Abstract
One of the most critical times in the human lifespan is the late embryonic/early postnatal period, due to the careful orchestration of numerous events leading to normal brain development. This period is also characterized by a heightened incidence of harmful events that act via the GABAergic system, including hypoxia-ischemia, seizures and drug exposure from maternal circulation (e.g. alcohol, barbiturates). Unfortunately, there are few effective means of attenuating damage in the immature brain. In the current investigation, we documented the effect of 17α-estradiol, a natural epimer of 17β-estradiol that has potent estrogen receptor-independent actions, on excessive GABAA receptor-induced damage to the neonatal brain. We observed that treatment with 17α-estradiol significantly attenuates the GABAA receptor-induced reduction in hippocampal volume and impaired hippocampal dependent performance on the Morris water maze and radial arm maze. 17α-estradiol mediated neuroprotection is hypothesized to be achieved by attenuating GABAA receptor-induced cell loss, assessed in primary hippocampal cultures using both the lactate dehydrogenase assay and TUNEL, with equivalent prevention of cell loss in the presence or absence of the estrogen receptor antagonist, ICI-182,780. These data highlight one of the initial investigations of the neuroprotective potential of 17α-estradiol in an in vivo model of injury to the immature brain.
Keywords: Development, Fetal Alcohol, GABA, Hippocampus, Hypoxia-Ischemia, Seizures, Steroid Hormones
The gonadal steroid 17β-estradiol, an important mediator of sexual differentiation of the developing brain (MacLusky and Naftolin, 1981; McEwen, 1981; Arnold and Gorski, 1984), is a potent neuroprotective agent in adult models of brain injury. 17β-estradiol attenuates neural injury due to stroke (Toung et al., 1998; Green et al., 2001; Dubal et al., 2006), seizures (Veliskova et al., 2000), excitotoxicity (Huang et al., 2004; Perrella et a., 2005; Zhao et al., 2005), oxygen-glucose deprivation (Harms et al., 2001) and oxidative stress (Behl et al., 1995; Mize et al., 2003). Protection occurs via receptor-dependent and receptor-independent mechanisms including increasing levels of Bcl-2 (Singer et al., 1998; Dubal et al., 1999; Stoltzner et al., 2001; Zhao et al., 2004), decreasing the production of reactive oxygen species and enhanced uptake of free radicals (Culmsee et al., 1999; Prokai et al., 2003), attenuation of calcium currents (Huang et al., 2004; Hilton et al., 2006), and activation of survival-promoting signal transduction cascades (Honda et al., 2000; Linford et al., 2000; Jover et al., 2002). While these studies represent a broad range of actions for 17β-estradiol, the effects of 17β-estradiol against injury (protective or otherwise) have not fully been explored in the developing central nervous system.
The developing central nervous system is particularly prone to injury (Volpe, 2001). Insults to the immature central nervous system, of a neural (e.g. stroke, seizure), environmental (e.g. umbilical cord strangulation or compression, physical abuse, fall), or maternal (e.g. pre-eclampsia, infection, diabetes, iron deficiency, drug abuse) origin, have a devastating and permanent effect on the brain and behavioral/cognitive abilities. Compounded upon this high-risk period for brain injury is the lack of currently available means of attenuation of damage.
Applying findings obtained from the mature central nervous system, we investigated the neuroprotective potential of 17β-estradiol against GABAA receptor-induced damage to the newborn rodent brain, our model of injury to the premature human infant brain (Nuñez and McCarthy, 2003). In stark contradiction to data obtained from the mature central nervous system, we documented that 17β-estradiol exacerbated damage via excessive enhancement of intracellular calcium levels and a prolongation of the excitatory actions of the neurotransmitter GABA (Nuñez et al., 2005). Upon closer examination, 17β-estradiol does afford a measure of neuroprotection by virtue of its rapid, potentially non receptor-mediated actions (Nuñez and McCarthy, 2003). However, we hypothesize that the longer acting receptor-mediated actions of 17β-estradiol overwhelm the shorter acting receptor-independent mechanism of neuroprotection.
In the current experiment, we were interested in the neuroprotective potential of 17α-estradiol in our in vivo model of injury to the human infant brain. This epimer of 17β-estradiol is noted to have sustained, rapid effects on intracellular signaling cascades (Singh et al., 1999; Toran-Allerand et al., 2005). 17α-estradiol is produced by the aromatization of epitestosterone, a naturally occurring epimer of testosterone (Finkelstein et al., 1981; Starka, 2003). Epitestosterone levels are elevated in the mother during pregnancy (Flint and Burrow, 1979), with the placenta having potent aromatization (epitestosterone to 17α-estradiol) capabilities (Higuchi and Villee, 1970; Horn and Finkelstein, 1971). With the ability of the epitesosterone to pass from the maternal placenta to the fetus (Finkelstein et al., 1981), the fetus encounters appreciable levels of 17α-estradiol during gestation (Toran-Allerand et al., 2005). Previous work has demonstrated neuroprotective actions in vitro for this endogenously present compound against hydrogen peroxide-induced damage to cultured human neuroblastoma SK-N-SH cells (Green et al., 1997; Wang et al., 2006), serum deprivation in cultured primary human fetal brain tissue (Hammond et al., 2001), amyloid β25–35 peptide exposure in cultured primary rat cerebro-cortical neurons (Cordey and Pike, 2005), and cultured primary basal forebrain neurons (Zhao and Brinton, 2006). By investigating cellular, anatomical and behavioral endpoints, we can more fully evaluate the neuroprotective potential of this agent as a practical means of intervention and attenuation of damage from insults to the immature brain.
Materials and Methods
Subjects
Animals were first generation descendants of Sprague-Dawley albino rats purchased from Charles River Lab (Wilmington, MA) and housed under a 12:12 hour light/dark cycle, with free access to food and water. Females were bred with male breeders in the Michigan State University animal colony. Pregnant females were checked every morning beginning at 7am, and every hour after that, for the presence of pups. Only females that gave birth after 7am were used. Day of birth was designated as postnatal day 0. All animal procedures were approved by the Michigan State University All-University Committee on Animal Use and Care, and followed National Institute of Health guidelines.
