Abstract
Paralysis of the diaphragm is a severe consequence of cervical spinal cord injury. This condition can be experimentally modeled by lateralized, high cervical lesions that interrupt descending inspiratory drive to the corresponding phrenic nucleus. Although partial recovery of ipsilateral diaphragm function occurs over time, recent findings show persisting chronic deficits in ventilation and phrenic motoneuron activity. Some evidence suggests, however, that spontaneous recovery can be enhanced by modulating neural pathways to phrenic motoneurons via synaptic circuitries which appear more complex than previously envisioned. The present review highlights these and other recent experimental multi-disciplinary findings pertaining to respiratory neuroplasticity in the rat. Translational considerations are also emphasized, with specific attention directed at the clinical and interpretational strengths of different lesion models and outcome measures.
Introduction
Among the more significant advances in spinal cord injury (SCI) research is growing recognition of the capacity for spontaneous recovery [1,2]. Intraspinal neuroplastic reserve in human subjects has been revealed by a range of outcome measures, and improvements can evolve months to years after trauma [3], even in cases of neurologically complete injury [4]. This has led to a greater appreciation for opportunities to therapeutically amplify natural recovery processes, as demonstrated by experimental studies [5,6].
Although most attention to spinal cord neuroplasticity has centered on locomotor function, it is well established that a significant potential for spontaneous recovery also exists in another motor domain referred to here as the phrenic motor system. Approximately half of spinal injuries occur at cervical levels [7], and in those cases involving diaphragm dysfunction, intensive post-SCI care and management are usually required [8–10]. Because such individuals are at risk of increased morbidity and mortality due to secondary pulmonary complications [8,11], continuing development of strategies to improve respiratory function is necessary.
Respiration after cervical SCI has thus attracted increasing scientific attention largely through integration of SCI and respiratory neurobiology expertise. As with locomotion, current literature suggests that respiratory neuroplasticity might also be responsive to therapeutic intervention [12–14]. In the present review, we first establish a clinical perspective with consideration of respiratory outcome measures, their interpretations and how some approaches might lend to clinical and laboratory application. Discussion then turns to recent experimental findings pertaining to mechanisms and neural substrates associated with phrenic motoneuron (PhMN) function and respiratory behavior after SCI. An additional emphasis of this review is on preclinical modeling considerations that can be pivotal in guiding future research and treatment approaches to optimize post-SCI respiratory neuroplasticity.
Clinical features of respiratory function following spinal cord injury
Respiratory compromise can result from trauma at any spinal segment from high cervical to midlumbar levels owing to impaired primary or accessory respiratory muscle activity [9,11]. A frequently cited example is injury sustained at high cervical levels which can result in diaphragm dysfunction due to interruption of bulbospinal respiratory drive to PhMN pools (C3–C5). In contrast, normal breathing and defensive mechanisms (e.g. cough) can be significantly compromised by more caudal trauma at lower cervical and thoracic levels. This can be attributed to damage affecting descending respiratory fibers to other motoneuron pools (e.g. intercostal and abdominal [15]) or direct damage to motoneurons themselves.
Despite their vulnerability after SCI, the networks controlling various respiratory muscles also possess resiliency. Irrespective of initial deficit, ventilatory improvements are often seen clinically months after injury [16,17]. However, the extent of recovery can vary due to lifestyle and other differences among individuals (e.g. age, history of smoking [16,18]). Spontaneously occurring improvements are often suboptimal, and many individuals who are ventilator weaned continue to experience shortness of breath, weakened phonation and impaired cough. The altered respiratory patterns of such individuals are generally characterized by increased breathing frequency and decreased tidal volume. This most likely reflects an attempt to increase respiratory drive to maintain blood-gas homeostasis. Unfortunately, this rapid shallow breathing pattern can lead to inefficient gas exchange [19], respiratory muscle fatigue [20] and eventually respiratory failure [8].
Although significant improvements in clinical management of respiratory dysfunction (e.g. respiratory muscle pacing [9]) are being made, therapeutic interventions to enhance intrinsic recovery mechanisms might provide a more effective long-term approach. However, the latter will require a better understanding of the interrelationships between different forms of respiratory recovery. For example, pulmonary improvements after SCI are often related to compensatory breathing strategies (e.g. recruitment of accessory respiratory muscles) [21] and altered respiratory muscle biomechanics [17]. Some observations also suggest that changes in PhMN activity might account for slowly evolving diaphragm recovery in some patients [22,23]. As discussed below, these are not necessarily mutually exclusive phenomena.
