dependence plus abuse) is 8.5% (Grant et al., 2004). The essential features of alcoholism are loss of control over consumption, obsessional thoughts about the next drink, and continuation of use despite knowledge of negative health and social consequences (American Psychiatric Association, 1994). A meta-analysis of twin studies has shown that the heritability of all addictive substances ranges from 40 – 70 %; the heritability of alcoholism, derived from nearly 10,000 twin pairs, is 50% (Goldman et al., 2005). Therefore genetic and environmental risk factors for alcoholism are almost equally important although they may differ in different populations.
Unlike other addictive drugs that are more specific, alcohol has widespread effects throughout the brain; it acts at a variety of targets within cell membranes and in intracellular signal transduction, inducing effects on neurotransmitter and neurohormone membrane receptors and receptor-gated and voltage-activated ion channels. Alcohol alters the balance between γ-aminobutyric acid (GABA), the primary inhibitory neurotransmitter, and glutamate, the major excitatory neurotransmitter. Genetic vulnerability to alcoholism is therefore likely to be due to numerous genes of small to modest effects in many neurotransmitter systems and signal transduction pathways.
GABAA receptors undergo allosteric modulation by several structurally unrelated drugs, most with their own binding sites, including ethanol, benzodiazepines (BZs), barbiturates, anesthetics and also endogenous neurosteroids. These drugs have similar anxiolytic, sedative-hypnotic, anticonvulsant, motor-incoordinating, and cognitive impairing effects. GABAA receptors are implicated in the acute and chronic effects of alcohol including tolerance, dependence and withdrawal, as discussed below. Chronic ethanol consumption results in cross-tolerance to BZs and barbiturates (Kumar et al., 2004). This cross-tolerance, together with the effectiveness of BZs in treating both anxiety and alcohol withdrawal, suggests that GABAA receptors may play an important role in vulnerability to alcoholism and anxiety, particularly in the mesolimbic dopamine (DA) reward pathway and interacting stress circuitry (Enoch et al., 2003; Enoch, 2007).
GABAA receptors: structure
GABAA receptors are composed of five subunits, each of which has several isoforms (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3) (Barnard et al., 1998). Most receptors consist of two α, two β and one γ subunits (Sigel et al., 2006). There are three main subunit combinations: α1β2γ2 (60%), α2β3γ2 (15%), and α3β3γ2 (10%) (Benke et al., 1994, Michels and Moss, 2007). Expression of the various subunit isoforms varies across brain locations and during development. The receptor subunit composition determines distinct pharmacological and electrophysiological properties (Minier and Sigel, 2004).
GABAA receptors are ligand-gated, chloride ion channels that confer fast synaptic inhibition. Channel opening is initiated by the extracellular association of agonists with discrete binding pockets leading to conformational changes that result in the opening of a central ion pore (Barnard et al., 1998; Connolly and Wafford, 2004; Wisden and Seeburg, 1992). GABAA receptors have structural and functional homology with a class of cys-loop ligand-gated ion channel receptors including glycine, 5-HT3 and nicotinic acetylcholine. The topology of this class of receptors consists of a large N-terminus, ligand binding, extracellular domain with a cysteine loop, four transmembrane (TM) domains forming a chloride-ion channel with a large intracellular loop between TM3 and TM4 and a short-extracellular C-terminus (Brejc et al., 2001; Michels and Moss, 2007). The GABAA receptor ion channel is lined by the TM2 segments from each of the five subunits that form the receptor. There appears be a pocket located between TM2 and TM3 of the GABAA α subunit that binds both alcohols and anesthetics. It has been shown that within this pocket of the α1 subunit, serine-270 and alanine-291 are essential not only for the binding of alcohols but also for alcohol-induced conformational changes within the GABAA receptor (Jung et al., 2005; Jung and Harris, 2006; Mascia et al., 2000; Mihic et al., 1997). However, these studies used very high concentrations of ethanol (200mM) that correspond to anesthetic concentration in vivo, and there is so far no evidence that ethanol binds to GABAA receptors at physiological doses. The exact mechanism by which ethanol enhances GABA responses remains unclear. The BZ binding site is at the interface of the γ2 subunit with α subunits excepting α4 and α6 (Wafford, 2005; Barnard et al., 1998). Binding sites for GABA (two copies) lies at the interface between α and β (Olsen et al., 2004). Ethanol- and stress-induced neurosteroids potentiate GABA at a binding site located in a cavity formed by α subunit TM domains (Hosie et al., 2006).
Presynaptic, postsynaptic and extrasynaptic receptors: phasic and tonic inhibition
GABAA receptors are ubiquitously distributed at synapses on dendrites, neuronal cell bodies and axons and are also found in extrasynaptic membranes. Most studies of GABA’s effects have focused on phasic inhibition; i.e. postsynaptic GABAA receptors are activated following brief exposure to a high concentration of GABA released from presynaptic vesicles. However GABA that escapes from the synaptic cleft can also activate receptors on pre-synaptic terminals. Accumulating evidence from recent studies has shown that in several brain regions including the cerebellum and the mesolimbic reward pathway, ethanol can enhance GABAergic transmission through effects at both pre- and postsynaptic GABAA receptors through complex mechanisms (Ming et al., 2006; Roberto et al., 2006; Siggins et al., 2005).
GABAA receptors can exist as either synaptic or extrasynaptic receptors and this is a dynamic state that may facilitate rapid changes in synaptic inhibition (Thomas et al., 2005). It has recently been shown that low concentrations of extracellular GABA may result in persistent or ‘tonic’ activation of extrasynaptic GABAA receptors in hippocampal interneurons, the cerebellum, and the dentate gyrus (Farrant and Nusser, 2005; Kullmann et al., 2005; Semyanov et al., 2003). Other than δ, no GABAA receptor subunits have so far been found to be exclusively extrasynaptic (Farrant and Nusser, 2005). Neurosteroids potentiate extrasynaptic GABAA receptors in nanomolar concentrations and decrease neuronal excitability by enhancing tonic conductance mediated by the δ subunit (Fodor et al., 2005; Stell et al., 2003). Some studies have shown that low concentrations of ethanol (1–30mM) potentiate extrasynaptic receptors (≤ 5% of all receptors), predominantly composed of the non-BZ sensitive α4, α6 and δ subunits, but have no effect on receptors with γ2 subunits, i.e. the vast majority of synaptic GABAA receptors (Hanchar et al., 2006; Krystal et al., 2006; Olsen et al., 2007; Sundstrom-Poromaa et al., 2002). This is relevant because these relatively low concentrations of ethanol are experienced in social drinking; for example, 17mM is the legal upper limit of intoxication in most US States (Lovinger and Homanics, 2007). These findings have not, however, been replicated in other labs (Borghese and Harris, 2007; Korpi et al., 2007). In addition, it is currently a subject of debate as to whether low concentrations of alcohol affect only α4, α6 and δ subunits (Lovinger and Homanics, 2007), For example, it has been shown that co-application of low concentrations of ethanol (17mM) influences channel potentiation of at least the α1β2γ2 receptor by neurosteroids (Akk et al., 2007). Moreover, α1δ subunit assemblies that are highly sensitive to low concentrations of ethanol and mediate tonic inhibitory currents have recently been detected in hippocampal interneurons (Glykys et al., 2007). Taken together, the importance of tonic inhibition for vulnerability to alcoholism has yet to be clearly elucidated. Moreover, the balance between tonic and phasic inhibition may shift towards the direction of phasic inhibition during chronic ethanol consumption. Rats exposed to the CIE model of binge drinking (described later) showed a net loss of extrasynaptic, and a gain of synaptic, hippocampal GABAA receptor responsiveness to ethanol concomitant with an increase in central synaptic localization of α4, but not δ subunits (Liang et al., 2006). The fact that this switch corresponds with the development of tolerance to the sedating / hypnotic effects of alcohol suggests that it may be important in the pathway to addiction (Liang et al., 2006).