Experiment 1: In vivo investigation
Treatment of animals
Rats were removed from their mothers within two hours of birth and placed on a heated pad to avoid a drop in body temperature. At this time, male and female rats were treated with either: 1) 17β-estradiol (50µg), 2) 17α-estradiol (50µg) or 3) sesame oil (vehicle). Due to the toxic nature of DMSO to animals, sesame oil was the vehicle for all in vivo experimentation. Within five minutes of removal, pups were returned to their mothers. Thirty minutes later, pups were once again removed from their mothers, with male and female rats treated with either: 1) the GABAA receptor agonist muscimol (10µg) or 2) saline (vehicle). Immediately following injections, pups were returned to their mothers. The muscimol or saline treatment was repeated six hours later. The entire injection procedure was repeated on the next postnatal day. Each animal was administered a total of four muscimol or saline injections, and two 17β-estradiol, 17α-estradiol or sesame oil injections. All drugs and vehicles were administered via a 0.05ml subcutaneous injection. Within each sex, there were a total of six groups: 1) vehicle (sesame oil + saline), 2) sesame oil + muscimol (10µg), 3) 17β-estradiol (50µg) + saline, 4) 17α-estradiol (50µg) + saline, 5) 17β-estradiol (50µg) + muscimol (10µg), or 6) 17α-estradiol (50µg) + muscimol (10µg). There were a total of five (for behavior) to six (for anatomy) animals in each group. The dose of estradiol used (both 17α- and 17 β-) in the present investigation is based on radioimmunoassay results that this dose of 17 β-estradiol results in high physiological levels of estradiol in hippocampal tissue (Amateau et al., 2004), and exacerbates muscimol induced hippocampal damage (Nuñez and McCarthy, 2003). Only one study has documented the neuroprotective potential of 17α-estradiol in vivo (Green et al., 2001). This study documented that 100µg/kg of the natural enantiomer of 17β-estradiol provided protection against damage in a model of middle cerebral artery occlusion. However, in order to document the equimolar actions of both 17α- and 17 β-estradiol, a dose of 50µg was used. For comparison to Green et al (2001), a separate set of animals (n=4) was administered 1µg 17α-estradiol prior muscimol administration. These animals only underwent anatomical investigation. All injections were made into the nape of the neck, with the injection sites were sealed with cyanoacrylate Vetbond Surgical Adhesive (3M Animal Care Products, St. Paul, MN), in order to avoid leakage of the injected solutions. Pups were marked depending upon the manipulation they underwent by injecting India ink into their paws.
Behavioral investigation and testing environment
Daily handling of all pups began on postnatal day (PN) 15. Handling took place for one to three minutes each day over postnatal days 15 to 60 in order to make the rats more familiar with the experimenter and more comfortable with the procedural components of the behavioral testing, thereby significantly reducing any potential confounding stress effects. Rat pups were weaned on PN20-23, with animals housed with one to two other same sex littermates. Behavioral testing began on PN60. Females were only tested during the diestrus phase of their estrous cycle in order to eliminate the potential confounding effect of cyclic changes in estradiol and progesterone. All testing took place in a single, isolated room that had numerous posters and objects hung on the walls. In order to minimize stress during behavioral testing, low-light levels were maintained throughout testing, and all testing was executed by the same experimenters (J.M. and J.L.N.). All behavioral testing occurred between 2 and 5pm (animals were on a 5am on: 5pm off light: dark cycle). Concern was given to the amount of time animals were not in their animal room (home environment). During testing, one experimenter (J.L.N.) sat at a computer station set up in an ante room; while an assistant (J.M.) was responsible for placing and retrieving animals. For data acquisition, an overhead low-light video camera connected to a computer with image analysis software (SMART video tracking system, San Diego Instruments, San Diego, CA) was used to track the movements of animals in all the various mazes.
Water maze
Pre-training
On PN60-65, all animals underwent one day of water maze pre-training. The water maze was purchased from San Diego Instruments (San Diego, CA). The maze, composed of blue industrial plastic, is circular with 30 inch high walls and a diameter of 6 feet. The maze was filled to a depth of 21 inches with 26 ± 1°C water rendered opaque with 150 grams of powdered skim milk. A white plastic platform (4 inch diameter and 10 inches tall) was placed in the center of the pool, sticking one inch above the surface of the water. Each animal was placed on the platform for 20 seconds, and then put into the pool at a random location. Animals were allowed 60 seconds to locate and escape onto the visible platform. If they did not find the platform in the allotted time, they were led there by the experimenter. Animals remained on the platform for twenty seconds. Each rat underwent three consecutive trials. After all trials were completed, animals were thoroughly dried and returned to their home cages.
Testing
One to two days following pre-training, rats underwent one day of water maze testing. Each animal was placed on the platform for 20 seconds, and then put into the pool at one of four start locations: north, south, east or west. The animals were allowed 60 seconds to locate and escape onto the submerged platform [a white plastic platform, 20 inches in height with a 4 inch diameter face, was used throughout the study and placed in the south-east corner of the pool (18 inches from the edge). The top of the platform was one inch below the surface of the water (therefore, the platform was invisible to the animals)]. If they did not find the platform in the allotted time, they were led there by the experimenter. Animals remained on the platform for ten seconds. Each rat underwent twelve trials. There was a 2 to 5 minute inter-trial interval, with a 30 minute rest period between the 8th and 9th trials. After all trials were completed, animals were thoroughly dried and returned to their home cages. Data was obtained using the SMART system tracking software. The following measures were recorded for all animals across all trials: 1) latency to find the escape platform, 2) path-length during the search for the escape platform, and 3) average swim speed for each individual trial.