Outcome measures of respiratory function and preclinical studies
With expanding interest in applied SCI research, emphasis has been placed on the need for sensitive and quantitative outcome measures to demonstrate the efficacy of novel therapeutic approaches in preclinical investigations and future clinical trials [24]. Although a variety of neurological and functional tests have been discussed [25], little attention has been given to respiratory assessment with translational relevance. Table 1 summarizes available outcome measures with commentary on their individual technical merits and interpretations.
Table 1.
Clinical and experimental measures of respiratory function
| Blood gases | |
| Use: clinical and some experimental | Considerations |
| - Measure of oxygen and carbon dioxide partial pressures, and pH from blood sample | - Repeated measures in animals can be limited by the small blood volume of most experimental animals |
| - As such, it is the most accurate reflection of respiratory efficiency (adequacy of gas exchange) | - In many cases, sampling from awake animals is difficult without causing stress (directly affecting respiration) |
| - Blood samples have potential for used in assessing biomarkers of injury, plasticity and repair | - Difficult to relate to underlying anatomy and might be influenced by circulatory dysfunction |
| Oximetry | |
| Use: experimental and clinical | Considerations |
| - Measure of hemoglobin saturation in arterial blood | - Oxygen saturation might not be compromised in common experimental SCI models, or might only be reduced following severe injury |
| - Noninvasive | - Cannot determine concentration of carbon dioxide or sodium bicarbonate, or pH |
| - Might not directly correlate with ventilation | |
| Spirometry | |
| Use: clinical | Considerations |
| - Uses a pneumotachograph to measure airflow to and from lungs | - Might reflect compensatory mechanisms by unaffected circuits (e.g. increased intercostal activity might offset diaphragm dysfunction, so parameters of ventilation are relatively unaffected). Thus, this method does not necessarily provide information about mechanisms of recovery. However, as respiration can be measured in absolute units, we can determine with some certainty whether the overall output of the respiratory system is altered by injury or a particular intervention. |
| - Routinely used clinically as a pulmonary function test to determine multiple parameters, including forced vital capacity (FVC), forced expiratory volume per second (FEV1.0), inspiratory capacity (IC) and peak expiratory flow (PEF) | - Requires voluntary control of respiration and therefore cooperation and motivation of the subject |
| - Noninvasive | |
| - The ‘sniff’ test can be used as an additional measure for assessment of diaphragm function under increased drive | |
| Plethysmography | |
| Use: experimental and clinical | Considerations |
| - Measure of ventilation (gross measure of respiratory behavior) in terms of breathing frequency (f), tidal volume (VT) and minute ventilation (VE) | - Can reflect compensatory mechanisms by unaffected circuits as described for spirometry |
| - Noninvasive | - Measure is minute ventilation and not alveolar ventilation. Thus, changes in pulmonary dead space could lead to misleading conclusions. |
| - Clinically, can determine functional residual capacity more accurately than spirometry | - Common interferences to ventilation need to be carefully controlled |
| Imaging techniques (including chest X-ray, CT, ultrasound, fluoroscopy); see Ref. [89] for discussion | |
| Use: experimental and in some clinical circumstances | Considerations |
| - Visual representation of dysfunction (chest X-ray and CT) | - Usually only suggestive of dysfunction and requires confirmation with additional outcome measures |
| - Ultrasonography assesses real-time movement of diaphragm | |
| - Can be used to determine diaphragm thickness and change in muscle thickness during respiratory activity | |
| Electromyography (EMG) | |
| Use: experimental and in some clinical circumstances | Considerations |
| - Measure of muscle activity | - Muscle activity does not necessarily reflect a behavioral effect |
| - Chronic placement of electrodes can be used for repeated measures | - EMG recordings cannot sample the activity of the entire pool of active motor neurons. Because there are regional differences in the activation of respiratory muscles (e.g. costal versus crural diaphragm), recording from a single site might not accurately reflect diaphragm function. |
| - Can be noninvasive depending on muscle of interest | - Muscle atrophy could alter the signal |
| - Clinical recording is usually made noninvasively, placing electrodes on skin surface, decreasing accuracy of recording | |
