Abstract
Objective
Although consumption of dietary supplements containing pomegranate extract by arthritis patients is on the rise, efficacy of such preparations in suppressing joint inflammation and damage is not known. The present study was designed to evaluate a standardized preparation of pomegranate extract (POMx) using collagen-induced arthritis in mice (CIA)-a widely used animal model of rheumatoid arthritis (RA).
Methods
CIA susceptible DBA/1 mice were fed POMx by gavage before and after immunization with chicken type-II collagen (CII). Severity of clinical arthritis was scored using a visual scoring system. Arthritic joints were analyzed by histopathology and graded. Lysates were generated from mouse joints and the levels of anti-type-II collagen IgG and inflammatory cytokines IL-1β, IL-6 and TNF-α were quantified by ELISA. Effect of POMx on LPS-induced NO production was determined by Griess reaction and MAP kinase activation was studied by Western immunoblotting in mouse macrophages.
Results
Consumption of POMx potently delayed the onset and reduced the incidence of CIA in mice. Severity of arthritis was also significantly lower in POMx -fed animals. Histopathology of the arthritic joints from POMx-fed mice demonstrated reduced joint infiltration by the inflammatory cells and the destruction of bone and cartilage were alleviated. Levels of IL-6 were significantly decreased in the joints of POMx-fed mice with CIA. In mouse macrophages, POMx abrogated multiple signal transduction pathways and downstream mediators implicated in RA pathogenesis.
Conclusions
Our studies suggest that inhibition of a spectrum of signal transduction pathways and the downstream pathogenic cellular response by POMx or compounds derived from it may be a useful approach for the prevention of onset and severity of inflammatory arthritis.
Keywords: Pomegranate, Collagen-induced arthritis, Rheumatoid Arthritis, IL-6, TNF-α
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that results from a complex interplay of both genetic and environmental factors. Clinically RA manifests itself as a systemic, chronic inflammatory disease characterized by synovial inflammation and erosion of bone and cartilage, which lead to the destruction of the affected joints. The normal synovial lining consists of 1–2 cell layers but increases to at least 10 cell layers in RA joints. Patients with RA have CII-reactive T cells and antibodies, which suggests that CII is a candidate autoantigen in disease pathogenesis (1). RA affects 0.5%-1% of the world population with more women being afflicted than men (2). The preclinical stages of RA pathogenesis are poorly understood, but it is now clear that inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 are over expressed in RA joints and play an important role in its pathogenesis (3, 4, 5). In addition the current view of the cytokine network in RA joints supports the notion that TNF-α activates a cytokine cascade characterized by the simultaneous production of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and IL-6, while anti-inflammatory cytokines such as IL-10 and soluble TNF receptor are suppressed (2, 6). In RA, joint infiltrating macrophages produce TNF-α and other inflammatory cytokines that potentiate inflammation (2, 4). TNF-α has been shown to play a pivotal role in synovitis and joint destruction in animal models of arthritis and in RA (6, 7). This is supported by published studies showing that the long term use of biological agents targeting TNF-α give rise to sustained improvements in symptoms and signs of RA and, furthermore, that TNF-α blockade protects joints from structural damage (8). While TNF-α has been hypothesized to be the “master cytokine” driving joint inflammation, about 50% of the RA patients fail to respond to anti-TNF therapy (9). Additionally, it has also been shown that mice lacking TNF-α gene can still develop severe joint inflammation and destruction demonstrating experimentally that TNF-α is not rate limiting for chronic inflammatory joint disease (10). The development of anti-TNF-α antibodies or soluble receptor molecules to treat RA emerged in part as a consequence of growing appreciation of the severity of this condition and in part due to significant progress made in understanding the important role TNF-α and inflammatory cytokine-signaling network play in the pathogenesis of this disease.
Although recently developed anti-TNF-α agents are well tolerated and have a good overall safety profile, pitfalls to the use of biological agents include the costs of these treatments, tuberculosis, and in some cases even malignancies (11, 12). Despite optimal use of currently available anti-rheumatic agents, most RA patients live with chronic pain and severe functional decline because these therapies focus primarily on preventing joint inflammation and soft tissue swelling, but are not effective in preventing cartilage breakdown and the joint destruction associated with RA.
The pomegranate has been used for centuries as a therapeutic agent for the treatment of inflammatory diseases and disorders of the digestive tract by the practitioners of the Ayurvedic and Unani systems of medicine (13). More recently standardized extracts of pomegranate have been shown to have anti-inflammatory and cartilage sparing effects in vitro, cancer preventing and cardiovascular diseases preventing effect in vivo (14, 15, 16, 17, 18, 19). The major effect of the pomegranate extract was shown to be the reduction of oxidative stress, inhibition of p38-mitogen-activated protein kinase (p38-MAPK) pathway and inhibition of the activation of transcription factor NF-κB. Activation of p38-MAPK and NF-κB is intimately associated with the increased gene expression of TNF-α, IL-1β, MCP1, iNOS and COX-2-agents that are critical mediators of joint inflammation and the pathogenesis of RA (20, 21).
Previous studies from our group have shown that a standardized pomegranate extract rich in hydrolysable tannins and anthocyanins was highly effective in exerting human cartilage sparing effects in vitro (19). In this study we have compared the efficacy of a commercial standardized preparation of pomegranate fruit extract (POMx) in a mouse model of RA. The animal model used-collagen-induced arthritis in mice (CIA)-is a well characterized animal model and has been used for more than 2 decades as an experimental model with which to study RA. Joint histopathology in this animal model shows features that are remarkably similar to those seen in RA joints. The significance of the model also lies in the fact that CII is the major constituent protein of the cartilage in the diarthrodial joints-the primary site affected in RA (22, 23). Because of many compelling similarities between CIA and RA, CIA is an excellent model to develop and test preventive and therapeutic approaches for the prevention and or treatment of arthritis in humans (24).
Materials and Methods
Mice
In the present study, we used 8-week-old male DBA/1 Lac J mice (Jackson Laboratory, Bar Harbor, ME) with an average body weight of 20.0 g. The mice were maintained under specific pathogen free (SPF) conditions with free access to tap water and commercial diet. All animal experiments were undertaken with the approval of the IACUC and were in accordance with the approved guidelines for care and use of laboratory animals.
Chemical Composition of the Pomegranate Extract (POMx)
POMx is a standardized pomegranate fruit extract provided as a dietary supplement. It is produced from pomegranates (Punica granatum L., Wonderful variety) grown in California by Paramount Farms. POMx is produced in a two step process: (1) extraction of fruit residue after pressing for juice and (2) solid phase extraction to produce a powder with a high concentration of polyphenols. Extraction is performed during fruit harvest using pressed pomegranate fruit and arils. The powder extract used in this study contains on average 86.0 % ellagitannins, 2.5 % ash, 3.2 % sugars, 1.9 % organic acids as citric acid equivalents, 0.8 % nitrogen and 1.2 % moisture. The approximate percent distribution of pomegranate polyphenols is as follows: 1) Ellagitannins as punicalagins and punicalins, 19 %; 2) free ellagic acid, 4%; and 3) oligomers composed of two to ten repeating units of gallic acid, ellagic acid, and glucose in different combinations, 77%.