Following water maze testing, the animals began food deprivation. Each animal was weighed, and fed 3 pellets (10 grams) per day. Over the next ten days, each animal’s body weight was reduced to 92–95% of the initial body weight. Along with the daily feeding, rats were given three pieces of whole grain cereal. The rats were encouraged to take the cereal from the experimenter. After a stable body weight was obtained, each animal began radial arm maze pre-training.
Radial arm maze
The radial arm maze was purchased from San Diego Instruments (San Diego, CA). The maze, composed of black plastic, consists of eight arms 20 inches in length and 4 inches in width, with 8 inch high walls. A central circular region (6 inches in diameter) joins the arms of the maze. The entire maze is elevated 20 inches off the ground.
Pre-training
Prior to each trial, the maze was cleaned thoroughly with a 70% alcohol solution. The pre-training trial began with the rat being placed in the central region of the maze. Each rat underwent one trial per day. On the first day of pre-training, a piece of the whole grain cereal was placed at the entrance to each of the eight arms. Animals were then allowed to eat the cereal, with the trial timed. After all cereal pieces were eaten, or 5 minutes had elapsed, each rat was removed and returned to its home cage. On the second day of pre-training, a piece of cereal was placed half-way down each of the eight arms, while for the third day of pre-training, a piece was placed at the end of each of the eight arms. In order to continue radial arm maze testing, animals had to eat all pieces of cereal within the allotted time. All animals attained asymptotic performance (all pieces of food eaten in the allotted time) after seven days of pre-training.
Testing
One day after radial arm maze pre-training ended, rats began testing. Prior to each trial, the maze was cleaned thoroughly with a soap solution, followed by 70% alcohol. The testing trial began with each rat being placed in the central region of the maze. Each rat underwent one trial per day, with testing lasting a total of five days. A small piece of whole grain cereal was placed in a dish at the end of five of the eight arms, to ensure the rat could not see the cereal from the center. Each trial lasted for 5 minutes, at which point the rat was removed and returned to its home cage. At the end of the testing phase, each animal has undergone twelve total trials – seven pre-training trials, and five testing trials. Data was obtained using the tracking software and supplemented by experimenter observations. The following measures were taken: 1) number of entries into arms that never contained food (reference memory error), 2) number of entries into arms that contained food, but it was already consumed on the same trial (working memory errors), and 3) amount of time required to complete the testing trials.
Following the termination of behavioral testing, the brains of these animals were collected for Golgi and electrophysiological investigation (discussed elsewhere).
Histological Processing and Stereological Analysis
A separate set of animals that did not undergo behavioral testing were euthanized on postnatal day 90 by barbiturate overdose, and their brains fixed overnight in 4% paraformaldehyde with 2.5% acrolein, then 24 hours in 4% paraformaldehyde. Brains were stored in 30% sucrose in paraformaldehyde for 72 hours, and then sectioned on a cryostat. Consecutive 60µm coronal sections were made through the entire hippocampus, with all tissue saved in tissue wells in the consecutive series. Tissue was processed for free-floating neuronal nuclear antigen (NeuN) immunocytochemistry. Following immunocytochemistry, consecutively labeled tissue sections were mounted onto gelatin coated slides. Using the StereoInvestigator program package (version 6.55, MicroBrightField, Colchester, VT) loaded onto a PC, with a Nikon Eclipse 80i microscope equipped with a motor-driven LEP stage and an Optronics Microfire color CCD camera, hippocampal volume was quantified.
Volumetric analysis using Cavalieri estimator
The anterior to posterior extent of the hippocampal formation on the tissue-mounted slides was recorded, with the anterior-most tissue section (section 0) containing the hippocampus and dentate gyrus recorded. The first section that underwent volumetric analysis using the Cavalieri estimator was a randomly chosen tissue section that was within 120µm (two tissue sections) of the anterior-most tissue section (section 0) containing the hippocampal formation. Each subsequent tissue section that underwent volumetric analysis was exactly 180µm (three tissue sections) from the previously analyzed tissue section. The last tissue section that underwent volumetric analysis was the last plane in which the hippocampal formation was present. There were a total of 8 to 10 tissue sections analyzed using the Cavilieri estimator for each animals. Cavalieri estimation was performed using a 40X objective and 75µm grid spacing. In the volumetric analysis, a distinction was made between the hippocampus proper and the dentate gyrus. The StereoInvestigator program package was used to convert the volume of each tissue section (tissue thickness multiplied by the surface area, as estimated using the Cavilieri method), sum the volumes across all tissue sections investigated (8–10 total), and through the entire depth of the hippocampus proper (between 1500 and 1920µm), in order to obtain total hippocampal and dentate gyrus volume.
Experiment 2: In vitro investigation
Preparation of primary hippocampal neuron cultures
Sprague-Dawley breeder females were bred with male breeders in the Michigan State University animal colony. On the day of birth, male pups were removed and used for primary cell culturing. Only males were used given the lack of difference between the sexes in the neuroprotective effects (both anatomically and behaviorally) afforded by 17α-estradiol. Animal use procedures were approved by the Michigan State University All University Committee on Animal Use and Care, and followed National Institute of Health guidelines. Hippocampi were dissected into HBSS+ [88ml sterile H2O, 10 ml Hank’s balanced salt solution (Ca2+ and Mg2+-free) 10X, 1 ml HEPES buffer, 1.0 M, pH 7.3, 1 ml Antibiotic/Antimycotic 100X liquid], then additional HBSS+ was added to the tube to a volume of 4.5 ml, with 0.5ml trypsin (2.5%), and incubated in a 37°C water bath for 15 minutes. The supernatant was discarded and the tissue washed with HBSS+. This procedure was repeated a second time. Cells were dissociated by trituration, with cell number and viability determined by trypan blue exclusion. Cells were plated on 25mm Poly-L-lysine (Sigma, St. Louis, MO, USA) coated cover slips at a density of 300,000 cells per coverslip, and placed in 60mm dishes containing 4ml plating medium [86ml MEM, 10 ml horse serum, 3 ml glucose (filter sterilized, 20%) 1ml pyruvic acid, 100mM]. Cells were allowed 4 hours to adhere to the coverslips in a 37°C, 5% CO2 incubator. The coverslips were removed from the plating dishes and placed into 60mm dishes filled with Neurobasal+ [1ml B-27 supplement, 1ml Antibiotic/Antimycotic 100X, 125µl L-Glutamine and filled to 50ml with Neurobasal (phenol red free)]. All cell culture chemicals and solutions were obtained from Invitrogen (Carlsbad, CA, USA).