GABAA receptors and the dopamine reward pathway
The mesolimbic dopamine (DA) system is implicated in the development of all addictions and is also stimulated by stress. This “reward” pathway originates in the ventral tegmental area (VTA) of the midbrain and projects to the nucleus accumbens (NAC), the limbic system and the orbitofrontal cortex. The amygdala, hippocampus and medial prefrontal cortex send excitatory projections to the NAC. The feeling of euphoria experienced by humans subsequent to drug ingestion is associated with increased synaptic DA in the reward pathway that is entwined with complex changes in numerous neurotransmitters including GABA, glutamate, serotonin (5-HT), opioid peptides and cannabinoids. GABAA receptors in the central nucleus of the amygdala appear to be important for oral ethanol self-administration in rodents (McBride, 2002).
GABAergic projections to the VTA come from several regions including the NAC and the ventral pallidum, however the primary inhibitory regulation of DA neurons is by GABAergic interneurons within the VTA (Johnson and North, 1992; Steffensen et al., 1998). It has been shown that VTA GABA neurons are coupled electrically via gap junctions (Stobbs et al., 2004). The predominant GABAA receptors expressed in the VTA are α2, α3, α4, β1, β3, and γ2 (Okada et al., 2004). In rats, acute ethanol exposure (20–80mM) excites DA neurons directly but also indirectly by reducing the firing rate of VTA GABAergic neurons (Gallegos et al., 1999; Xiao et al., 2007) and by influencing NMDA receptor-mediated excitation (Stobbs et al., 2004). Chronic exposure to ethanol enhances baseline activity of GABA neurons and induces tolerance to ethanol inhibition of the firing rate (Gallegos et al., 1999).
Human and animal studies implicate the opioid system, particularly β-endorphin and its receptor, µ opioid, in sensitivity to the rewarding or reinforcing effects of ethanol. The β-endorphin released by acute ethanol consumption is suppressed in chronic consumption and may be a factor in craving or negative reinforcement (Oswald and Wand, 2004). In the VTA, µ opioid receptors are expressed not on DA neurons but on GABA interneurons. Hyperpolarization of these GABAergic neurons by opiates results in increased firing of DA neurons (Johnson and North, 1992).
In non-drug dependent rodents, opiates can produce their acute rewarding effects through a DA-independent system mediated through brainstem reward circuits (Laviolette et al., 2004) however, in addicted animals the motivational effects of opiates derive from the mesolimbic DA system (Laviolette et al., 2002; Nader et al., 1997). GABAA receptors in the VTA control this bi-directional reward signaling; chronic opiate exposure and withdrawal induces CREB phosphorylation in a subset of GABAA receptors that switches their functional conductance properties from an inhibitory to an excitatory signaling mode (Laviolette et al., 2004). This intriguing switching system has been shown to have considerable plasticity in rats and it may have implications for the switch from controllable heavy drug use to addiction in humans.
Plasticity of GABAA receptor subunits in chronic ethanol consumption and withdrawal
Studies using animal models of addiction or human postmortem brain samples have shown that long-term ethanol use results in differential alteration in GABAA receptor subunit expression in different brain regions.
The human superior frontal cortex is selectively damaged in chronic alcohol abuse with neuronal loss. Two RT/PCR studies that were controlled for age and post-mortem delay found elevated or trend α1 mRNA expression in the superior frontal cortex of alcoholics compared with controls but no difference for α2, α3 or α4 expression (Lewohl et al., 1997; Mitsuyama et al., 1998). Moreover, the total concentration of α subunits was greater in alcoholics compared with controls, in line with findings of increased GABAA (but not NMDA) binding in alcoholic frontal cortex reported in an earlier study (Dodd et al., 1992). There was one positive study for increased β3 mRNA expression in alcoholics in frontal cortex (Mitsuyama et al., 1998) but another similar study was negative for both β2 and β3 (Buckley and Dodd, 2004).
In contrast, a consistent finding in rodents is that chronic ethanol consumption decreases cortical mRNA and peptide levels of α1 subunits but increases α4 levels (Devaud et al., 1996, 1997; Grobin et al., 1998; Matthews et al., 1998). Cortical peptide levels for β2/3 and γ1 and mRNA for γ2S increase in ethanol-dependent rats (Devaud et al., 1997).
Cynomolgus macaques who self-administered freely available alcohol at 2g/kg/day on average for 18 months showed significant alterations in current desensitization in the basolateral amygdala that were accompanied by significantly decreased mRNA levels of α2 and α3 subunits, a trend decrease of α1 but no impact on α4 (Floyd et al., 2004). Moreover mRNA expression of β1 and γ2 was reduced in total amygdala samples (Anderson et al., 2007). It has been shown that after 14 days of chronic alcohol consumption there was a decrease of α1 and α4 subunit peptide expression in the amygdala, a decrease of α4 in the NAC but no change in subunit expression in the VTA (Papadeas et al., 2001).
In rats, the chronic intermittent ethanol (CIE) model (≥ 60 doses), with persistent cycles of self-administration and withdrawal, mimics ‘binge’ drinking in humans (Olsen et al., 2005). CIE rats show GABAA receptor changes specifically in the hippocampus, including decreased α1 and δ subunit peptide expression (Cagetti et al., 2003) and elevated mRNA levels of α4, γ1 and the short splice variant (γ2S) of γ2 (Cagetti et al., 2003; Mahmoudi et al., 1997; Petrie et al., 2001). A model of chronic ethanol consumption (40 days) has also been shown to increase α4 subunit (but no other subunit) peptide expression in the hippocampus (Matthews et al., 1998).
A single-photon emission computed tomographic scan (SPECT) imaging study showed that at one week of abstinence from alcohol, binding of 123I-iomazenil to GABAA BZ receptors was higher throughout the brain, including the hippocampus and amygdala, but particularly in the frontal cortex, in alcoholic non-smokers compared with controls (Staley et al., 2005). This difference normalized after one month of abstinence. Frontal isotope uptake corresponded with severity of alcohol withdrawal and the number of days since the last drink (Staley et al., 2005). Therefore either the expression of GABAA receptors increased with chronic drinking in line with the findings in postmortem brain samples (Lewohl et al., 1997) and subsequently declined with abstinence, or GABAA receptors increased in acute withdrawal before gradually declining. In line with these results, a proton magnetic resonance spectroscopy (1H-MRS) study has shown that non-smoking alcoholics had higher GABA levels at one week of abstinence than controls but after one month, GABA levels were the same (Mason et al, 2006).