| Nerve conduction recording using diaphragm CMAP (compound motor action potential) | |
| Use: Clinical | Considerations |
| - Noninvasive (electrode placed on skin) | - Electrode placement might not detect solely the nerve of interest |
| - Indirect measure of nerve function by assessing nerve integrity | - High-intensity stimulus is required, which might cause discomfort to subject |
| - Uses either electrical or magnetic stimulation | |
| Direct neurophysiological recording (exemplified by nerve recording described here) | |
| Use: Experimental (animal models) only | Considerations |
| - Direct measure of nerve function and relative motoneuron output | - Most neurophysiological recordings (e.g. from phrenic nerve) are terminal experiments |
| - The amplitude of the nerve burst reflects: | - Nerve activity does not necessarily reflect muscle activity |
| (i) the number of active motoneurons | - Nerve output does not necessarily reflect behavior, particularly because most studies are done under anaesthesia |
| (ii) the frequency of motoneuron discharge | - The amplitude of the nerve burst is also dependent on: |
| - Can compare between output from nerves on each side | (iii) electrode placement (proximity of the electrode to axons of active motoneurons) |
| - In the standard preparation (e.g. anesthetized, vagotomized, ventilated, etc.), many factors influencing breathing can be limited, and thus the output of a particular motor pool (e.g. phrenic) can be studied under carefully controlled conditions | (iv) the configuration of the recording system |
| - Can directly assess effect of treatment (serotonin agonists) and/or challenge (hypoxia) on motoneuron output | - Quantifying nerve output is problematic and there is no universally accepted method for quantifying the burst amplitude |
| - The peak height of the integrated phrenic inspiratory burst is highly correlated with peak airway pressure. Accordingly, many researchers have used this as an index of ‘respiratory neural drive.’ | - Comparing the burst amplitude between two nerves in the same rat (e.g. IL versus CL) or across rats can be problematic(e.g. even output in uninjured animals can differ between sides) |
| - Normalizing phrenic bursting to a maximum (e.g. asphyxic bursting) can remove physiologically meaningful differences between groups, as maximum bursting is reduced after SCI | |
Summary of some of the outcome measures used to assess respiratory function experimentally and/or clinically. The extent and detail of information provided by each test and necessary considerations are addressed. The most effective assessment of respiratory function is likely to come from use of multiple functional tests (e.g. nerve recording to assess function at the circuitry level, relative muscle activity assessed with EMG, plethysmography to measure overall breathing patterns and blood-gas analysis to determine respiratory efficiency).
The most frequently used clinical assessment of respiratory dysfunction is spirometry, which measures ventilation under elevated respiratory drive (e.g. forced expiratory volume; see Table 1). Unfortunately, this approach requires subject cooperation and is therefore not feasible in animal studies. Plethysmography is a clinically relevant alternative and has the experimental advantage that even small deficits can be detected by increasing the animal’s respiratory drive during brief periods of hypercapnic or hypoxic challenge [26–28].
In addition to plethysmography, assessment of respiratory function in experimental models has focused predominantly on neurophysiological recordings of phrenic nerve activity (also known as phrenic neurograms or compound motor action potentials; CMAP) and muscle electromyography (EMG). Such techniques more accurately detect deficits at identifiable levels of individual respiratory circuits. Recent advances in clinical testing have provided similar methods (e.g. CMAP and EMG) for assessing respiratory circuitry in humans [29–31] (Table 1). Furthermore, some of these tests can predict the long-term functional outcome of injured individuals [31]. However, the information provided by each outcome measure is very different (Table 1) and not always causally related. For example, a change in phrenic nerve or diaphragm muscle activity might not necessarily reflect a change in ventilation, which is a complex respiratory behavior involving the activity of multiple muscle groups (e.g. phrenic, intercostal, abdominal). Likewise, there can be recovery of PhMN activity without a concomitant change in diaphragm [32]. As demonstrated by the recent trend in experimental studies, combined testing approaches provide a more comprehensive and accurate evaluation of respiratory function [26,27,33].