Cultured cell treatment
Hippocampal cultures were treated on days in vitro (DIV) 0 and 1 with one of six treatment paradigms: 1) 1nM 17β-estradiol, 2) 1nM 17α-estradiol, 3) 1µM ICI-182,780 (estrogen receptor antagonist), 4) 1µM ICI-182,780, followed 30 minutes later by 1nM 17β-estradiol, 5) 1µM ICI-182,780, followed 30 minutes later by 1nM 17α-estradiol or 6) vehicle alone (DMSO). Due to the lack of solubility of sesame oil in the culture medium, all drugs were dissolved in DMSO (less than 0.001%). Beginning on DIV 2, cultures were treated with either 10µM muscimol (GABAA receptor agonist) or sterile saline (vehicle). The treatment was repeated four hours later, and the muscimol or saline treatment schedule was repeated on DIV 3, for a total of four muscimol treatments.
Lactate dehydrogenase assay
Beginning two hours after the last muscimol treatment, cell culture medium was collected for lactate dehydrogenase (LDH) assay, a measure of cellular injury following exposure to a cytotoxin. Samples were made at 2, 8, 24, 48 and 72 hours after the last muscimol administration. There were a total of six culture dishes from two separate culture runs collected for each treatment group and at each time point. At the collection time points, 200µl of culture supernatant was removed from each hippocampal culture treatment group, along with the various LDH assay controls (background control; “high” control – cultures treated with 2µl Triton X-100; “low” control – untreated cultures) and stored at −80°C until assayed. Immediately following the withdrawal of medium from the “test” cultures, 200µl of medium from cultures in the same treatment group, but which were not used for the LDH assay, was replaced into the “test” cultures. The LDH assay was performed using a microplate reader (Model 680, Bio Rad, Hercules, CA) and the Cytotoxicity Detection Kit (Roche, Indianapolis, IN). The ELISA reader determines the absorbance for each sample, and then the percent cytotoxicity was calculated using the equation:Cytotoxicity = (treatment group – “low” control)/ (“high” control – “low” control) *100
TUNEL assay
Twenty four hours after the last muscimol treatment, hippocampal cultures were fixed by immersion in 4% paraformaldehyde for 25 minutes at room temperature. Cultures were then washed three times in PBS for five minutes, permeabilized by immersion in 0.2% Triton X-100 solution in PBS, and stained using the standard protocol provided in the DeadEnd Colorimetric TUNEL system (Promega, Madison, WI) and TUNEL immunoreactive cells visualized using diaminobenzidine (DAB). Cells were lightly counter-stained with the Nissl stain cresyl violet, dehydrated and mounted on slides. TUNEL immunoreactive (TUNEL-ir) and cresyl stained cells were counted on each coverslip using the fractionator technique in conjunction with the StereoInvestigator program (version 6.55, MicroBrightField, Colchester, VT). An unbiased disector frame was randomly placed throughout each coverslip, with one hundred fields sampled in order to obtain an estimate (number of TUNEL-ir cells and number of cresyl stained living cells) from each coverslip. A total of five coverslips were sampled in each treatment group. To ensure that cell distribution was uniform across all coverslips and treatments, we reported the number of TUNEL-ir cells per 100 living cells.
Statistical Analysis
Two way analyses of variance for the effect of drug (vehicle, muscimol) and hormone (vehicle, 17α-estradiol, and 17β-estradiol) on volumetric quantification were performed. Males and females were analyzed separately. Repeated measures two way analyses of variance were run on water maze and radial arm maze data. LDH data was analyzed by three way analysis of variance. For all ANOVAs, post-hoc (Tukey) tests were performed, with a level of p<0.05 required to obtain statistical significance. The non-parametric χ2 test was performed on the number of TUNEL-ir cells per 100 living cells. All analyses were performed using the SYSTAT Program (version 11.0).
Results
Behavioral Analysis
Morris Water Maze
Pretraining
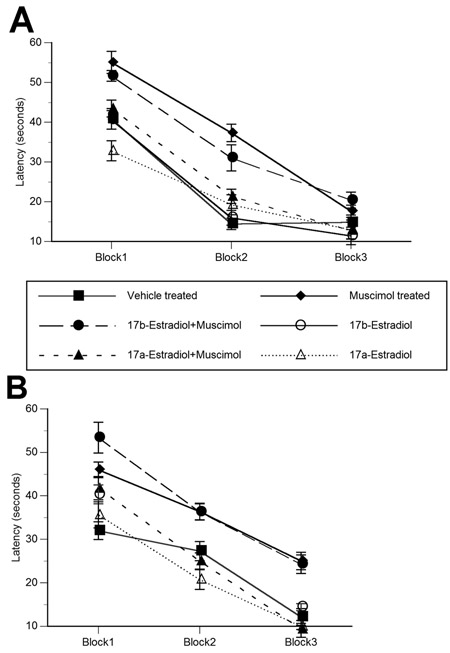
There were no significant treatment, hormone or sex effects on latency to find the visible platform across the three trials (Figure 1A and 1B). This indicates that all animals were able to acquire the procedural components of the task, without problems brought about by motor or sensory deficits.
Figure 1.
Pretreatment with 17α–estradiol attenuates neonatal muscimol-induced impairment on water maze task. Neonatal muscimol administration resulted in deficits in water maze performance in both males (A) and females (B). While 17β-estradiol pretreatment was without effect on the muscimol-induced deficits in performance, 17α–estradiol attenuated the deleterious effects of muscimol in animals of both sexes. On all trial blocks, 17α–estradiol + muscimol treated animals displayed performance equivalent to vehicle treated animals. Data represent the mean latency to find the “hidden” escape platform ± SEM values, obtained from 5 animals in each group. Data is grouped into trial blocks, with each trial block representing four individual trials. * indicates significant difference from vehicle treated animals (Tukey; p<0.05).