Studies have shown that the clinical course of alcoholism differs in smokers and non-smokers. Amongst current alcoholics, 35% are also nicotine dependent (Grant et al., 2004). Either drug may increase the rewarding effects and/or reduce the aversive effects (such as withdrawal) of the other. In contrast to the non-smoking alcoholics described above, GABAA receptor binding and GABA levels in alcoholic smokers did not differ from controls at one week of abstinence (Mason et al., 2006; Staley et al., 2005). Moreover, a 1H-MRS study in non-alcoholics showed no difference in cortical GABA levels between smokers and non-smokers (Epperson et al., 2005). These results suggest that smoking may ameliorate some of the effects of chronic alcohol consumption, perhaps in part by influencing GABA synthesis and the density of GABAA receptors that share structural and functional homology with nicotinic acetylcholine receptors. Nicotine is known to stimulate GABA neuronal activity, at least in the hippocampus (Barik and Wonnacott, 2006). One mechanism for this may be that α7 nicotinic receptors frequently occur close to synaptic GABAA receptors and α7 nicotinic agonists are implicated in the postsynaptic modulation of GABAA receptors in the hippocampus (Wanaverbecq et al., 2007; Zhang and Berg, 2007). However, other studies have shown that neurobiological recovery in alcoholics (gauged by common brain metabolite concentrations) in the first few weeks of abstinence is adversely affected by chronic smoking (Durazzo et al., 2006). Taken together, these studies suggest that GABAA receptor subunit plasticity may not underlie the damage observed in the frontal lobes of severe alcoholics. Further studies are needed in this area.
During the peak of behavioral withdrawal (1 day) after chronic ethanol treatment in rats, mRNA expression for β2, β3 and γ1 significantly increase whereas α1, α4 and γ2S expression rebounds back to control levels (Devaud et al., 1996). However, in the CIE model with numerous cycles of drinking and withdrawal that more closely mimic binge drinking in humans, subunit changes (decreased α1, δ, elevated α4, γ1 and γ2S) persist beyond acute withdrawal (2 days) (Cagetti et al., 2003). GABRG2 has also been associated with alcohol withdrawal severity in mice (Buck and Finn, 2001; Hood et al., 2006).
Taken together, the studies in rodents indicate that chronic ethanol consumption decreases cortical and hippocampal α1 subunit levels but increases α4 , γ1 and γ2S levels however there are regional differences in receptor adaptations, such as in the amygdala and the NAC. There are conflicting results between studies in rodents and human postmortem brain: the latter show increased α1 mRNA expression in the cerebral cortex. However these postmortem results were from severe alcoholics with considerable phenotypic and comorbidity variability and are not likely to be comparable to controlled animal models.
Mechanisms for the effects of chronic ethanol on GABAA subunits include modification of gene transcription or post-translation modification, synaptic or extracellular localization, and ethanol induced neurosteroid changes (Kumar et al., 2004). For example, the ethanol-induced internalization or endocytosis of cortical α1 leads to decreased cell surface and increased intracellur receptors (Kumar et al., 2003). Also, within rat hippocampus chronic ethanol consumption induces down-regulation of tyrosine kinase phosphorylation of α1 subunits, up-regulation of β2 subunits and no change in γ2 subunits (Marutha Ravindran et al., 2007).
At the moment, there is little evidence from animal models to determine whether receptor plasticity induced by chronic alcohol consumption reverts to pre-drinking levels after protracted withdrawal. On the other hand, the spectroscopy study in humans described above (Mason et al, 2006) showed that after one month of abstinence GABA levels in recovering alcoholics reverted to the same levels as in controls. This would suggest that GABAA receptor changes induced by chronic ethanol consumption may not be markers of addiction. Further animal and human studies of long-term withdrawal are required before this issue can be addressed.
GABAA receptors, stress and ethanol
Alcohol may be consumed in excess as a coping mechanism for stress and the altered homeostasis subsequent to addiction can result in stress upon withdrawal (Thomas et al., 2003; Wand, 2005). GABAergic neurotransmission is likely to be important in addiction-associated stress because GABA modulates emotion and response to stress. GABA inhibits, whereas glutamate activates, the hypothalamic-pituitary-adrenal axis (HPA) responses to stress (Herman et al., 2004). Acute stress immediately reduces GABA-stimulated chloride influx in the frontal cortex and amygdala (Martijena et al., 2002). Corticotropin releasing hormone (CRH), the primary mediator of the mammalian neuroendocrine stress response, is localized and co-synthesized within GABAergic neurons in the central amygdala, and in this location CRH1 receptors have been shown to mediate ethanol enhancement of GABAergic synaptic transmission (Nie et al., 2004). GABAA receptors in this region play an important role in ethanol self-administration in rodents (McBride, 2002).
Socially isolated rats exhibit anxious behavior accompanied by increased plasma corticosterone together with diminished levels of neurosteroids and brain GABAA receptor function (Serra et al., 2000). Adult rats that have been subjected to early life stress (maternal separation / handling) have a more active stress response (Hsu et al., 2003). Epigenetic effects on receptors implicated in stress, such as glucocorticoid and GABAA, have been demonstrated in rodents. For example, BZ receptor levels in the adult rat central nucleus of the amygdala are highly correlated with the frequency of maternal licking/grooming over the first week of life (Caldji et al., 1998). Early life stress in rats permanently alters GABAA receptor subunit expression in the hippocampus such that α2 subunits predominate in the stressed animals whereas α1 predominates in emotionally healthy animals (Hsu et al., 2003). Early life maternal neglect also results in increased levels of α3 and α4 in the adult rat amygdala whereas enriched maternal care in the first week of life results in increased α1 and β3 mRNA levels almost everywhere, including the hippocampus and amygdala, and increased β2 and γ2 in the amygdala. These effects can be reversed by cross-fostering (Caldji et al., 2003).
Early life stress affects ethanol consumption and preference in adult male, but not female, rats (Moffett et al., 2007). It is not known whether similar epigenetic effects exist in humans however severe childhood stressors, especially emotional, physical and sexual abuse, have been associated with increased vulnerability to adult psychopathology (Verona and Sachs-Ericsson, 2005; Wilsnack et al., 1997).
Ethanol and neurosteroids
In vivo, GABAA receptors are exposed to endogenous neurosteroids that include progesterone and its metabolites: 3α-5 α-THP (allopregnanolone or ALLO) and 3α-5 α-THDOC (allotetrahydrodeoxycorticosterone) (Finn et al., 2004a). The effects of ALLO appear to be primarily mediated via its interactions with GABAA receptors (Herd et al, 2007). Ethanol and stress both stimulate neurosteroid synthesis from cholesterol in the brain. Neurosteroids bind to GABAA receptors at low (nanomolar) concentrations and potentiate GABA currents (Biggio et al., 2007). Consistent with this action, acute exposure to progesterone or ALLO is anxiolytic (Gulinello and Smith, 2003). In healthy humans, ethanol induces changes in ALLO concentration that correlates with alcohol liking and desire for more alcohol (Pierucci-Lagha et al., 2006). Pre-treatment with finasteride (5α-reductase inhibitor) that reduces the synthesis of ALLO and THDOC, diminishes the subjective response to a moderate dose of alcohol in social drinkers (Pierucci-Lagha et al., 2005). Several studies in rodents have shown that ethanol-induced neuroactive steroids contribute to the acute behavioral effects of ethanol such as sedative-hypnotic (Van Doren et al., 2000; Khisti et al., 2003), anti-depressant (Hirani et al., 2002) and anxiolytic (Hirani et al., 2005). Moreover a relationship exists between the time course of ethanol-induced neurosteroids and specific behavioral and neural effects of ethanol (Morrow et al., 2001). Thus neuroactive steroids contribute to ethanol sensitivity and may influence the risk for addiction (Morrow et al., 2006).