Type-II collagen, antibodies and ELISAs
Chicken type-II collagen was purchased from Chondrex, Seattle, WA. Antibodies were purchased from Cell Signaling Technology, MA, and Santa Cruz Biotechnology, Santa Cruz, CA. Kits for performing cytokine ELISAs were purchased from eBiosciences, San Diego, CA and R & D Systems, St. Paul, MN. Anti-type-II collagen antibody ELISA kit was purchased from Chondrex (Seattle, WA). All other chemicals were from Sigma-Aldrich, St Louis, MO.
Immunization of mice and induction of CIA
After 1 week of acclimatization, animals were randomly divided into 3 groups of 8 animals each: Group-1, water-fed, Control (C), Group-2 (mice given 13.6 mg/Kg POMx), Group-3 (mice given 34 mg/Kg POMx). Mice were given the stated dose of POMx dissolved in water by gavage for 10 days prior to the induction of arthritis with animals in the control group receiving equal amount of water the same way. This regimen was continued after immunization with type-II collagen for the induction of arthritis. CIA was induced in all 3 groups of mice by immunization with chicken type-II collagen (Chondrex) as previously described (1). In brief, mice received intradermal immunization with 100 µg of chicken type-II collagen emulsified in complete Freund’s adjuvant (CFA) and were boosted 3 weeks later with Chicken type-II collagen emulsified in incomplete Freund’s adjuvant (IFA). Mice were scored for the severity of arthritis using the previously described visual scoring system (25). Briefly, grade 0, no swelling or erythema; grade 1, mild swelling and erythema or digital inflammation; grade 2 moderate swelling and erythema confined distal to the mid paw; grade 3, more pronounced swelling and erythema with extension to the ankle; grade 4, severe swelling, erythema, and joint rigidity of the ankle, foot and digits. Thus a mouse could have a maximum arthritis severity score of 16.
Histopathology of the joints
Affected limbs were harvested and dissected free of soft tissue as much as possible. These were then decalcified prior to embedding in paraffin. Sections were stained with H & E and scored by an investigator blinded to the treatment for synovitis, pannus formation and bone and cartilage destruction. The sections were scored as previously described (26). Briefly, grade 0, normal; grade 1, mild inflammation, mild hyperplasia of the synovial lining layer, mild cartilage destruction without bone erosion; grades 2–4, increasing degrees of cellular infiltration, synovial lining hyperplasia, pannus formation, and bone and cartilage destruction.
Mouse macrophages and stimulation
Mouse macrophages (RAW 264.1, ATCC) were cultured in complete MEM (InVitrogen) with 10% FBS. Confluent (80%) macrophage cultures were serum starved and then pretreated with POMx (20 µg/ml) for 2 hr and stimulated with LPS (50 ng/ml) for different periods of time. Cell lysates were prepared and used for Western Immunoblotting and culture supernatants were used for the measurement of cytokines by ELISA. POMx concentrations used in these studies were derived from pilot studies employing RAW 264.1 cells.
Determination of nitric oxide production
Culture supernatants from LPS stimulated macrophages were used for the determination of nitrite levels by Griess reaction as an indicator of NO production using a commercially available kit (Molecular Probes-InVitrogen).
Protein Determination
Total protein content was determined using the Bio-Rad detergent compatible protein assay kit (Bio-Rad) according to the instructions. BSA (Sigma) was used as standard.
Enzyme-linked Immunosorbent Assay (ELISA)
Harvested limbs were dissected free of soft tissue as much as possible and then snap frozen in liquid nitrogen. Cell free lysate was then prepared as previously described (1). Total lysate protein or culture supernatant was used to measure the levels of anti-type-II collagen antibodies (Chondrex), IL-6 (eBiosciences), IL-1β, and TNF-α (R & D Systems) using sandwich ELISAs according to the instructions of the manufacturer. The limit of detection (sensitivity) of the IL-6 kit was 4 pg/ml and for IL-1 and TNF-α it was 7.8 and 5.1 pg/ml respectively. ELISA results were quantitated by the absorbance at 450 nm using a Synergy HT microplate reader (BioTek Instruments, VT).
Western Immunoblotting
Cell lysates were prepared from harvested joints and stimulated macrophages (lysis buffer: 50 mM Tris:HCl, pH 7.4, NaCl 150 mM, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, Complete Protease inhibitor cocktail [Roche], phosphatase inhibitor cocktail [Roche]) as previously described (1, 27). Total lysate protein (25 µg/lane) was resolved by SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad), blocked with skim milk and probed with primary and secondary antibodies (Cell Signaling Technologies, Santa Cruz Biotechnology). Immunoreactive proteins were visualized by enhanced chemiluminescence (GE Healthcare) as previously described (28). Images were captured and analyzed using the Alpha-Innotech Imaging System and software.
Luciferase Reporter Assay
Mouse macrophages were seeded at 3×105 cells/ml in 6 well plates in MEM growth medium (with FBS and without antibiotic), day before transfection. Next day, cells were transiently transfected using the NF-κB luciferase reporter gene construct (Panomics, Fremont, CA) and Lipofectamine 2000 according to protocol provided by the manufacturer (InVitrogen). Fresh growth media with 10 % FBS was added 5h after the incubation of cells and incubation continued for 18 h in a tissue culture incubator (total incubation time 23 hr). Cells were then starved for 6 h in MEM without FBS and stimulated by LPS (100 ng/ml) for 18 hours. To determine the effect of POMx, cells were pretreated with PomX (40ug/ml) for 2 h prior to stimulation with LPS. Cells were then harvested and washed with PBS, lysed by adding 200 ul of lysis buffer and the cell lysates was prepared according to the instructions provided with the kit (Luciferase Assay kit, Promega, Madison, WI). Luciferase activity in the cell lysates was measured using a tube luminometer (Promega) and kit supplied substrate and the results were expressed as Relative Light Units (RLU). Macrophages stimulated with LPS in the presence of PolymyxinB (PMB,10 µg/ml) were used as positive controls for the LPS-induced activation of NF-κB.
Statistical Analysis
Arthritis scores and histology scores were compared by the Mann-Whitney U test using the GraphPad statistical software package. Cytokine and NO levels were compared using the unpaired 2-tailed Student’s ‘t’ test (GraphPad Software). P values less than 0.05 were considered significant.