Testing
There was a significant main effect of drug treatment and drug treatment by hormone interaction on water maze performance in both males and females (Between group analyses – Drug – Male: F1, 28=17.836, p<0.001; Female: F1, 28=12.248, p<0.001, Drug X Hormone – Male: F2, 28=7.63, p<0.01; Female: F2, 28=5.48, p<0.01) (Figure 1A and 1B). Muscimol and 17β-estradiol + muscimol treated animals had the longest latencies as compared to all other groups (p<0.05). A similar pattern of results was obtained from path-length data (not shown). Vehicle and 17α-estradiol + muscimol treated males and females had almost equivalent latencies across all trial blocks. All animals were able to learn the task (Within group analyses - Male: F2, 56=128.49, p<0.001; Female: F2, 56=112.34, p<0.001). The effect of muscimol treatment was the most pronounced in the first two trail blocks. All animals with the exception of muscimol treated females, and 17β-estradiol + muscimol treated males and females attained asymptotic performance by the third trial block. Swim speed was unaffected by neonatal treatment (data not shown).
Radial Arm Maze
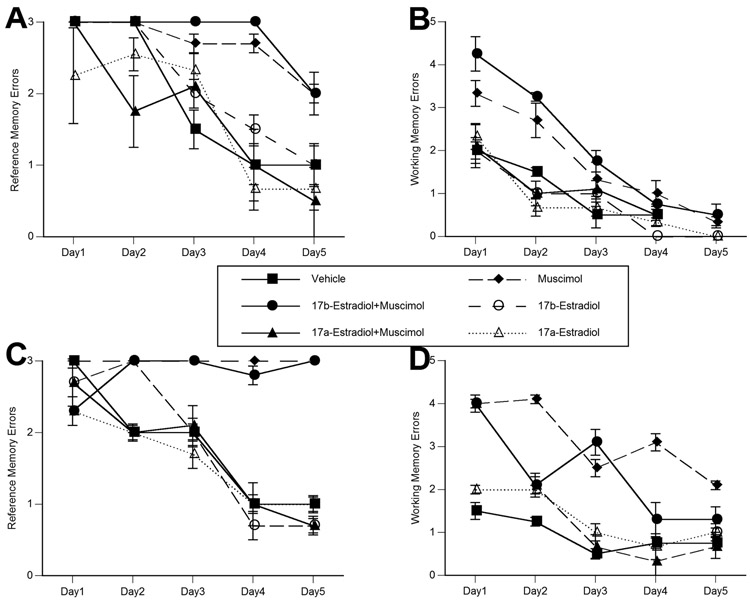
There was a significant main effect of drug treatment, and drug treatment by hormone interaction on radial arm maze performance (reference memory task) in both males and females (Between group analyses – Drug – Male: F1,24=18.137, p<0.001; Female: F1, 24=21.741, p<0.001, Drug X Hormone – Male: F2,24=9.334, p<0.003; Female: F2, 24=6.993, p<0.008) (Figure 2A and 2C). Consistent with Morris water maze performance, muscimol and 17β-estradiol + muscimol treated animals had the most number of errors as compared to all other groups (p<0.05). Within group analyses indicated that while all groups learned the task (Male: F4, 96=78.331, p<0.001; Female: F4, 96=29.243, p<0.001), there was a significant drug treatment effect on the rate of learning across trial blocks in both sexes (Male: F4, 96=11.112, p<0.001; Female: F4,96=7.186, p<0.002). Muscimol and 17β-estradiol + muscimol treated females did not improve in task performance across the five days of testing. Acquisition was also significantly impaired in muscimol and 17β-estradiol + muscimol treated males. All other groups attained asymptotic performance by the fourth day of testing.
Figure 2.
Pretreatment with 17α-estradiol significantly attenuates the neonatal muscimol-induced alterations in males (A) and females (C) on the reference memory component of the radial arm maze, and in males (B) and females (D) on the working memory component of the radial arm maze. Consistent with deficits on water maze task, 17β-estradiol was ineffective against the deleterious actions of muscimol. Data represent the mean number of reference memory errors (A and C) (visiting arms that never had food), and working memory errors (B and D) (visiting an arm in which the food was previously consumed) ± SEM values, obtained from three animals in each group. Testing took place over a five day period, with data representing an individual five minute trial performed on a single day.
There was a significant main effect of treatment in females, and a drug treatment by hormone interaction on the number of working memory errors in both males and females (Between group analyses – Drug – Female: F1, 24=6.111, p<0.03, Drug X Hormone – Male: F2,24=5.917, p<0.01; Female: F2, 24=5.363, p<0.02) (Figure 2B and 2D). Muscimol and 17β-estradiol + muscimol treated males and females had the most number of working memory errors (p<0.05). On all days in males and females (except day one in females), 17α-estradiol + muscimol treated animals made 40–50% fewer working memory errors than muscimol alone treated animals. Vehicle and 17α-estradiol + muscimol treated animals made equivalent number of working memory errors across all trial blocks. All animals displayed improved performance across the consecutive training days (Within group analyses - Male: F4, 96=51.874, p<0.001; Female: F4, 96=16.924, p<0.001). Muscimol and 17β-estradiol + muscimol treated males and females had the slowest rates of obtaining asymptotic performance on the task. The effects of treatment were the most pronounced in the first three training days, with all animals attaining near perfect performance by the fifth training day. Maximal performance was achieved by day three in control and 17α-estradiol + muscimol treated males and females.
There were no drug treatment or hormone effects on the time to complete the radial arm maze trials (data not shown). This is surprising given that muscimol and 17β-estradiol + muscimol treated males and females made significantly more working and reference memory errors across the testing trials. On day one, animals took between 130 and 185 seconds to successfully complete the task. This improved to 70 to 100 seconds by the second day of testing. By the fifth day of testing, all animals were performing the task in 50 to 70 seconds.