Basal brain and plasma ALLO levels are higher in females than males and are influenced by hormonal fluctuations however stress can elevate male ALLO levels to female levels (Barbaccia et al., 2001). It is therefore not surprising that sexually dimorphic effects have been noted in the interaction of ethanol and neurosteroids. ALLO pretreatment significantly increases voluntary ethanol consumption in male but not female mice (Sinnott et al., 2002) and intra-cranial injection of ALLO modulates the onset and maintenance of ethanol self- administration but does not affect appetitive (ethanol seeking behaviors) (Ford et al., 2007). In contrast, very high pre-treatment doses of ALLO suppresses ethanol intake (Ford et al., 2005a). Chronic (17 days) self administration of limited access ethanol has been shown to elevate endogenous brain ALLO levels in male but not female mice (Finn et al., 2004b).
Rats withdrawing from ethanol are sensitized to the anticonvulsant effects of neurosteroids. Potentiation of GABA currents is enhanced up to 50% in withdrawal by ALLO and THDOC (Devaud et al., 1996). THDOC decreases neuronal excitability by enhancing tonic extracellular inhibitory conductance mediated by the δ subunit (Stell et al., 2003). Ethanol withdrawal-prone mice exhibit decreased ALLO levels during withdrawal plus tolerance to ALLO’s anticonvulsant effect (Finn et al., 2004a). Pretreatment with finasteride increases acute withdrawal seizures in female mice but decreases withdrawal severity in male mice (Gorin-Mayer et al., 2007). Finasteride initially diminished ethanol consumption in male mice that were established drinkers but heavy drinking eventually re-established indicating compensatory changes in neurosteroid modulation of GABAergic tone (Ford et al., 2005b). Ethanol-induction of neurosteroids is diminished in tolerant and dependent rodents (Morrow et al, 2001). These studies indicate that neurosteroids, synthesized in response to ethanol consumption with effects primarily mediated through GABAA receptors, appear to ameliorate some of the negative aspects of ethanol, particularly during withdrawal. However, the effects on neurosteroids may be more pronounced in males.
Protein regulation of GABAA receptors
GABAA receptors are regulated by several signaling proteins including protein kinase C and A (PKC and PKA) (Chen and Olsen, 2007; Kumar et al., 2004). GABAA receptor function can be modulated by protein phosphorylation at sites thought to be located in the intracellular domain between TM3 and TM4. The PKC family of serine-threonine kinases influences receptor sensitivity to positive allosteric modulators such as ethanol, neurosteroids and BZs (Hodge et al., 1999; Kumar et al., 2004; Song and Messing, 2005) and has been implicated in animal models of alcohol addiction (Newton and Ron, 2007). PKA regulation of GABA-activated currents occurs through phosphorylation at highly conserved sites in the intracellular domain of β subunits, the effect depending on the particular β subunit isoform (McDonald et al., 1998). PKA has been implicated in anxiety and drinking behavior (Wand, 2005). A range of proteins that associate with individual GABAA subunits and play important roles in regulation have recently been found and are described elsewhere (reviewed in Chen and Olsen, 2007).
Association between GABAA receptor genes and alcoholism in humans
Genes for the GABAA receptor subunit isoforms are clustered in several chromosomal regions, including 4p13-q11 (α2, α4, β1, γ1), 5q34–q35 (α1, α6, β2, γ2) and 15q11–q13 (α5, β3, γ3) as well as Xq28 (α3, β4, ε1) (Barnard et al., 1998). Alternative splicing has been demonstrated for most of the GABAA receptor subunit isoforms indicating complexity in gene expression (Jin et al., 2004; Tian et al., 2005). From Figure 1 it can be seen that there is considerable conservation and linkage disequilibrium extending across long stretches of sequence in the chromosome 4 and 5 clusters but less so in the chromosome 15 cluster. Do these gene clusters have significance? The most abundant GABAA receptor subunit complex (α1, β2, γ2 (60%)) that has widespread distribution in adult brain originates from the chromosome 5 gene complex. The mRNAs from the chromosome 4 cluster genes predominate in rat embryo but these genes are generally down regulated in the adult rat except in the hippocampus, the majority of DA neurons in the substantia nigra and the VTA where they are highly expressed (Okada et al., 2004; Steiger and Russek, 2004; Wisden et al., 1992). Thus the chromosome 4 cluster of genes are likely to be important in addiction and anxiety and may be vulnerable to epigenetic effects in early development, as indicated above for GABRA2 (Hsu et al., 2003). Moreover, the anxiolytic effects of BZs appear to be mediated in part by GABRA2; mice with a GABRA2 knock-in point mutation are insensitive to BZs’ anxiolytic effects (Dias et al., 2005; Low et al., 2000).
FIGURE 1. Chromosomal clusters for GABAA receptor genes in the HapMap Caucasian population.
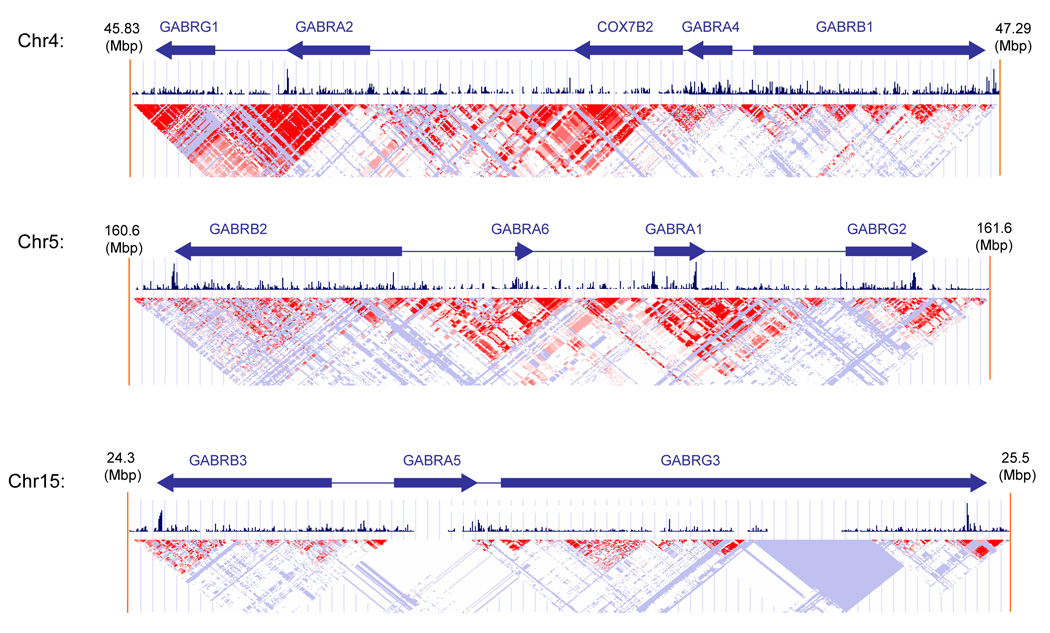
Arrows indicate gene position, size and direction of transcription. Red triangles show areas of linkage disequilibrium with D’ ≥ 0.8. Conservation (vertical lines) across 17 vertebrate species, from zebrafish to humans is show across the chromosomal regions.
GABAA receptor chromosome 4 gene cluster
Earlier genomewide scans in American Indians and Caucasians have provided evidence for linkage of alcohol dependence and relapse-associated β EEG to chromosome 4p at the location of the GABAA gene cluster (Ghosh et al., 2003; Long et al., 1998; Porjesz et al., 2002; Reich et al., 1998; Zinn-Justin and Abel, 1999). Subsequently, several studies, nearly all in Caucasians, have found haplotype and SNP associations between GABRA2 and alcoholism. All these studies, together with HapMap (Figure 2), have identified the same two GABRA2 haplotype blocks, at least within Caucasians, American Indians and Asians. The significant association signals have been within the haplotype block that extends downstream from intron 3. Within this block numerous SNPs are in allelic identity resulting in two major yin-yang haplotypes of similar frequency that account for nearly all of the haplotype diversity in Caucasians and Asians (Figure 3).