Results
Consumption of POMx reduces the incidence and severity of CIA in mice
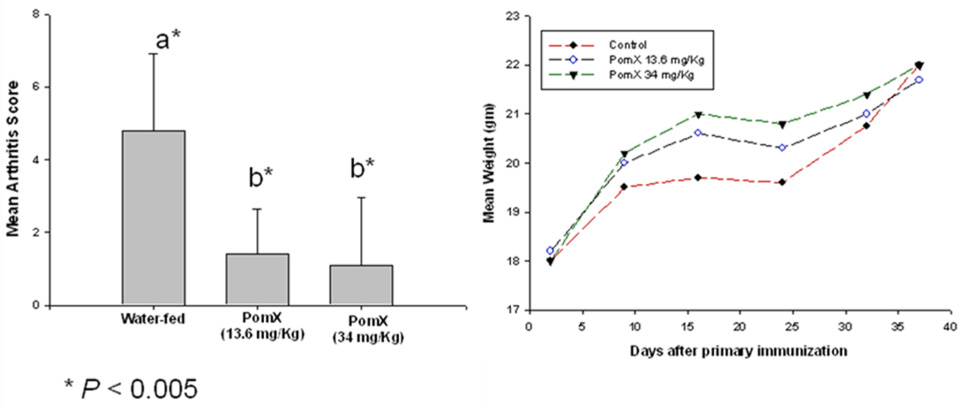
In these experiments we determined the ability of POMx to prevent the development of inflammatory arthritis in DBA/1 mice. Arthritis was induced by immunizing DBA/1 mice with hetrologous type-II collagen (C-II) emulsified in CFA followed by a booster dose three weeks later with C-II emulsified in incomplete Freund’s adjuvant (IFA). Mice were dosed orally with POMx 10 days prior to immunization for the induction of arthritis and then continued to receive POMx orally until the termination of the experiment. Mice orally fed either 13.6 mg/Kg or 34 mg/Kg POMx displayed significant reductions in disease incidence (Figure-1A) and delay in the onset of disease (Figure-1B). Clinical severity of arthritis was also reduced in mice fed POMx (Figure-1C) based on reduced paw swelling, erythema and joint rigidity as determined by the mean visual arthritis score (P <0.05). There was no apparent toxicity or weight loss in mice receiving POMx orally (Figure-1D). Control mice (water-fed) showed a loss in body weight at 3 weeks but the difference in body weight did not reach statistical significance when compared to the body weights of POMX-fed mice at any of the time periods used in this study (P > 0.05).
Figure 1. Consumption of POMx prevents and delayed the onset of CIA in susceptible mice.
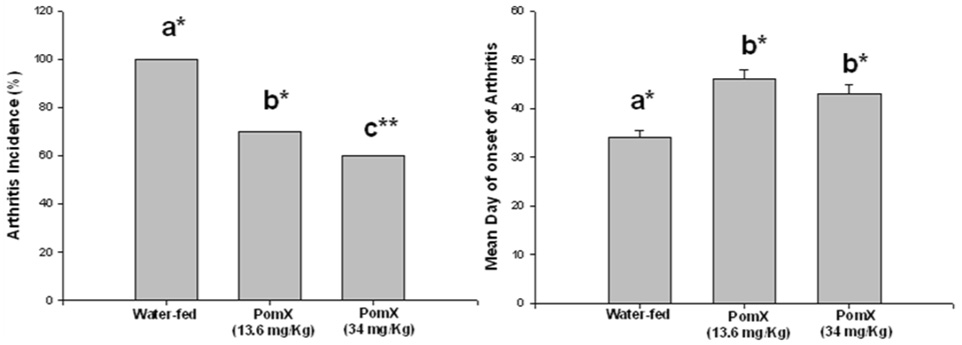
CIA susceptible DBA/1 mice (10 mice/group) were administered either 13.6 mg/Kg or 34 mg/Kg POMx dissolved in water or plain water orally every day starting 10 days prior to immunization with chicken type-II collagen to induce arthritis. (A) The incidence of arthritis at the termination of the experiment; (B) The mean day of onset of arthritis in different groups of mice; (C) severity of arthritis was assessed using subjective scoring system; and (D) Mean weights of mice in each group are shown. Values shown are Mean ± SE and differ without a common letter; *P <0.05; **P<0.005.
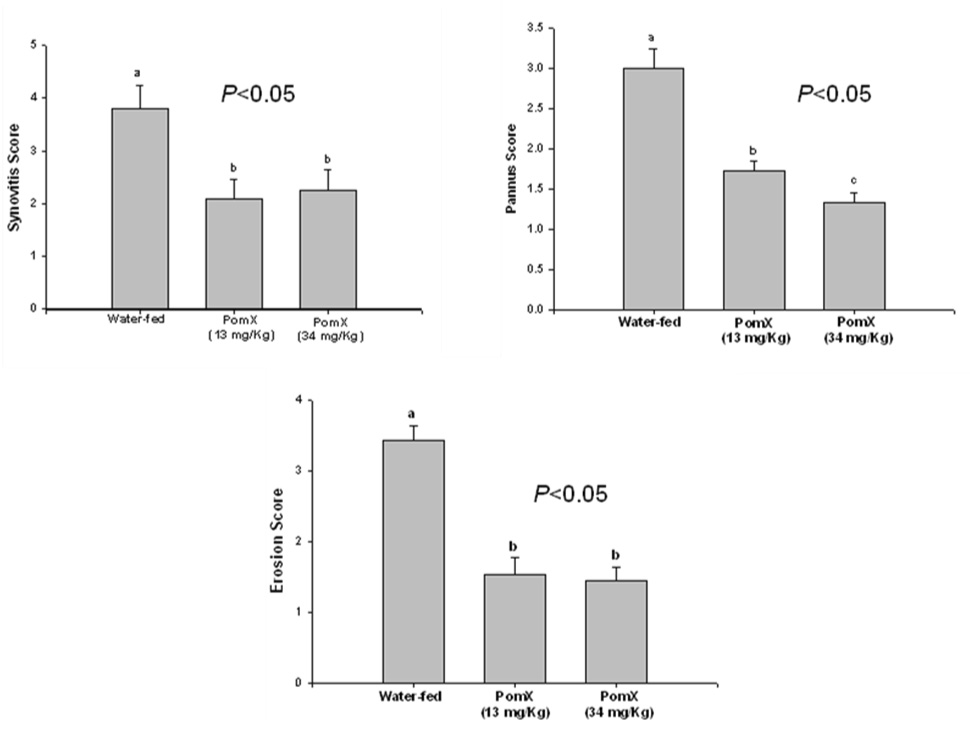
Histopathological analysis was performed on affected paws (fore or hind paws) harvested from control mice (no treatment) and mice with CIA receiving water or POMx. Representative images of H & E stained joint sections from these groups are shown in Figure-2 A. These data show that in treatment groups oral consumption of POMx resulted in statistically significant reductions in synovitis, pannus and bone erosion scores when compared to water-fed mice immunized the same way to get arthritis (Figure-2B).
Figure 2. (A). POMx inhibits synovitis, pannus formation and joint degradation in CIA.