Anatomical Investigation
Volume
There was a significant effect of drug treatment, and a drug treatment by hormone interaction on the volume of the hippocampus in both males (Drug Treatment: F1, 15=38.982, p<0.001; Drug Treatment X Hormone: F2, 15=10.963, p=0.001) and females (Drug Treatment: F1, 16=4.501, p=0.050; Drug Treatment X Hormone: F2, 16=9.607, p=0.002) (Figure 3A and 3B).
Figure 3.
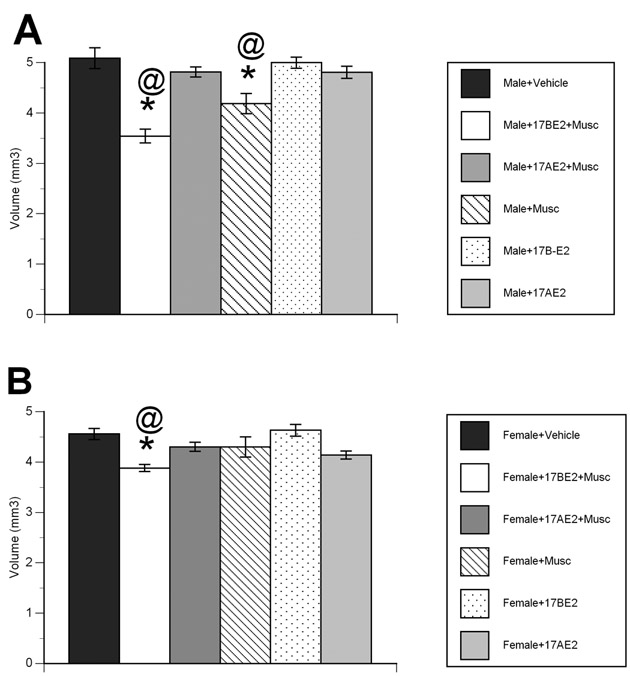
Pretreatment with 17α-estradiol attenuated muscimol-induced decrement in hippocampal volume in the (A) male and (B) female rat. Muscimol administration led to a significant decrease in hippocampal volume in males, with the combination of 17β-estradiol + muscimol resulting in further decrement in hippocampal volume in both males and females. In contrast, the hippocampal volumes of animals treated with 17α-estradiol prior to muscimol was equivalent to that of vehicle treated animals. Data represent the mean hippocampal volume (mm3) ± SEM values, obtained from six animals in each group. * indicates significant difference from vehicle treated animals (Tukey; p<0.05). @ indicates significant difference from 17α-estradiol + muscimol treated animals (Tukey; p<0.05).
In males, muscimol administration resulted in a 14.5% reduction in hippocampal volume, with a further reduction to 30% smaller than controls in 17β-estradiol + muscimol treated animals (p<0.05 for each comparison). Conversely, the hippocampal volume of 17α-estradiol + muscimol treated animals was only 5.4% smaller than controls. 17β-estradiol + muscimol treated animals had smaller hippocampal volumes than all other groups (p<0.05).
In females, both muscimol alone and the combination of 17α-estradiol + muscimol were without effect on hippocampal volume, resulting in a modest 4% decrement as compared to controls. However, 17β-estradiol + muscimol treated animals had hippocampal volumes 14% smaller than controls, and significantly smaller than all groups (p<0.05 for each comparison).
A subset of males and females were administered 1µg 17α-estradiol prior muscimol treatment. Hippocampal volumes in 17α-estradiol+musicmol treated males and females were not significantly different from control animals of the same sex, yet significantly greater than muscimol alone treated animals. There was no significant difference in hippocampal volume between 17α-estradiol (1µg) + muscimol and 17α-estradiol (50µg) + muscimol treated animals (both males and females). Given the lack of difference, only 17α-estradiol (50µg) + muscimol animals were investigated.
In vitro Investigation
LDH
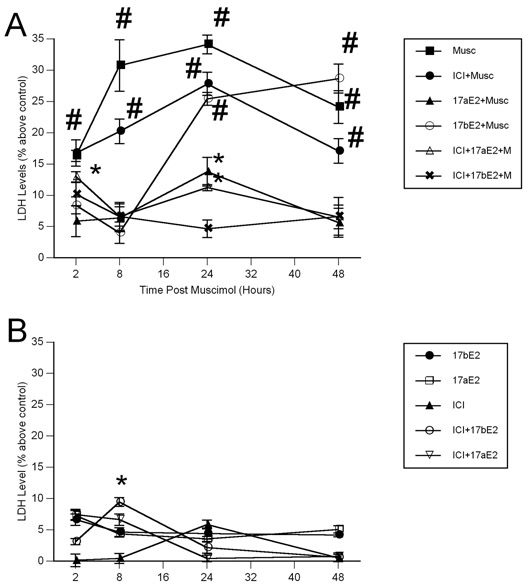
There was a significant effect of drug treatment, and a drug treatment by hormone interaction on lactate dehydrogenase levels in the primary hippocampal cultures (Between group analyses – Drug: F1, 60=109.727, p<0.001, Drug X Hormone: F2,60=19.326, p<0.001) (Figure 4A and 4B). Across all time points examined, muscimol alone treated cultures displayed elevated levels of LDH as compared to all groups except ICI-182,780 + muscimol treated cultures (p<0.05 for each measure). Also, muscimol alone treated cultures displayed elevated LDH levels relative to 17β-estradiol + muscimol treated cultures at 2, 8 and 24 hours post muscimol, but equivalent at 48 hours post muscimol (p<0.05 for each comparison). 17β-estradiol + muscimol treated cultures had elevated LDH levels relative to controls, 17α-estradiol + muscimol, ICI-182,780 + 17α-estradiol + muscimol, and ICI-182,780 + 17β-estradiol + muscimol treated cultures at 24 and 48 hours post muscimol application (p<0.05 for each comparison). Consistent with the data on hippocampal volume, 17α-estradiol was protective against muscimol-induced damage, with only modest initial protection (only between 2 to 8 hours post muscimol) afforded by 17β-estradiol. The protective effects of 17α-estradiol appear to be independent of the classical nuclear estrogen receptor, given there was no effect of the estrogen receptor antagonist ICI-182,780.