FIGURE 2. GABRA2 gene structure.
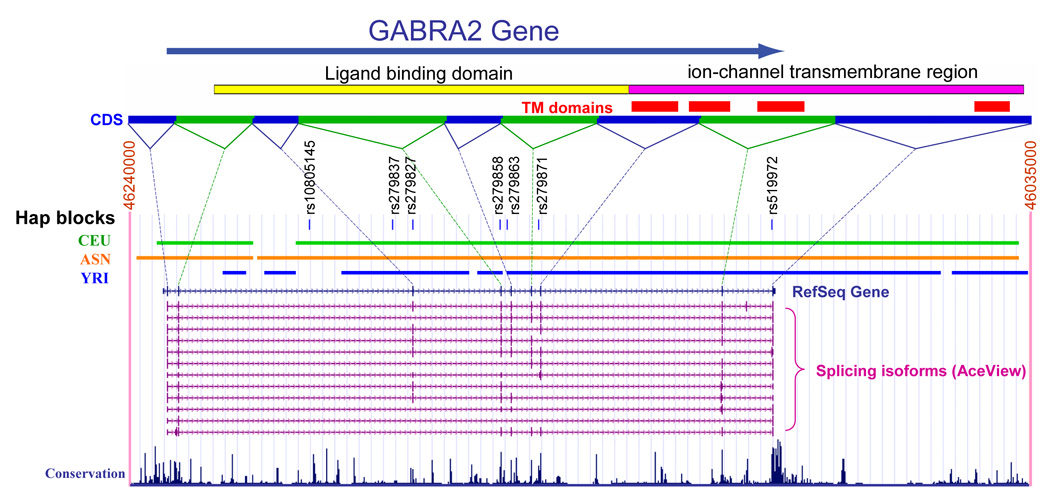
The large arrow indicates the direction of transcription. CDS = coding sequence are shown, linked by dotted lines to the relevant exons. TM domains = transmembrane domains, numbering 1 through 4 from left to right. The locations of the TM domains within the coding sequences are shown. HapMap population blocks are indicated: CEU = Caucasian; ANS = Asian; YRI = African. The structure of 12 isoforms is shown. Conservation across 17 vertebrate species, from zebrafish to humans is indicated. Information derived from HapMap and the UCSC genome browser: www.genome.ucsc.edu
FIGURE 3. GABRA2 common haplotypes for HapMap Asian, African and Caucasian samples.
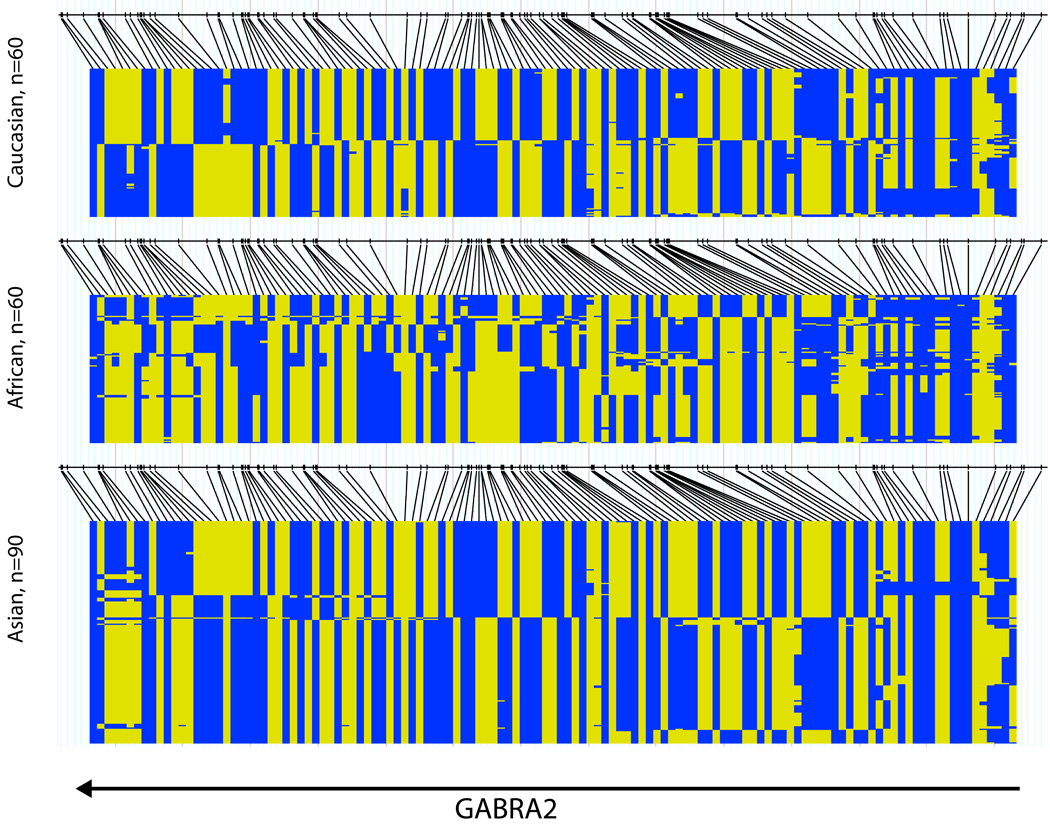
Black lines indicate the genotyped SNPs; the two colors indicate the two alleles for each SNP. All 3 populations show 2 haplotype blocks with the block boundary in intron 3. The Asian and Caucasian populations both have 2 common haplotypes in the distal haplotype block. The Africans show more haplotype diversity.
The first GABRA2 association study came from the Collaborative Study on the Genetics of Alcoholism (COGA) that has a dataset compiled from six centers across the USA. COGA performed a family-based association study on 2282 individuals, 41% with lifetime alcoholism and 29% with lifetime illicit drug dependence, from 262 multiplex families with a high density of alcoholism, genotyped across the whole chromosome 4 gene cluster. Probands were treatment-seeking alcoholics. Using the pedigree disequilibrium test, Edenberg et al. (2004) found that the more abundant of the two common haplotypes was a significant risk factor for alcohol dependence and was also associated with β EEG power, an intermediate phenotype for alcoholism. Two subsequent studies using the same COGA dataset showed that the signal for the earlier reported association came only from the alcoholics with comorbid illicit drug dependence (Agrawal et al., 2006) and that GABRA2 was significantly associated with childhood conduct disorder symptoms, but not alcohol dependence symptoms, in children (Dick et al., 2006a). A recent case-control study in German treatment-seeking alcoholics supports the COGA finding of an association between the more abundant haplotype and alcoholism (Soyka et al., 2008).