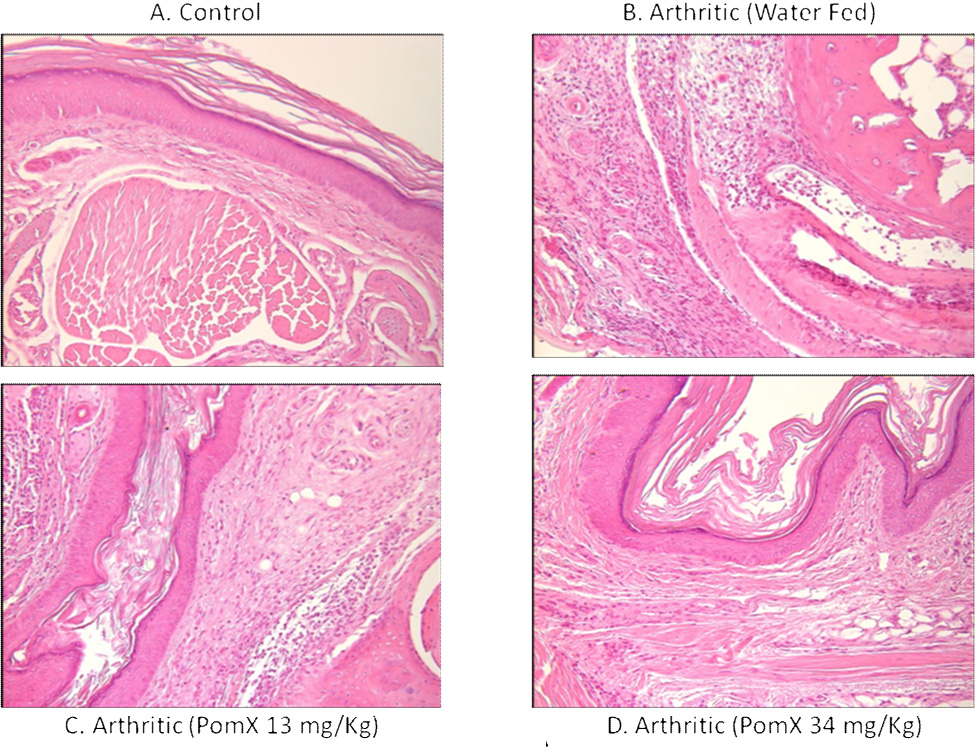
Representative H & E stained joint tissue sections from (A) control DBA/1 mice (no treatment); (B) water-fed DBA/1 mice immunized to get arthritis; (C) DBA/1 mice orally fed with POMx (13.4 mg/Kg) daily and immunized to get arthritis; and (D) DBA/1 mice orally fed with POMx (34 mg/Kg) daily and immunized to get arthritis.
(B) Histopathological scores of synovitis, pannus formation and bone and cartilage erosions in CIA susceptible DBA/1 mice. Values are Mean ± S.E. and differ without a common letter (P < 0.05)
POMx inhibits macrophage production of inflammatory mediators
It has previously been shown that activated macrophages infiltrate the joint and produce several inflammatory mediators in the joint microenvironment (reviewed in 29). One of these mediators is the NO which has been shown to be produced by activated macrophages and high levels of NO have been detected in arthritic joints (29). Based on this knowledge we characterized the effect of POMx on the activation of mouse macrophages. Mouse macrophages were cultured, pretreated with POMx and then stimulated with LPS and the production of the second messenger nitric oxide (NO) in the culture supernatant was determined. POMx at the concentration used dramatically inhibited the production of NO (P < 0.001) compared to the cultures stimulated with LPS alone (Figure-3). As high level expression of NO impact the cellular response to injury and its high levels can be pathogenic, these results suggest that POMx may inhibit joint damage by suppressing the production of NO.
Figure 3. POMx inhibits the LPS-induced production of NO in mouse macrophages in vitro.
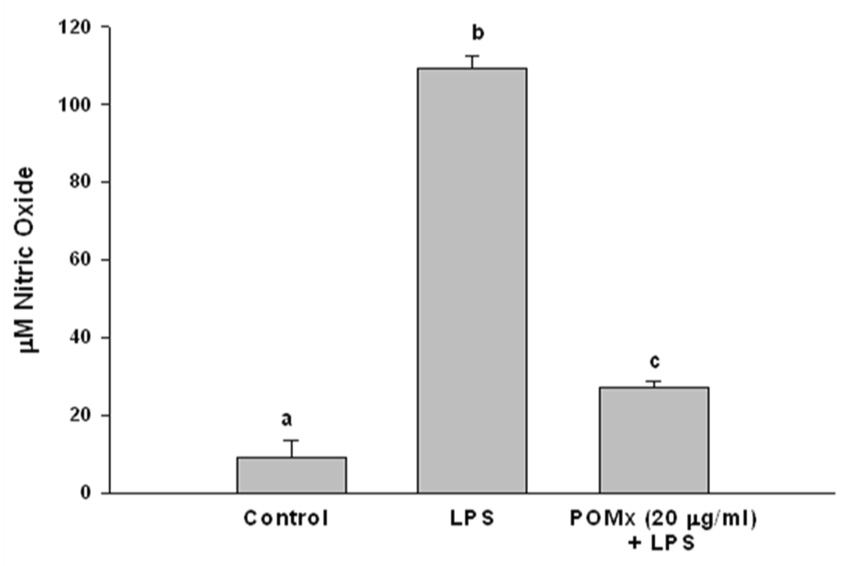
Mouse macrophages were treated with 20 µg/ml of POMx only, with LPS only or pre-treated with 20 µg/ml of POMx and then stimulated with LPS (50 ng/ml) for 24 h. At the end of stimulation 100 µl of the culture medium was removed and used for the estimation of the nitrite production. Values are Mean ± S.E. and derived from 3 independent experiments and differ without a common letter (P < 0.005).
POMx effect on the production of anti-type-II collagen antibodies in mice
We also determined the presence and level of anti-type-II collagen antibodies by CII-specific ELISA in the arthritic joints of POMx-fed mice and non-POMx-fed mice with arthritis. There was no statistically significant difference in the levels of anti-CII-specific antibodies in any of the groups (P > 0.05, results not shown). This suggests that the reduced incidence and severity of arthritis in POMx-fed mice could be due to the modulation mainly of cellular immune response.
POMx effect on inflammatory cytokines in arthritic joints
Since the cytokine milieu is a critical mediator of the pathogenesis of arthritis, the production of IL-1β, IL-6 and TNF-α was examined by ELISA in the arthritic joints lysate prepared from joints isolated from POMx-fed and non-POMx-fed mice with CIA. Previous studies have suggested that these cytokines play a pivotal role in RA (30, 31, 32, 33). Our results (Figure-4) showed that in arthritic joints isolated from non-POMx-fed mice levels of the inflammatory cytokine IL-6 were higher in the joints of non-POMx-fed mice compared to the levels present in arthritic joints of POMx-fed mice. Although the levels of IL-1β and TNF-α were low in the arthritic joints of POMx-fed mice the difference was not statistically significant when compared to the levels detected in the joints of non-POMx-fed mice. None of these cytokines were detected in the joint lysates of untreated control mice (results not shown). Low dose POMx feeding was more effective in inhibiting the production of IL-1β while the higher dose tested was more effective in suppressing the production of TNF-α and IL-6 (Figure-4). The reason behind this observation is not clear but suggests a window beyond which the consumption of pomegranate extract may not be useful. Taken together, these results suggest that the reduced incidence and severity of CIA may be related to the lower level of IL-6 in POMx-fed mice. Absence of significant differences in the levels of TNF-α in the arthritic joints of untreated and POMx treated mice suggests that POMx constituents exert their joint sparing effects independently of the suppression of TNF-α, possibly by modulating the production of other inflammatory mediators in arthritis.