Experimental models of cervical SCI
The term ‘neuroplasticity’ is not commonly used in the clinical literature in association with recovery of respiratory function after SCI. Therefore, experimental demonstrations of this concept could facilitate future therapeutic developments. Essential to this are preclinical models approximating the human condition or capable of providing fundamental proof-of-principle information. Of all experimental cervical spinal lesion paradigms used to assess respiratory function after SCI (Figure 1; Table 2), the ‘crossed phrenic phenomenon’ (CPP) [12] has provided the most evidence for enduring functional changes with associated synaptic remodeling after injury. In this model, complete lateral hemisection (HMx) at the second cervical segment (i.e. C2) interrupts descending inspiratory drive on one side of the spinal cord and results in ipsilateral diaphragm paralysis (Figure 1a). Although it has been reported that incomplete lateral hemisection (with some medial white matter sparing) results in a similar functional outcome [32,34], as these prior reports acknowledged, further elucidation of the underlying anatomy is required. Although some studies have noted the presence of bulbospinal-phrenic pathways in the ventromedial white matter [35,36], it is possible that these fibers do not subserve PhMN function [32]. Phrenic neurograms and diaphragm EMG (Table 1) have shown reactivation of ipsilateral PhMNs, either spontaneously following lateral C2HMx [26,27, 37–40] or as a consequence of subsequent contralateral phrenicotomy [12,41]. Recovery has been attributed to activation of a preexisting latent pathway originating from intact contralateral bulbospinal axons that cross at the level of the phrenic nucleus and terminate monosynaptically on ipsilateral PhMNs (i.e. the CPP). Synaptic remodeling occurs in the phrenic nucleus after C2HMx and appears to precede expression of the CPP (reviewed in Ref. [12]). As indicated in Table 2, the CPP has been demonstrated in many species. This includes recent demonstration of the CPP in mice [42,43], which opens the possibility for genetically probing mechanisms of respiratory neuroplasticity.
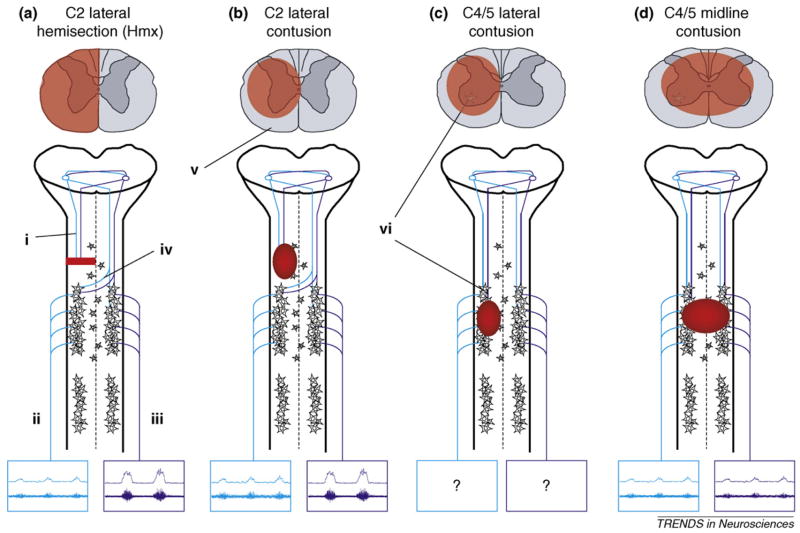
Figure 1.
Schematic diagrams outlining cervical injury models that have been used to examine respiratory function. Diagrams of spinal cord cross-sections demonstrate general morphological features (highlighted in red) of each injury type. Emphasis is given to descending pathways from the medulla (i) to PhMNs, which control diaphragm function via the phrenic nerve. Examples are presented of how each injury type affects efferent burst activity (both raw [top] and integrated [bottom] neurograms) recorded in the phrenic nerves (ii, iii) on each side of the spinal cord. The C2HMx (a) is the most frequently examined model and has provided the best evidence for respiratory neuroplasticity post-SCI. Following interruption of ipsilateral input from the medulla, spontaneous recovery of ipsilateral phrenic nerve activity (ii) has been observed. This recovery, known as the crossed phrenic phenomenon (CPP), is attributed to latent pathways (iv) that cross the spinal midline at the level of PhMNs. Note that following lateral injuries (a,b), in addition to ipsilateral recovery, contralateral phrenic activity is increased relative to normal. As contusive injury occurs more frequently in human SCI, some studies have begun to compare lateral C2 contusions (b) to the C2HMx model. However, depending on the extent of injury, contusive lesions usually result in some degree of white matter sparing (v). Therefore, in addition to the crossed, contralateral pathways of the CPP, some ipsilateral input from the medulla might be spared. As the majority of human SCIs occur in the midcervical region (C4/5), some studies have also begun to address the effects of such injuries on respiration (c, d). How lateral injuries at this level affect phrenic output is yet to be determined. However, as indicated, lesions at the midcervical levels also result in loss of PhMNs (vi). Furthermore, lesions that extend toward the spinal midline (d) are likely to interrupt crossing pathways associated with the CPP.