In contrast to the above studies, the less frequent of the two yin-yang haplotypes was found to be significantly more abundant in treatment-seeking alcoholics than controls in several case-control studies: U.S. Caucasians (Covault et al., 2004), Russians (Lappalainen et al., 2005), and Germans (Fehr et al., 2006). Covault et al. (2004) found that the association was strongest in the alcoholics who did not have drug dependence or major depressive episode; this was opposite to the COGA finding (Agrawal et al., 2006). Fehr et al. (2006) showed that the frequency of the risk haplotype was positively correlated with alcoholism severity, gauged by withdrawal seizures and early onset of dependence. The G allele of a synonymous exonic GABRA2 SNP rs279858, a tag allele for this same less frequent alcoholism risk haplotype, was associated with a higher daily probability of drinking and heavy drinking during the 12-week treatment and 12-month post-treatment period in alcoholics from the Project MATCH study (Bauer et al., 2007). This GABRA2 rs279858 G allele was also associated with decreased pleasurable effects for alcohol in social drinkers (Pierucci-Lagha et al., 2005). Furthermore, the subjective experiences of the social drinkers who had the rs279858 protective AA genotype could be manipulated by pre-treatment with finasteride, a metabolism blocker of the endogenous neurosteroids ALLO and THDOC,that diminished their subjective experiences of alcohol (Pierucci-Lagha et al., 2005). There was no effect of neurosteroid blockade on individuals with the rs279858 G risk allele, possibly because of a floor effect.
The results from the studies described above appear to indicate that both of the two abundant, yin yang haplotypes can be risk factors for alcoholism, at least in Caucasians. Supportive evidence for this apparent paradox comes from a study in non-treatment seeking alcoholics in two population isolates, Finnish Caucasians and Plains American Indians (Enoch et al., 2006). This study showed that although there was no haplotype association with alcoholism per se, alcoholics with high trait anxiety (TPQ harm avoidance) had the highest frequency of the more abundant haplotype, alcoholics with low anxiety had the highest frequency of the less abundant haplotype and non-alcoholics had intermediate frequencies (Enoch et al., 2006). Alcoholics, including those with antisocial personality disorder, have been shown to have higher trait anxiety than non-alcoholics (Ducci et al., 2007; Goodwin and Hamilton, 2003). If trait anxiety does indeed play a role in mediating linkage of GABRA2 haplotypes with alcoholism then the Enoch et al. (2006) study would predict that COGA alcoholics have higher trait anxiety than the treatment seeking alcoholics of other studies (Covault et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005). Indeed, it has been demonstrated that COGA alcoholics have higher trait anxiety than non-alcoholics (Ducci et al., 2007). Moreover, supportive evidence comes from the fact that in the Covault et al. (2004) study the effect got stronger when alcoholics with major depression (who are likely to have high trait anxiety) were removed. It is noteworthy that the linkage of a disorder with both alleles has been found previously, for example alcoholism has been associated with both the L and S alleles of the serotonin transporter promoter polymorphism HTTLPR (Feinn et al., 2005; Hu et al., 2005).
A few studies have found no GABRA2 association with alcoholism, either in U.S. Caucasians (Covault et al., 2008 (Project MATCH dataset); Drgon et al., 2006; Matthews et al., 2007) or African Americans (Covault et al., 2008). Some of these negative findings may be due to sample selection or lack of power (Drgon et al., 2006; Matthews et al., 2007) although the Project MATCH study included over 700 alcoholics. Another issue may be sexual dimorphism. As described above, Enoch et al. (2006) found significant anxiety-mediated GABRA2 haplotype associations with alcoholism in Finnish Caucasian and American Indian men but found no association in American Indian women. The negative study in African Americans (Covault et al., 2008) may be attributable to the fact that in this group the two yin yang risk haplotypes are present at much lower frequencies (29% and 27%) compared with other populations (Enoch, unpublished data; Figure 3). Finally, population stratification may be an issue.
Although further studies that include alcoholism subtypes, different ethnic groups and also women are clearly needed, there appears to be good evidence for a fundamental association between GABRA2 and alcoholism. Many of the SNPs in allelic identity, such as rs279858 (discussed above) and rs279863 (Figure 2), are conserved across species indicating the likelihood of selective pressure for the GABRA2 region distal to intron 3. One recent study has shown that GABRA2 mRNA levels in post-mortem prefrontal cortical tissue differed significantly according to genotype (Haughey et al., 2007). However, no functional polymorphism has as yet been found There are numerous alternative splicing isoforms, most of which differ in their 3’ end after exon 3 (Figure 2) and these may be implicated in function. Within the brain there are four major isoforms with alternative 5’ (exon 1A or IB) and 3’ exons (exon 9A or 10) as well as minor isoforms lacking exon 4 or exon 8 (Tian et al, 2005; Jin et al, 2004). The functional significance of these isoforms is at present unknown. SNP rs279827 (Figure 2) is near the acceptor site of intron 4 and thus might affect splicing efficiency.
Studies in the COGA dataset have found no association between alcohol or illicit drug dependence and GABRA4 (5 SNPs), GABRB1(6 SNPs) or GABRG1(6 SNPs) (Edenberg et al., 2004; Agrawal et al., 2006). However two GABRG1 SNPs in one COGA study showed trend level association with alcoholism (Edenberg et al., 2004). A recent study has shown significant haplotype and SNP association with alcoholism in a haplotype block that extends from the intergenic region between GABRA2 and GABRG1 (Figure 1) up to GABRG1 intron 3 in two large groups of U.S. Caucasians but not in African Americans (Covault et al., 2008). As the HapMap block structure indicates, there is extended LD between GABRA2 and GABRG1 (Figure 1). Covault et al. (2008) concluded that their earlier GABRA2 association (Covault et al., 2004) may in part be secondary to LD with risk-related variants in GABRG1 although there may be separate contributions to alcoholism risk from the two genes. One study found an association between a GABRB1 intron 8 tetranucleotide repeat and alcoholism (Parsian and Zhang, 1999). A COGA study found consistent, albeit weak, LD between GABRB1 and alcoholism (Song et al., 2003). A recent study found GABRB1 haplotype linkage to alcoholism in both Caucasians and American Indians in both proximal and distal haplotype blocks of this large gene (Enoch et al., 2005).
GABAA receptor chromosome 5 and chromosome 15 gene clusters
The cluster 5 and cluster 15 genes (Figure 1) are not as strong candidates for alcoholism as are the cluster 4 genes for reasons discussed above. Indeed, the results of association studies have been mixed. Although COGA initially found no linkage between chromosome 5 genes and alcoholism (Dick et al., 2005; Song et al., 2003) a secondary analysis showed association of GABRA1 with measures of drinking severity: history of blackouts, age at first drunkenness, level of response to alcohol (Dick et al., 2006b). A study in two population isolates: Finnish Caucasians and Southwestern American Indians, found sibpair linkage of alcohol dependence to GABRG2 in the Finns and associations in both populations with GABRB2 and GABRA6 SNPs (Radel et al., 2005). Markers in GABRA6 and GABRB2 have been associated with alcohol dependence as well as Korsakoff’s psychosis in a Scottish study (Loh et al., 1999). The Pro385Ser substitution in GABRA6 has been associated with a lower level of response to the sedating effects of alcohol (Hu et al., 2005), and to reduced sensitivity to the effects of diazepam in children of alcoholics (Iwata et al., 1999) and a SNP in the 3’ UTR (T1521C) has been associated with variation in physiological response to psychological stress (Uhart et al., 2004). There has been a positive association with GABRG2 and antisocial alcoholism in Japanese (Loh et al., 2000) but negative studies for GABRB2 and GABRG2 in Germans (Sander et al., 1999)
Studies using the COGA dataset found evidence of haplotype and SNP association between alcohol dependence and GABRG3 (Dick et al., 2004) and evidence for imprinting in that paternal, but not maternal, transmission of GABRA5 and GABRB3 alleles showed association with alcoholism (Song et al., 2003).