Figure 4. POMx inhibits inflammatory cytokine production in ankle joints of CIA susceptible DBA/1 mice.
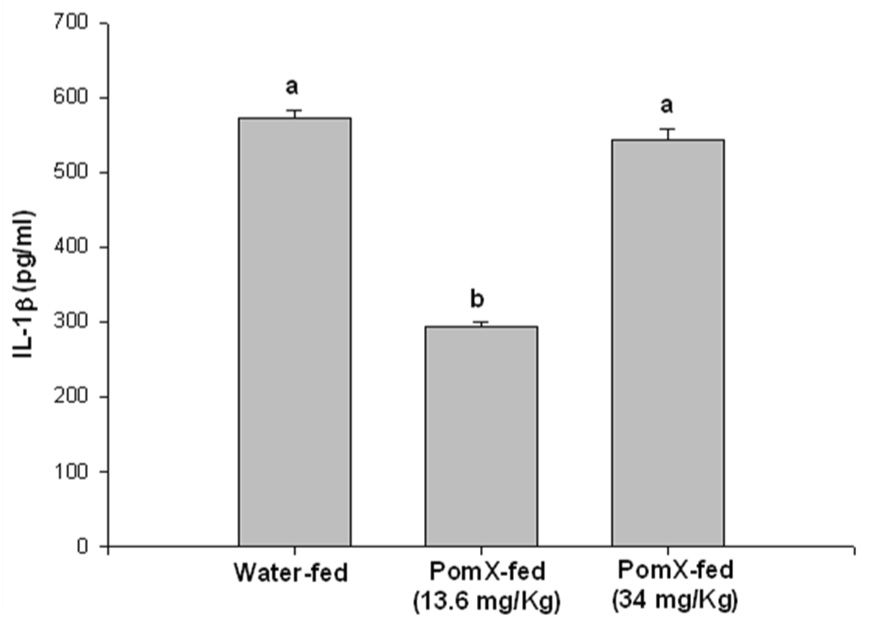
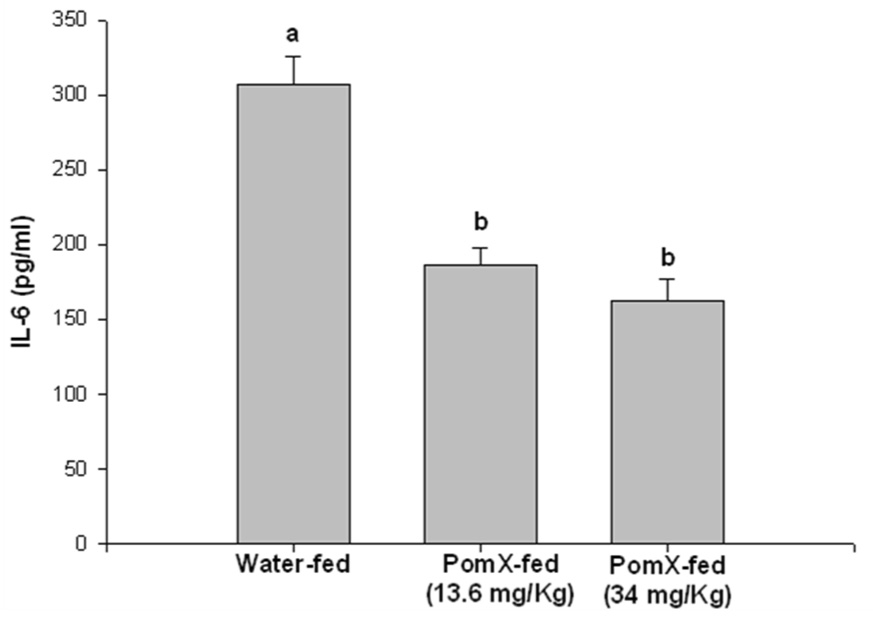
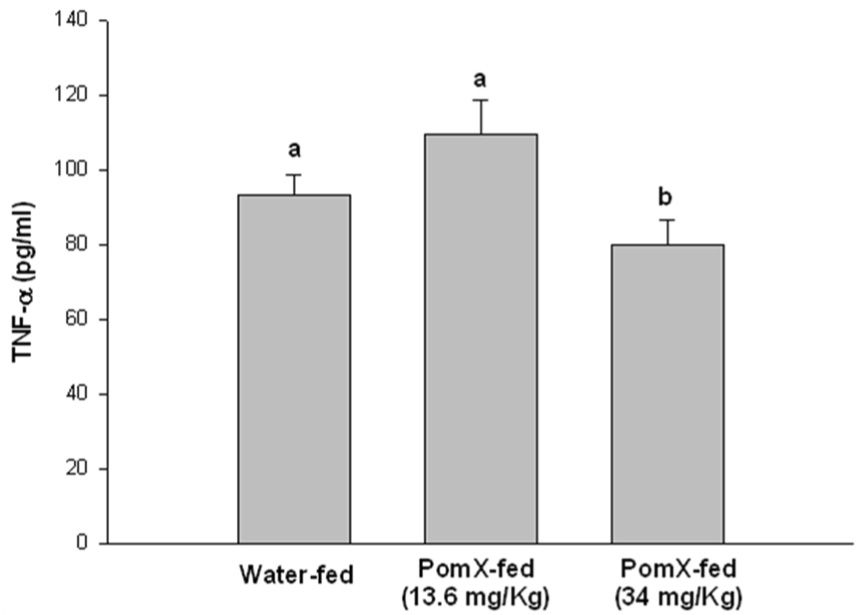
Arthritic mice given water only or orally fed different doses of POMx were killed at the end of the experiment and ankle joints were isolated from each mouse, snap frozen in liquid nitrogen and ground to a fine powder. Lysis buffer at room temperature was added to the powder, homogenized and the lysates were centrifuged and the supernatant was used to measure the levels of (A) IL-1β, (B) IL-6 and (C) TNF-α using ELISA kits. Values are Mean ± S.E. and differ without a common letter (P < 0.05). Water-fed with arthritis n = 3, POMx-fed (13.4 mg/Kg) n = 5; POMx-fed (34 mg/Kg) n = 3.