Table 2.
Experimental models of SCI used to assess respiratory dysfunction
| Spinal level | Injury model | Recovery | Species |
|---|---|---|---|
| C1 | Complete transection | Spontaneous (but temporary) | Dog [90,91], cat [92], rat [93] |
| C2 | Complete transection | Spontaneous (but temporary) | Dog [90,91], rabbit [94] |
| Lateral hemisection | CPP: induced by contralateral rhizotomy | Dog [41,95], cat [95], rabbit [41,95], rat [96], mouse [42,43], woodchuck [95], guinea pig [58] | |
| Lateral hemisection | CPP: spontaneous | Cat [97], rabbit [97], rat [26,27,37–40] | |
| Incomplete lateral section | Spontaneous phrenic nerve activity, but no diaphragm EMG recovery | Rat [32,34] | |
| Complete transection | Induced by anastomosis and innervation by spared systems | Rat [98] | |
| Lateral hemicontusion | Spontaneous recovery | Rat [44] | |
| Lateral hemicontusion | No recovery | Rat [45] | |
| C4/5 | Midline contusion | Spontaneous recovery | Rat [44] |
| C5 | Lateral hemicontusion | Spontaneous recovery, but enhanced via serotonergic agonists | Rat [28] |
| T8 | Midline contusion | Induced by administration of serotonergic agonists | Rat [54,64] |
This table summarizes some of the injury models that have been used to examine respiratory dysfunction, with reference to potential mechanisms of recovery and the animals used. Note that these examples reflect studies in both male and female animals, at a range of weights and ages. The C2 lateral hemisection (C2HMx) is the most extensively documented model and has provided the most comprehensive evidence for neuroplasticity in the injured respiratory circuitry.
An acknowledged translational shortcoming of the CPP model is that laceration-type injuries analogous to C2HMx are rare in humans. Spinal contusions instead represent the most frequent lesion type and present different pathophysiological and histological conditions. Following on an earlier study of cervical contusion injuries [44], Baussart et al. [45] recently examined respiratory consequences of lateralized C2 contusions (Figure 1b). As in the case of C2HMx, severe lateral contusions will interrupt respiratory drive to PhMNs. However, any white matter sparing (dependent on injury severity) is a critical interpretational consideration owing to lateral and ventromedial distribution of bulbospinal respiratory fibers [36] and the different functional outcomes [44,45] (see Figure 1b,v).
The lateralized C2 contusion approach is an important complement to the C2HMx model, and clinical reports of such injuries have been made [46]. The practical relevance of such lesions, however, still appears modest, as most injuries of that nature in the human are near midline and often exhibit only moderate asymmetry (Figure 1d). In addition, there is a propensity for contusions to occur at midcervical levels (e.g. [47]). Midcervical contusion injury models have been extensively characterized [48–50], with some reports addressing respiration after either lateralized or midline C4–C5 contusions [28,44] (Figure 1c,d). However, the pathological features of these lesions differ strikingly from conditions associated with high cervical injuries as discussed below. The C2HMx model is nevertheless a more consistent lesion affecting an identified population of descending respiratory axons. As such, the CPP provides a fundamental example of respiratory neuroplasticity in which the underlying circuitry can be more clearly defined and its functional role in recovery analyzed. These considerations lead to an important question, namely, to what extent does the CPP account for any improvements in breathing?
Patterns of breathing after cervical SCI
Previous work suggests the CPP has little impact on breathing behavior during the first 5 weeks post-C2HMx [27]. Plethysmography studies of unanesthetized rats demonstrated that minute ventilation was maintained but with a rapid, shallow breathing pattern under room air (i.e. ‘quiet breathing’) conditions, similar to what is seen in humans (see above). Moreover, persistent deficits in ventilation were observed during conditions of increased respiratory drive, and parallel studies in anesthetized rats indicated minimal recovery of ipsilateral phrenic output (i.e. the CPP) [27].