Figure 4.
Muscimol-induced enhancement in lactate dehydrogenase (LDH) levels is completely attenuated by pretreatment with 17α-estradiol (A), but unaffected by 17α-estradiol alone (B) in primary hippocampal cultures. 17α-estradiol, alone and in combination with the estrogen receptor antagonist ICI-182,780, blocked muscimol-induced elevations in LDH levels. In contrast, while 17β-estradiol afforded protection against muscimol-induced toxicity at two and eight hours post application. Data represent the mean lactate dehydrogenase levels ± SEM values, obtained from six individual cultures dishes (two separate culture runs) in each group. # indicates significant difference from vehicle treated control cultures, 17α-estradiol + muscimol, ICI-182,780 + 17α-estradiol + muscimol and ICI-182,780 + 17β-estradiol + muscimol treated cultures at the same time point (Tukey; p<0.05). * indicates significant difference from control (vehicle treated) cultures at the same time point (Tukey; p<0.05).
TUNEL-ir cell number
Consistent with the LDH data, we have documented that muscimol application significantly increased the proportion of dying/dead cells (TUNEL-ir) relative to vehicle treated hippocampal neurons (χ2 = 5.43; p=0.021). Vehicle – 1.824±0.05, Muscimol – 9.250±0.24, 17β-Estradiol + Muscimol – 14.326±1.40, 17α-Estradiol + Muscimol – 4.426±0.66, ICI-182,780 + 17α-Estradiol + Muscimol – 5.901±0.47, ICI-182,780 + 17β-Estradiol + Muscimol – 5.649±0.16. All values are number of TUNEL positive cells per 100 living cells. Both the LDH and TUNEL-ir findings are consistent with previous work (Nuñez et al., 2003; Nuñez et al., 2004). Pretreatment with 17β-estradiol significantly increased the proportion of TUNEL-ir cells (χ2 = 17.14; p=0.001) – while muscimol alone led to a four-times increase in the proportion of TUNEL-ir cells, 17 β-estradiol + muscimol treated cultures had an almost seven fold increase in the proportion of TUNEL-ir cells. In stark contrast, pretreatment with 17α-estradiol significantly attenuated TUNEL-ir cell death compared to both muscimol alone (χ2 = 6.28; p=0.011) and 17 β-estradiol + muscimol treated cultures (χ2 = 10.73; p=0.009). The protective effect of 17α-estradiol appears to be independent of the classical nuclear estrogen receptor, given that there is equivalent protection from muscimol-induced damage in both ICI-182,780 + 17α-estradiol treated cultures (χ2 = 3.17; p=0.041) and ICI-182,780 + 17β-estradiol treated cultures (χ2 = 3.09; p=0.042).
Discussion
In the present study we have documented that 17α-estradiol is a potent neuroprotective agent against excessive GABAA receptor activation induced damage to the newborn rat, our in vivo model of brain injury in human infants. We have previously documented that muscimol, the selective GABAA receptor agonist, induces cell loss in the immature hippocampus that persists through adolescence (Nuñez et al., 2003a,b). In the present study, muscimol administration resulted in a 4–15% reduction in the volume of the hippocampus. Prior administration of 17α-estradiol resulted in near complete attenuation of muscimol-induced hippocampal damage. Behavioral performance on two hippocampal-dependent tasks was likewise spared the deleterious effects of muscimol by pretreatment with 17α-estradiol. The present findings are consistent with the wealth of data on the neuroprotective effects of 17α-estradiol against serum deprivation, amyloid β25–35 peptide, glutamate, and hydrogen peroxide exposure in vitro (Green et al., 1997; Hammond et al., 2001; Cordey and Pike, 2005; Wang et al., 2006; Zhao and Brinton, 2006).
17α-estradiol is a naturally occurring estrogen that has potential as a neuroprotective agent. Although only investigated in a handful of studies, 17α-estradiol has been demonstrated to attenuate brain lipid peroxidation (Green et al., 2001), and induce sustained activation of the survival promoting MAPK/ERK and phophatidylinositol 3-kinase-Akt intracellular signaling cascades (Singh et al., 1999; Cordey and Pike, 2005; Toran-Allerand et al., 2005). In vivo, 17α-estradiol has been documented to attenuate mortality and reduce ischemic lesion volume following middle cerebral artery occlusion in the adult female rat (Simpkins et al., 1997; Green et al., 2001). A combination of these mechanisms may be responsible for the protection afforded by 17α-estradiol in the current paradigm.
Circulating 17α-estradiol results from the aromatization of epitestosterone, a naturally occurring epimer of testosterone (Finkelstein et al., 1981; Starka, 2003). It is of interest that epitestosterone levels significantly increase during pregnancy (Flint and Burrow, 1979), with the human placenta having potent aromatization (epitestosterone to 17α-estradiol) capabilities (Higuchi and Villee, 1970; Horn and Finkelstein, 1971). Given the presence of significant levels of epitestosterone in males, females (Havlikova et al., 2002), and pregnant females (Finkelstein et al., 1981), we hypothesize that the actions of 17α-estradiol investigated in the present study may represent an under-appreciated and endogenous mechanism of neuroprotection in the human brain.
Muscimol administration to the newborn rat results in a marked decrease in the volume of the male and female hippocampus. We also report an exacerbation of muscimol-induced damage in the hippocampal formation by pretreatment with high physiological levels of 17β-estradiol, consistent with previous work from our lab (Nuñez et al., 2003a,b; Nuñez and McCarthy, 2003). The decrement in hippocampal volume following muscimol administration is attributed to enhanced cell death. In support of this hypothesis, we have dpcumented a significant increase in lactate dehydrogenase (LDH) release and the number of TUNEL-positive cells per living cells following muscimol application in primary hippocampal cultures, two indicators of cell death. As further confirmation of the long-term deleterious effects, muscimol administration resulted in attenuated performance on the Morris water maze and the reference memory component of the radial arm maze, two hippocampal dependent tasks, in adulthood.