POMx inhibits MAPK signal transduction pathways in mouse macrophages
High levels of pro-inflammatory cytokines are present in RA joints and can induce the production of joint degrading mediators by macrophages and other cell types thereby exacerbating arthritis (29). Activation of MAPK signal transduction pathways plays an important role in the production of such molecules in rheumatoid arthritis (34, 35). To determine whether POMx affects inflammatory stimuli-mediated MAPK signal transduction in mouse macrophages we used immunoblotting to characterize lysates generated from mouse macrophages. Mouse macrophages were pre-treated with POMx (20 µg/ml), stimulated with 50 ng/ml of LPS for different periods of time and lysates were prepared for analysis. Results of studies employing immunoblotting with highly specific antibodies showed that when mouse macrophages were stimulated with LPS alone, phosphorylation of JNK was rapid and intense, reached the peak between 15 and 30 min post stimulation and then declined (Figure-5A). On the other hand stimulation of mouse macrophages stimulated in the presence of POMx, LPS-induced phosphorylation of JNK was delayed with weakly phosphorylated JNK being detected at 30 min post-stimulation and then barely detectable at 120 min post-stimulation (Figure-5A). Also, the phosphor-JNK/Total JNK analysis showed that phosphorylation of JNK molecules was higher in cells stimulated with LPS alone (Figure-5B) compared to cells stimulated with LPS in the presence of POMx (Figure-5C).Taken together these data indicate that POMx inhibited the LPS-induced activation of JNK pathways while levels of total JNK were not affected (Figure-5A and results not shown). Phosphorylation of the downstream signaling molecule ATF-2, a target of the SAPK/JNK signaling pathways (21), was also inhibited in mouse macrophages pre-treated with POMx (not shown). These data provide support to the hypothesis that POMx exert its anti-inflammatory effect by modulating the activation of JNK pathway in macrophages.
Figure 5. POMx inhibition of JNK activation in mouse macrophages.
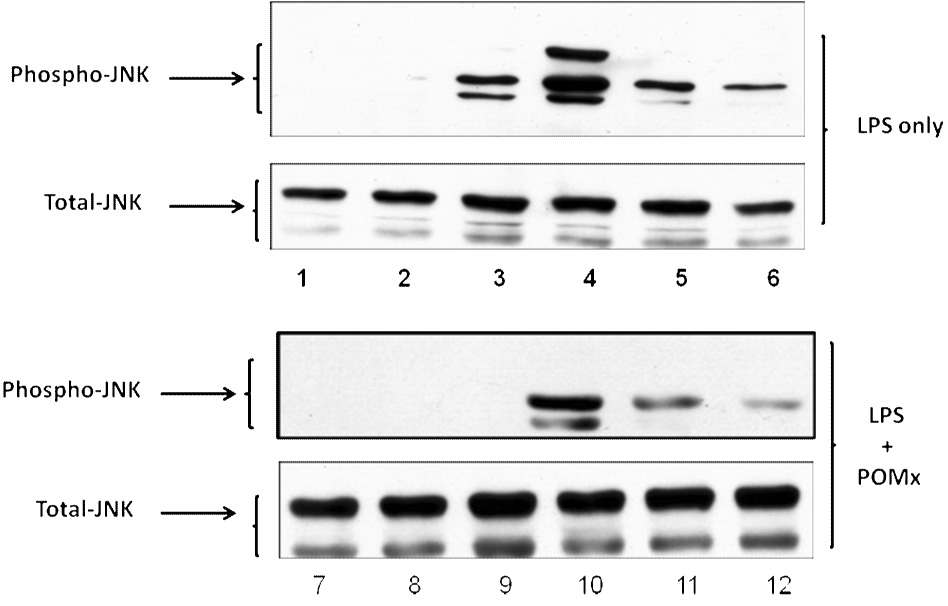
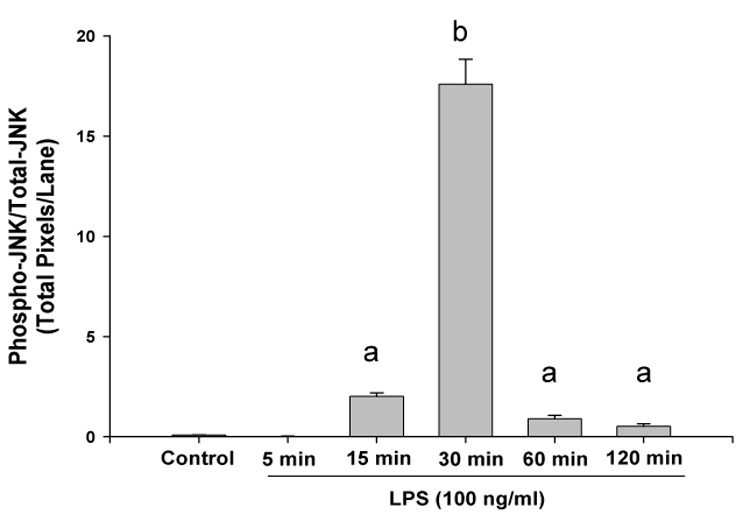
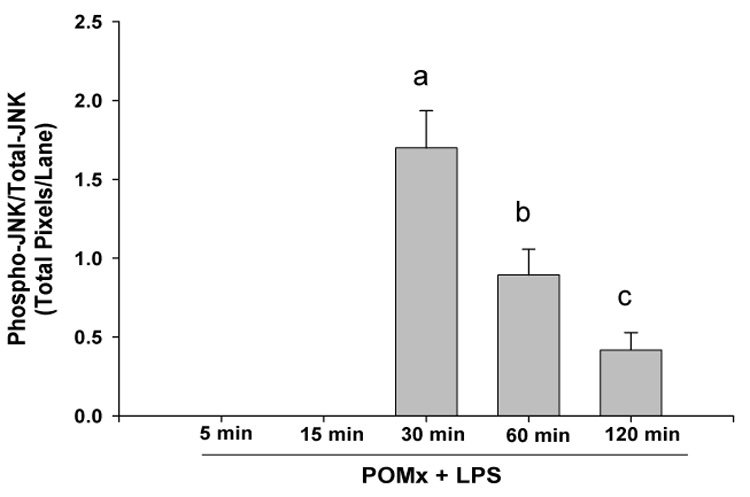
RAW 264.1 mouse macrophages were stimulated with LPS alone, or pre-treated with POMx and then stimulated with LPS for different time periods and cell lysates were prepared for immunoblotting. Immunoblots were probed with antibodies specific for JNK and phospho-JNK. (A) Lanes 1–6, control and cells stimulated with LPS for 5, 15, 30, 60 and 120 min; Lanes 7–12, control, cells pre-treated with 20 µg/ml POMx and then stimulated with LPS for 5, 15, 30, 60 and 120 min. (B & C) Phospho-JNK/Total JNK ratio for results shown in 5A.