A more recent investigation extending assessment to 12 weeks post-C2HMx showed gradual, but continual, improvement in ventilation during baseline and hypercapnic conditions [26]. These findings might reflect increased reliance on the CPP, but the nature of these outcome measures (specific neurophysiological activity versus gross respiratory behavior) does not permit direct correlation between results, for reasons noted earlier (see also Table 1). Indeed, the observation that ipsilateral phrenic activity recovers after C2HMx, but before ventilatory improvement is observed, brings into question the contribution of the CPP to overall respiratory outcome. However, Golder et al. [51] demonstrated that activation of ipsilateral PhMN pool after C2HMx does contribute to ventilation under certain conditions. Specifically, there was a significant reduction in the volume of augmented breaths (i.e. ‘sighs’) and decreased tidal volume when recovered PhMN activity was prevented from reaching the diaphragm by an ipsilateral phrenicotomy. The augmented breaths described require elevated neural drive and increased PhMN recruitment [52,53]. The functional outcome of the CPP might thus only be detectable under conditions of respiratory challenge and greater respiratory drive. Collectively, existing data suggest that neuroplasticity associated with the CPP might contribute to respiratory recovery after C2HMx, but this mechanism remains insufficient to promote full respiratory recovery.
More recently, Choi et al. [28] examined breathing patterns following lateralized C5 contusions of graded severity. That study showed that, under room air conditions, the injured rats had reduced tidal volumes (Vt) and increased respiratory frequency (f) during the first 2 weeks post-SCI, and the magnitude of these changes was positively correlated with injury severity. Thereafter, however, rapid shallow breathing transitioned to a normal respiratory pattern. Alterations in breathing during hypercapnic respiratory challenge (e.g. reduced Vt and f) were more persistent but, by 6 weeks post-injury, breathing in contused rats became comparable to controls under these conditions [28]. This more prolonged deficit correlated with global motoneuron loss at the lesion epicenter. Therefore, respiratory outcome measures following midcervical contusion in rats are likely to be at least partially determined by the relative degree of PhMN loss, as has been suggested in humans [30].
Spontaneously improved diaphragm function is likely to be an important part of the recovery process, but in what context neuroplasticity might play a role in midcervical injury models and humans is unclear. First, given midline tissue damage even with a lateralized contusion, a CPP-like contribution might be impaired, resulting from damage to the crossed ‘CPP pathway’ (Figure 1c,d). Second, midcervical contusions will also entail variable white matter damage on both sides (e.g. [50]). Third, some uni- or bilateral loss of PhMNs will occur which has been correlated with ventilatory function [54]. How such relative disruption of white or gray matter affects respiratory neuroplasticity following contusive injury is yet to be defined. It is thus likely that plasticity in the phrenic motor system can be differentially expressed depending upon the segmental level and severity of the injury. Whereas severe midcervical contusion injuries would presumably show greater respiratory dysfunction, such trauma in rodents (i.e. approximating a ventilator-dependent human) is not immediately feasible [28]. The preclinical value of experimental midcervical injuries must therefore be gauged by what clinically relevant features can be defined. For example, it is conceivable that deficits observed during conditions of increased respiratory drive in animals might reflect what is experienced by a ventilator-weaned individual whose overall respiratory capacity is still weak. By contrast, any change in diaphragm function seen experimentally and clinically might reflect neuroplasticity via mechanisms other than the CPP. This might entail reorganization of neuromuscular junctions at the level of the diaphragm [55,56], altered recruitment of remaining PhMNs [55], intrinsic changes in PhMNs themselves [33,57] or changes in pre-phrenic circuitries above or below the injury site, among other possibilities. In addition, some functional recovery observed following midcervical SCI might be attributed to compensatory mechanisms such as recruitment of alternative respiratory pathways unaffected by injury. Such changes have been observed with SCI at other vertebral [58] and brainstem [40] levels, and might represent another expression of respiratory plasticity [40]. For example, Golder et al. [40] demonstrated that C2HMx in the rat also resulted in concomitant changes in hypoglossal nerve activity. The overall pattern of ventilation observed following cervical SCI could thus be a result of the combined effects of neuroplasticity within the phrenic circuit and compensatory recruitment of unaffected, accessory respiratory circuits.
Finally, although the focus of the present review is predominantly on descending respiratory drive, one consideration is that spinal cord injury might also disrupt afferent input to spinal respiratory cells. Furthermore, several studies have shown that both ipsilateral and contralateral afferent input to phrenic neurons can influence expression of the CPP [34,59]. Goshgarian [59] demonstrated that acute contralateral rhizotomy following a lateral C2HMx elicited an earlier and more robust activation of the ipsilateral phrenic motor pool than would be seen via the spontaneous CPP alone. However, the underlying mechanisms and neural circuitries are poorly understood.