GABAA receptors as therapeutic targets for alcoholism
Benzodiazepines are commonly used to ameliorate the symptoms of acute withdrawal from alcohol, however there is an increasing trend to instead use anti-convulsants such as carbamezepine that are more effective in preventing rebound withdrawal symptoms and reducing post-treatment drinking than BZs (Malcolm et al., 2002; Mueller et al., 1997). The sedative-anticonvulsant chlomethiazole that may act at the anesthetic binding site on GABAA receptors (Usala et al., 2003) is widely used in Europe in acute withdrawal. Other, newer anti-convulsants that are considered to act primarily via an effect on GABA may prove useful in the treatment of alcoholism (Czuczwar and Patsalos, 2001). For example, topiramate, which has a complex effect on GABAA receptors (Gordey et al., 2000) has been shown to reduce the percentage of heavy drinking days and other drinking outcomes in recovering alcoholics (Johnson et al., 2007). Finally, as this review has indicated, neuroactive steroids are likely to be promising new drug targets for the treatment of alcoholism.
Conclusion
The modulation of GABAA receptors by ethanol is complex and involves numerous interactions including with neurosteroids, protein regulators, stress hormones and neurotransmitters such as opioids. Although substantial progress has been made in recent years we are still far from understanding the GABAA receptor changes associated with the switch from heavy drinking to alcohol addiction. Recent studies are beginning to shed light on associations between GABAA receptor genes and alcoholism although functional polymorphisms have yet to be determined. The relatively new field of epigenetics is emerging and studies of gene × environment interactions are likely to yield further insights into the role that GABAA receptors play in the development of alcoholism.
ACKNOWLEDGEMENTS
I am grateful to Dr Qiaoping Yuan for assistance with bioinformatics and preparation of the figures. This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, et al. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- Akk G, Li P, Manion BD, Evers AS, Steinbach JH. Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the alpha1beta2gamma2L GABAA receptor. Mol Pharmacol. 2007;71:461–472. doi: 10.1124/mol.106.029942. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–628. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, et al. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcohol Clin Exp Res. 2007;31:1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. Distribution, prevalence, and drug binding profile of gamma-aminobutyric acid type A receptor subtypes differing in the beta-subunit variant. J Biol Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–171. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van der Oost J, Smit AB, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Finn DA. Genetic factors in addiction: QTL mapping and candidate gene studies implicate GABAergic genes in alcohol and barbiturate withdrawal in mice. Addiction. 2001;96:139–149. doi: 10.1046/j.1360-0443.2001.96113910.x. [DOI] [PubMed] [Google Scholar]
- Buckley ST, Dodd PR. GABAA receptor beta subunit mRNA expression in the human alcoholic brain. Neurochem Int. 2004;45:1011–1020. doi: 10.1016/j.neuint.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U.S.A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem Soc Trans. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet (Neuropsychiatr Genet) 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′-Region of GABRG1 Associate to Alcohol Dependence and are in Linkage Disequilibrium with Markers in the Adjacent GABRA2 Gene. Neuropsychopharmacology. 2008;33:837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuczwar SJ, Patsalos PN. The new generation of GABA enhancers. Potential in the treatment of epilepsy. CNS Drugs. 2001;15:339–350. doi: 10.2165/00023210-200115050-00001. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, et al. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Kuperman S, Schuckit M, et al. Association of GABRG3 with alcohol dependence. Alcohol Clin Exp Res. 2004;28:4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Hesselbrock V, Schuckit M, et al. No association of the GABAA receptor genes on chromosome 5 with alcoholism in the collaborative study on the genetics of alcoholism sample. Am J Med Genet B Neuropsychiatr Genet. 2005;132:24–28. doi: 10.1002/ajmg.b.30058. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006a;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Plunkett J, Wetherill LF, Xuei X, Goate A, Hesselbrock V, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006b;30:1101–1110. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Thomas GJ, Harper CG, Kril JJ. Amino acid neurotransmitter receptor changes in cerebral cortex in alcoholism: effect of cirrhosis of the liver. J Neurochem. 1992;59:1506–1515. doi: 10.1111/j.1471-4159.1992.tb08467.x. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Funt S, Virkkunen M, Albaugh B, Goldman D. Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol. 2007;41:3–12. doi: 10.1016/j.alcohol.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. Pharmacogenomics of alcohol response and addiction. Am J Pharmacogenomics. 2003;3:217–232. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Enoch M, Schwartz LS, Albaugh B, Virkkunen M, Goldman D. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Linkage of GABRB1 haplotypes to alcoholism in Caucasians and American Indians. Program No. 409.4. Online. [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A. Genetics, Stress and the Risk for Addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. New York: Elsevier Neuroscience; 2007. pp. 127–146. [Google Scholar]
- Epperson CN, O'Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004a;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004b;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Fodor L, Biro T, Maksay G. Nanomolar allopregnanolone potentiates rat cerebellar GABAA receptors. Neurosci Lett. 2005;383(1–2):127–130. doi: 10.1016/j.neulet.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005a;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005b;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–1053. [PubMed] [Google Scholar]
- Ghosh S, Begleiter H, Porjesz B, Chorlian DB, Edenberg HJ, Foroud T, et al. Linkage mapping of beta 2 EEG waves via non-parametric regression. Am J Med Genet (Neuropsychiatr Genet) 2003;118B:66–71. doi: 10.1002/ajmg.b.10057. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Hamilton SP. Lifetime comorbidity of antisocial personality disorder and anxiety disorders among adults in the community. Psychiatry Res. 2003;117:159–166. doi: 10.1016/s0165-1781(02)00320-7. [DOI] [PubMed] [Google Scholar]
- Gordey M, DeLorey TM, Olsen RW. Differential sensitivity of recombinant GABA(A) receptors expressed in Xenopus oocytes to modulation by topiramate. Epilepsia. 2000;41 Suppl 1:S25–S29. [PubMed] [Google Scholar]
- Gorin-Meyer RE, Wiren KM, Tanchuck MA, Long SL, Yoneyama N, Finn DA. Sex differences in the effect of finasteride on acute ethanol withdrawal severity in C57BL/6J and DBA/2J mice. Neuroscience. 2007;146:1302–1315. doi: 10.1016/j.neuroscience.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW, et al. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE. The human GABA(A) receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 2007 Nov 13; doi: 10.1111/j.1601-183X.2007.00369.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3 alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3alpha-hydroxy-5alpha-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, et al. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hood HM, Metten P, Crabbe JC, Buck KJ. Fine mapping of a sedative-hypnotic drug withdrawal locus on mouse chromosome 11. Genes Brain Behav. 2006;5:1–10. doi: 10.1111/j.1601-183X.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U.S.A. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Iwata N, Cowley DS, Radel M, Roy-Byrne PP, Goldman D. Relationship between a GABAA alpha 6 Pro385Ser substitution and benzodiazepine sensitivity. Am J Psychiatry. 1999;156:1447–1449. doi: 10.1176/ajp.156.9.1447. [DOI] [PubMed] [Google Scholar]
- Jin P, Fu GK, Wilson AD, Yang J, Chien D, Hawkins PR, Au-Young J, Stuve LL. PCR isolation and cloning of novel splice variant mRNAs from known drug target genes. Genomics. 2004;83:566–571. doi: 10.1016/j.ygeno.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Akabas MH, Harris RA. Functional and structural analysis of the GABAA receptor alpha 1 subunit during channel gating and alcohol modulation. J Biol Chem. 2005;280:308–316. doi: 10.1074/jbc.M409871200. [DOI] [PubMed] [Google Scholar]