POMx inhibits activation of NF-κB in mouse macrophages
The activation of NF-κB is essential for the induction and expression of many pro-inflammatory genes, such as inducible nitric oxide synthase (iNOS, NOS2), IL-6, which may be relevant to the pathogenesis of arthritis. Activation of NF-κB typically requires the phosphorylation of the inhibitory protein IκB by the IκB kinase (IKK) complex which results in the degradation of IκB. This releases NF-κB and allows it to translocate to the nucleus where it binds to the promoters of target genes. Since our results showed that treatment with POMx inhibited the LPS-induced production of NO by mouse macrophages, we determined whether POMx also inhibit the activation of NF-κB in mouse macrophages. We used the NF-κB-Luc reporter vector to transfect the mouse macrophages, treat the transfected macrophages with POMx and then these cells were stimulated with LPS. Activation of NF-κB was determined by measuring the activity of the reporter enzyme in the macrophages. These results are shown in Figure-6 and clearly demonstrate that POMx was effective in suppressing the LPS-induced activation of NF-κB in mouse macrophages in vitro.
Figure 6. POMx inhibition of NF-κB activation in mouse macrophages.
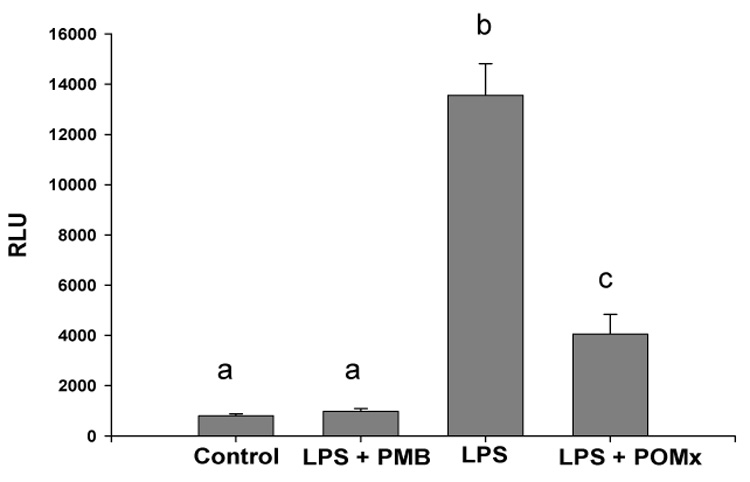
Mouse macrophages were transfected with the reporter vector, treated with POMx and then stimulated with LPS for 18 hrs. Luciferase enzyme activity was measured using a luminometer. Experiments were repeated and each assay was performed in triplicate. Results expressed in RLU are Mean ± SE and differ without a common letter (P < 0.001).
Discussion
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the connective tissue and is characterized by synovial hyperplasia with local invasion of bone and cartilage. The disease is largely driven by the recruitment of activated T cells and B cells and macrophages to the afflicted joints. Pro-inflammatory cytokines such as IL-6, TNF-α produced by these activated cells contribute to the irreversible joint damage seen in RA. In view of the historical use and currently reported anti-inflammatory effects of pomegranate extract in several model systems (14–19), we explored the potential of consumption of POMx for preventing joint damage in RA. In this study we demonstrate that consumption of POMx robustly prevents the development of CIA in mice (an animal model of RA) by selectively inhibiting a spectrum of signal transduction pathways and cytokines critical to the development and maintenance of inflammation in RA. Our results demonstrate that consumption of POMx reduces the incidence and severity of CIA in mice (Figure-1). We also demonstrate that POMx abrogates the phosphorylation of JNK and production of pro-inflammatory cytokines and inhibits cytokine-induced NO production in mouse macrophages. The c-Jun N-terminal protein kinase mitogen-activated protein kinases (JNK) is a sub-group of the evolutionarily-conserved family of serine/threonine protein kinases. JNK is activated by treatment of cells with inflammatory cytokines (such as TNF-α) and by exposure of cells to endotoxin. Previous studies have demonstrated that inhibition of JNK inhibits joint damage in inflammatory arthritis (36). Thus our in vitro and in vivo data collectively indicate that POMx potently inhibits a range of cellular responses, including the activation of JNK, that play critical roles in driving synovitis, pannus formation, and joint destruction in RA. In all experiments there were no signs of toxicity in any of the groups as evidenced by weight gain (Figure-1D), ALT or serum creatinine levels (data not shown). No mortality due to POMx in any of the groups was observed.
Dietary supplements are taken orally and hence our use of gavage feeding was chosen to maximize the delivery so that bioactivity of the administered dose could be determined independently of other factors. Examination of the data reveals that this regimen was effective in protecting mice from the development of CIA as statistically significant arthritis inhibition and joint sparing effects were demonstrated in POMx-fed mice compared to water-fed mice (Figure-1 & Figure-2). These results provide the first in vivo evidence of antiarthritic efficacy of a chemically complex mixture of pomegranate fruit that is commercially available. Because in these studies pretreatment was required to inhibit the incidence and severity of joint inflammation, these data support the use of POMx for arthritis prevention and not for arthritis treatment in the face of active inflammation. Future studies will address the disease modifying aspect of POMx consumption.
Macrophages are present in RA joints and play an important role in the pathogenesis of RA (reviewed in 37). These activated cells produce TNF-α, NO and other inflammatory mediators that exacerbate RA. Using biochemical techniques and immunoblotting we demonstrate that POMx inhibits LPS-induced activation of the JNK and NF-κB signal transduction pathways and downstream activation of transcription factors and the production of NO in mouse macrophages. NO is synthesized from L-arginine by the enzyme NO synthase (NOS). After induction of the inducible isoform of NOS (iNOS) by endotoxin or inflammatory cytokines, NO is overproduced resulting in the cyto- and bacteriotoxic effects. Role of NO in inflammation is also evident from its inhibitory effects on T cell activity, chemotaxis of monocytes and migration of neutrophils, production of PGE2 and thromboxane in macrophages (reviewed in 38). Studies with animal models of inflammatory arthritis and in patients with rheumatoid arthritis have demonstrated an important role of high levels of NO in the pathogenesis of the disease (38). Our novel data presented here suggest thats POMx-mediated inhibition of macrophages could contribute to its efficacy in inhibiting CIA as shown in this study and possibly in human RA.
Transcription factors NF-κB are ubiquitously expressed and can both induce and repress gene expression by binding to κB elements present in the promoters of target genes including the genes that control apoptosis, adhesion, inflammation, and tissue remodeling (reviewed in 39). Activation of NF-κB involves the phosphorylation of its inhibitory protein IκB by IκB kinase (IKK) complex which results in its degradation and release of NF-κB. It has been shown that the incidence and severity of CIA were decreased significantly in transgenic mice constitutively expressing IκB compared with nontransgenic littermates (40). Similar results were obtained when mice immunized to get CIA were treated with a small molecule inhibitor of NF-κB (41). Attenuation of murine CIA by administration of a selective small molecule inhibitor of NF-κB correlated with the reduction in the levels of inflammatory cytokines such as IL-6 in the paw tissues of arthritic mice (42). These data provide support to the results presented here showing that POMx inhibited the endotoxin-induced activation of NF-κB in mouse macrophages. Significantly low levels of IL-6 detected in the arthritic joints of POMx-fed mice compared to control mice (P <0.005) may be related to the in vivo inhibitory effect of POMx on NF-κB activation. However, this remains to be investigated.