Neuroprotection from the debilitating anatomical and behavioral alterations afforded by 17α-estradiol is hypothesized to result from attenuated hippocampal neuron loss. Our findings in vitro demonstrate diminished release of lactate dehydrogenase following 17α-estradiol + muscimol application and fewer TUNEL-immunopositive cells in 17α-estradiol + muscimol-treated primary hippocampal cultures as compared to muscimol alone treated cultures. Equivalent protection was afforded by 17α-estradiol in vitro in the presence and absence of antagonism of the classical nuclear estrogen receptor, indicating the importance of the non-genomic actions of 17α-estradiol.
We conclude that the natural enantiomer of 17β-estradiol, 17α-estradiol, is neuroprotective against anatomical and behavioral deficits in an in vivo model of early brain injury. Equivalent protection by 17α-estradiol in vitro was documented in the presence and absence of the estrogen receptor antagonist ICI-182,780. Likewise, there was no difference in the neuroprotective capabilities of 17α-estradiol + ICI-182,780, 17β- estradiol + ICI-182,780 and 17α-estradiol alone administered prior to muscimol. While the nuclear receptor dependent effects of 17β-estradiol led to an exacerbation of damage (both anatomically and behaviorally), the classic nuclear receptor independent effects of both 17β-estradiol and 17α-estradiol resulted in protection. Antagonism of the nuclear estrogen receptor was not performed in vivo due to the mixed effects of currently available drugs. We hypothesize that 17α-estradiol attenuates muscimol-induced damage via its non-genomic actions in the developing hippocampus. 17α-estradiol reduces the amount of muscimol-induced cell loss, which in turn may lessen the reduction in hippocampal volume and concomitant behavioral deficits. While recent work has documented that 17α-estradiol is present in the postnatal and adult male and female mouse brain, and has potent action on the estrogen receptor in the adult female mouse uterus, an investigation of 17α-estradiol binding to estrogen receptors in the newborn mouse hippocampus was not performed (Toran-Allerand et al., 2005). The current data speak to the neuroprotective actions of 17α-estradiol in the developing rat hippocampus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences in regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- Cordey M, Pike CJ. Neuroprotective properties of selective estrogen receptor agonists in cultured neurons. Brain Res. 2005;1045:217–223. doi: 10.1016/j.brainres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M, Weidenfeld J, Ne'eman Y, Samuni A, Mizrachi Y, Ben-Uzilio R. Comparative studies of the aromatization of testosterone and epitestosterone by human placenta aromatase. Endocrinology. 1981;108:943–947. doi: 10.1210/endo-108-3-943. [DOI] [PubMed] [Google Scholar]
- Flint AP, Burrow PV. Epitestosterone in the plasma of the goat during pregnancy and at parturition. J Endocrinol. 1979;82:287–291. doi: 10.1677/joe.0.0820287. [DOI] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW. 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci. 1997;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber JR, Hortnagl H. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlikova H, Hill M, Hampl M, Starka L. Sex- and age-related changes in epitestosterone in relation to pregnenolone sulfate and testosterone in normal subjects. J Clin Endocrinol Metab. 2002;87:2225–2231. doi: 10.1210/jcem.87.5.8499. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Villee CA. Aromatization of epitestosterone by human placenta. Endocrinology. 1970;86:912–913. doi: 10.1210/endo-86-4-912. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. Estradiol modulation of kinainic acid-induced calcium elevationin neuronal hippocampal neurons. Endocrinology. 2006;147:1246–1255. doi: 10.1210/en.2005-1258. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Horn H, Finkelstein M. Aromatization of epitestosterone by human placenta. Endocrinology. 1971;88:271–273. doi: 10.1210/endo-88-1-271. [DOI] [PubMed] [Google Scholar]
- Huang Y, Huang YL, Zhang S, Zhu YC, Yao T. Estradiol acutely attenuates glutamate-induced calcium overload in primarily cultured rat hippocampal neurons through a membrane receptor-dependent mechanism. Brain Res. 2004;1026:254–260. doi: 10.1016/j.brainres.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford N, Wade C, Dorsa D. The rapid effects of estrogen are implicated in estrogen-mediated neuroprotection. J Neurocytol. 2000;29:367–374. doi: 10.1023/a:1007113323582. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:294–302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Neural gonadal steroid actions. Science. 1981;211:1303–1311. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage: I. GABAA receptor activation induces cell death in developing rat hippocampus. Exper Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage: II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exper Neurol. 2003;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm infant brain injury. Endocrinology. 2003;144:2350–2359. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- Perrella J, Bhavnani BR. Protection of cortical cells by equine estrogens against glutamate-induced excitotoxicity is mediated through a calcium independent mechanism. BMC Neurosci. 2005;6:34. doi: 10.1186/1471-2202-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez EJ, Liu R, Simpkins JW. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc Natl Acad Sci. U. S. A. 2003;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins C, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starka L. Epitestosterone. J Steroid Biochem Molec Biol. 2003;87:27–34. doi: 10.1016/s0960-0760(03)00383-2. [DOI] [PubMed] [Google Scholar]
- Stoltzner SE, Berchtold NC, Cotman CW, Pike CJ. Estrogen regulates bcl-x expression in rat hippocampus. Neuroreport. 2001;12:2797–2800. doi: 10.1097/00001756-200109170-00009. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;6:S30–S35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez E, Liu R, Yang R, Covey DF, Simpkins JW. Neuroprotective effects of 17beta-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formation of conjugated equines estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci. 2006;7:1–13. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca(2+) influx, dendritic and nuclear Ca(2+) rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: A potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]