- Jung S, Harris RA. Sites in TM2 and 3 are critical for alcohol-induced conformational changes in GABA receptors. J Neurochem. 2006;96:885–892. doi: 10.1111/j.1471-4159.2005.03617.x. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O'Buckley T, Morrow AL. Neuroactive steroid 3 alpha- hydroxy-5 alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–265. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, et al. Does ethanol act preferentially via selected brain GABAA receptor subtypes? The current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, et al. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Nader K, van der Kooy D. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res. 2002;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Expression of the alpha 1, alpha 2 and alpha 3 isoforms of the GABAA receptor in human alcoholic brain. Brain Res. 1997;751:102–112. doi: 10.1016/s0006-8993(96)01396-0. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh EW, Smith I, Murray R, McLaughlin M, McNulty S, Ball D. Association between variants at the GABAAbeta2, GABAAalpha6 and GABAAgamma2 gene cluster and alcohol dependence in a Scottish population. Mol Psychiatry. 1999;4:539–544. doi: 10.1038/sj.mp.4000554. Erratum in: Mol Psychiatry 2000 Jul;5(4):452. [DOI] [PubMed] [Google Scholar]
- Loh EW, Higuchi S, Matsushita S, Murray R, Chen CK, Ball D. Association analysis of the GABA(A) receptor subunit genes cluster on 5q33–34 and alcohol dependence in a Japanese population. Mol Psychiatry. 2000;5:301–307. doi: 10.1038/sj.mp.4000719. [DOI] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? High-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABA(A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Myrick H, Roberts J, Wang W, Anton RF, Ballenger JC. The effects of carbamazepine and lorazepam on single versus multiple previous alcohol withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17:349–355. doi: 10.1046/j.1525-1497.2002.10201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martijena ID, Rodriguez Manzanares PA, Lacerra C, Molina VA. Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse. 2002;45:86–94. doi: 10.1002/syn.10085. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of tyrosine kinase phosphorylation of the GABAA receptor subunits in the rat brain. Neurochem Res. 2007;32:1179–1187. doi: 10.1007/s11064-007-9288-y. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, et al. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The Role of the GABRA2 Polymorphism in Multiplex Alcohol Dependence Families With Minimal Comorbidity: Within-Family Association and Linkage Analyses. J Stud Alcohol Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minier F, Sigel E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:7769–7774. doi: 10.1073/pnas.0400220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama H, Little KY, Sieghart W, Devaud LL, Morrow AL. GABA(A) receptor alpha1, alpha4, and beta3 subunit mRNA and protein expression in the frontal cortex of human alcoholics. Alcohol Clin Exp Res. 1998;22:815–822. [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TI, Stout RL, Rudden S, Brown RA, Gordon A, Solomon DA, et al. A double-blind, placebo-controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcohol Clin Exp Res. 1997;21:86–92. [PubMed] [Google Scholar]
- Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Ron D. Protein kinase C and alcohol addiction. Pharmacol Res. 2007;55:570–577. doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts J, Madamba G, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Okada H, Matsushita N, Kobayashi K, Kobayashi K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem. 2004;89:7–14. doi: 10.1111/j.1471-4159.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Chang CS, Li G, Hanchar HJ, Wallner M. Fishing for allosteric sites on GABA(A) receptors. Biochem Pharmacol. 2004;68:1675–1684. doi: 10.1016/j.bcp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochem Res. 2005;30:1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the "one glass of wine" receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(-) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Parsian A, Zhang ZH. Human chromosomes 11p15 and 4p12 and alcohol dependence: possible association with the GABRB1 gene. Am J Med Genet. 1999;88:533–538. doi: 10.1002/(sici)1096-8628(19991015)88:5<533::aid-ajmg18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Petrie J, Sapp DW, Tyndale RF, Park MK, Fanselow M, Olsen RW. Altered GABAA receptor subunit and splice variant expression in rats treated with chronic intermittent ethanol. Alcohol Clin Exp Res. 2001;25:819–828. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, et al. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, et al. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci USA. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radel M, Vallejo RL, Iwata N, Aragon R, Long JC, Virkkunen M, et al. Haplotype-based localization of an alcohol dependence gene to the 5q34 {gamma}-aminobutyric acid type A gene cluster. Arch Gen Psychiatry. 2005;62:47–55. doi: 10.1001/archpsyc.62.1.47. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, et al. Actions of acute and chronic ethanol on presynaptic terminals. Alcohol Clin Exp Res. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Ball D, Murray R, Patel J, Samochowiec J, Winterer G, Rommelspacher H, Schmidt LG, Loh EW. Association analysis of sequence variants of GABA(A) alpha6, beta2, and gamma2 gene cluster and alcohol dependence. Alcohol Clin Exp Res. 1999;23:427–431. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Boulineau N, Minier F. Impact of subunit positioning on GABAA receptor function. Biochem Soc Trans. 2006;34:868–871. doi: 10.1042/BST0340868. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62(2):119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Song J, Koller DL, Foroud T, Carr K, Zhao J, Rice J, et al. Association of GABA(A) receptors and alcohol dependence and the effects of genetic imprinting. Am J Med Genet B Neuropsychiatr Genet. 2003;117:39–45. doi: 10.1002/ajmg.b.10022. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Staley JK, Gottschalk C, Petrakis IL, Gueorguieva R, O'Malley S, Baldwin R, et al. Cortical gamma-aminobutyric acid type A-benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005;62:877–888. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JL, Russek SJ. GABAA receptors: building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol Ther. 2004;101:259–281. doi: 10.1016/j.pharmthera.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Randall CL, Carrigan MH. Drinking to cope in socially anxious individuals: a controlled study. Alcohol Clin Exp Res. 2003;27:1937–1943. doi: 10.1097/01.ALC.0000100942.30743.8C. [DOI] [PubMed] [Google Scholar]
- Tian H, Chen H-J, Cross TH, Edenberg HJ. Alternative splicing and promoter use in the human GABRA2 gene. Brain Res Mol Brain Res. 2005;137:174–183. doi: 10.1016/j.molbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Uhart M, McCaul ME, Oswald LM, Choi L, Wand GS. GABRA6 gene polymorphism and an attenuated stress response. Mol Psychiatry. 2004;9:998–1006. doi: 10.1038/sj.mp.4001535. [DOI] [PubMed] [Google Scholar]
- Usala M, Thompson SA, Whiting PJ, Wafford KA. Activity of chlormethiazole at human recombinant GABA(A) and NMDA receptors. Br J Pharmacol. 2003;140:1045–1050. doi: 10.1038/sj.bjp.0705540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Sachs-Ericsson N. The intergenerational transmission of externalizing behaviors in adult participants: the mediating role of childhood abuse. J Consult Clin Psychol. 2005;73:1135–1145. doi: 10.1037/0022-006X.73.6.1135. [DOI] [PubMed] [Google Scholar]
- Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G. The anxious amygdala: CREB signaling and predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2697–2699. doi: 10.1172/JCI26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack SC, Vogeltanz ND, Klassen AD, Harris TR. Childhood sexual abuse and women’s substance abuse: national survey findings. J Stud Alcohol. 1997;58:264–271. doi: 10.15288/jsa.1997.58.264. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. GABAA receptor channels: from subunits to functional entities. Curr Opin Neurobiol. 1992;2:263–269. doi: 10.1016/0959-4388(92)90113-y. [DOI] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjevic K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn-Justin A, Abel L. Genome search for alcohol dependence using the weighted pairwise correlation linkage method: interesting findings on chromosome 4. Genet Epidemiol. 1999;17 Suppl 1:S421–S426. doi: 10.1002/gepi.1370170771. [DOI] [PubMed] [Google Scholar]