Additionally, low levels of inflammatory cytokines detected in the arthritic joints of the POMx-fed mice also support the hypothesis that the observed reduction in joint inflammation scores was likely due to the inhibition of inflammatory cytokine production in the joints. In contrast to the ineffectiveness of high dose POMx on the production of IL-1β in arthritic joints, low dose feeding of POMx was effective in suppressing the production of IL-1β (Figure-4A) while high dose was needed to suppress the production of TNF-α (Figure-4C). These results suggest that the constituents of POMx are needed in different concentrations to suppress the damaging effects of IL-1β and TNF-α in arthritis and other diseases associated with abnormal production of these cytokines. Identification of these components of POMx will open new avenues for the development of effective preventive therapies against arthritis. Moreover, our demonstration of arthritis preventive and joint protective effects of POMx consumption provide support to the use of POMx or pomegranate containing dietary supplements for the prevention of inflammatory arthritis.
Conclusions
Results of these translational studies and studies reported previously (19) together provide strong and compelling evidence to support further clinical testing of POMx for the prevention of RA and osteoarthritis. However, this must be mentioned that to our knowledge no clinical trials in RA patients have yet been done to document the safety and efficacy of POMx or other extracts of pomegranate available for over-the-counter use.
Acknowledgements
This work was supported in part by USPHS grants R01 AR048782 (NIH/NIAMS), R01 AT003627 (NIH/NCCAM) and a grant from The Lynda and Stewart Revocable Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci U S A. 1999;96:4524–4529. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moller B, Villiger PM. Inhibition of IL-1, IL-6 and TNF-α in immune-mediated inflammatory diseases. Springer Semin Immunopathol. 2006;27:391–408. doi: 10.1007/s00281-006-0012-9. [DOI] [PubMed] [Google Scholar]
- 5.Arend WP, Dayers JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor-α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 6.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 7.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 8.Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, et al. Etanercept therapy in rheumatoid arthritis. A randomized controlled trial. Ann Intern Med. 1999;130:478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 9.Feldman M, Maini RM. Anti-TNF-α therapy of rheumatoid arthritis: what have we learned. Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 10.Campbell IK, O’Donnell K, Lawlor KE, Wicks IP. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF-α. J Clin Invest. 2001;107:1519–1527. doi: 10.1172/JCI12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd DJ, Costenbader KH, Weinblatt ME. Abatacept in the treatment of rheumatoid arthritis. Int J Clin Pract. 2007;61:494–500. doi: 10.1111/j.1742-1241.2007.01289.x. [DOI] [PubMed] [Google Scholar]
- 12.Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006;33:2398–2408. [PubMed] [Google Scholar]
- 13.Palaniswamy R. A guide to Medicinal Plants of Asian Origin and Culture. Newbury, UK: CPL Press; 2003. Southgate MT. 2007. JAMA 297:781. [Google Scholar]
- 14.de Nigris F, Williams-Ignarro S, Sica V, Lerman LO, D'Armiento FP, Byrns RE, et al. Effects of a pomegranate fruit extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc Res. 2007;73:414–423. doi: 10.1016/j.cardiores.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Esmaillzadeh A, Tahbaz F, Gaieni I, Alavi-Majd H, Azadbakht L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int J Vitam Nutr Res. 2006;76:147–151. doi: 10.1024/0300-9831.76.3.147. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblat M, Volkova N, Coleman R, Aviram M. Pomegranate byproduct administration to apolipoprotein e-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J Agric Food Chem. 2006;54:1928–1935. doi: 10.1021/jf0528207. [DOI] [PubMed] [Google Scholar]
- 17.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005;135:2096–2102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden MS, Ghosh S. Siganling to NF-κB. Genes Develop. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 21.Schieven GS. The biology of p38 kinase: A central role in inflammation. Curr Topics Med Chem. 2005;5:921–928. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- 22.Cook AD, Stockman A, Brand CA, Tait BD, Mackay IR, Muirden KD, et al. Antibodies to type-II collagen and HLA disease susceptibility markers in rheumatoid arthritis. Arthritis Rheum. 1999;42:2569–2576. doi: 10.1002/1529-0131(199912)42:12<2569::AID-ANR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- 24.Anthony DD, Haqqi TM. Collagen-induced arthritis in mice: an animal model to study the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:240–244. [PubMed] [Google Scholar]
- 25.Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 27.Miller MJ, Bobrowski P, Shukla M, Gupta K, Haqqi TM. Chondroprotective effects of a proanthocyanidin rich amazonian genonutrient reflects direct inhibition of matrix metalloproteinases and upregulation of IGF-1 production by human chondrocytes. 2007. J Inflamm (Lond) 2007;14(41):16. doi: 10.1186/1476-9255-4-16. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hafeez BB, Ahmed S, Wang N, Gupta S, Zhang A, Haqqi TM. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol Appl Pharmacol. 2006;216:11–19. doi: 10.1016/j.taap.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Burmester GR, Stuhlmuller B, Keyszer G, Kinne RW. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997;40:5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- 30.Neidhart M, Gay RE, Gay S. Anti-interleukin-1 and anti-CD44 interventions producing significant inhibition of cartilage destruction in an in vitro model of cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2000;43:1719–1728. doi: 10.1002/1529-0131(200008)43:8<1719::AID-ANR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–343. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Tarner IH, Muller-Ladner U, Gay S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2007;3:336–345. doi: 10.1038/ncprheum0506. [DOI] [PubMed] [Google Scholar]
- 33.Asquith DL, McInnes IB. Emerging cytokine targets in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:246–251. doi: 10.1097/BOR.0b013e3280eec78c. [DOI] [PubMed] [Google Scholar]
- 34.Westra J, Limburg PC. p38 mitogen-activated protein kinase (MAPK) in rheumatoid arthritis. Mini Rev Med Chem. 2006;6:867–874. doi: 10.2174/138955706777934982. [DOI] [PubMed] [Google Scholar]
- 35.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 38.Stichtenoth DO, Frolich JC. Nitric oxide and inflammatory joint diseases. Br J Rheumatol. 1998;37:246–257. doi: 10.1093/rheumatology/37.3.246. [DOI] [PubMed] [Google Scholar]
- 39.Perkins ND. Integrating cell signaling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 40.Seetharaman R, Mora AL, Nabozny G, Boothby M, Chen J. Essential role of T cell NF-kappa B activation in collagen-induced arthritis. J Immunol. 1999;163:1577–1583. [PubMed] [Google Scholar]
- 41.Gerlag DM, Ransone L, Tak PP, Han Z, Palanki M, Barbosa MS, Boyle D, Manning AM, Firestein GS. The effect of a T cell-specific NF-kappa B inhibitor on in vitro cytokine production and collagen-induced arthritis. J Immunol. 2000;165:1652–1658. doi: 10.4049/jimmunol.165.3.1652. [DOI] [PubMed] [Google Scholar]
- 42.Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, Mellor GW, Evans C, Roshak AK. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl) amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J Pharmacol Exp Ther. 2005;312:373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]