Enhancement of respiratory recovery
Whereas the clinical approach to respiratory dysfunction post-SCI has primarily focused on management and assisted ventilation, other approaches have been employed to enhance post-SCI pulmonary function such as respiratory muscle strengthening (reviewed in Ref. [60]). In addition, experimental evidence has shown that ipsilateral phrenic nerve activity post-C2HMx can be significantly enhanced by surgical [59,61] or physiological [37,39] approaches. Such findings suggest that chronic respiratory deficiency might not be an insurmountable condition. This view has been more firmly anchored by studies showing that a variety of neurotransmitters/neuromodulators (Table 3) can enhance neuroplasticity in the phrenic motor system. For instance, it has been shown that increased expression or activation of serotonin and 5HT receptors might mediate the onset and extent of respiratory plasticity post-injury [28,54,62–64]. Contralateral cervical rhizotomies, as discussed above, also increase serotonergic input onto PhMNs [65]. Pharmacological studies have revealed a likely role for glutamatergic receptors in mediating plasticity post-C2HMx [43,66]. These alterations in neurotransmitter/receptor expression enhance motoneuron excitability [67]. Recent studies have shown a role of adenosine receptors in respiratory plasticity post-SCI, with particular focus on pharmacological manipulation of receptor activity [33,68,69]. Activation of the central adenosine receptor A2a induces a persistent phrenic motor facilitation and enhances synaptic strengthening at the spinal level in uninjured animals [33]. Furthermore, administration of adenosine agonists following C2HMx significantly increases tidal volume [33].
Table 3.
Pharmacological mediators of respiratory plasticity post-SCI
| Molecule | Receptor | Expression post-injury | Anatomical location of expression | Effect on plasticity |
|---|---|---|---|---|
| Serotonin | 5HT-1A | Increased | Dorsal horn [62,64] | Enhancing |
| PhMN [64] | ||||
| 5HT-2A | Increased | Medulla [99] | Enhancing | |
| Spinal cord [99], PhMN [63] | Enhancing | |||
| Glutamate | AMPA-GluR1 | Increased [66] | PhMN [66] | Enhancing |
| AMPA-GluR2 | Decreased [66] | PhMN [66] | Enhancing | |
| NMDA–2A | Increased [66] | PhMN [66] | Enhancing | |
| GABA | GABA-A | – | Dorsal horn [71] | Inhibiting |
| GABA-B | – | Inhibiting | ||
| Adenosine | Adenosine–A1 | – | Carotid bodies [69] | Inhibiting |
| Adenosine–A2a | – | Carotid bodies [69] | Enhancing | |
| PhMN [33] |
Summary of some of the reported molecules and receptors that can influence the onset and extent of respiratory plasticity following spinal cord injury. Identification of these neuromodulators, and an increasing interest in their role following injury, has led to the development of several therapeutic approaches to improving respiratory function post-SCI.
Respiratory neuroplasticity post-SCI also appears to involve molecular changes downstream to activated neurotransmitter receptors by mechanisms (e.g. involvement of trophic factors [57]) similar to what has been described in other cellular models of neuroplasticity. It has become evident that expression and regulation of neuromodulators and neurotrophins are intimately related to each other. Recently, Baker-Herman et al. [70] showed serotonin-dependent BDNF synthesis in cervical ventral gray matter containing the phrenic nucleus that was associated with intermittent hypoxia and long-term facilitation.
Finally, inhibitory neurotransmitters have been implicated in mediating plasticity post-SCI [71]. Inhibition of ipsilateral PhMN activity by contralateral afferents has been demonstrated in several animal models, attributed to γ-aminobutyric acid (GABA) [71]. Therefore, the onset and extent of recovery associated with plasticity post-SCI represents a balance between mechanisms that can both inhibit and excite respiratory motor output.
Collectively, these findings reveal several potential targets for promoting plasticity, and some treatment strategies exploiting neuromodulatory actions post-injury have been explored (e.g. plasminogen activator [43]). In addition, there has been growing interest in strategies designed to promote axonal growth following injury that might also enhance respiratory recovery post-SCI (e.g. cell/tissue transplants [72,73], increased cAMP [74]) or attenuate molecules inhibitory to axonal growth (e.g. chondroitin sulfate proteoglycans [75]).
Neural circuitry and respiratory recovery
To date, enhanced respiratory-related synaptic plasticity has been attributed to changes primarily at the level of the PhMN, as exemplified by neurophysiological studies [37,76], Some synaptic plasticity might also entail respiratory-associated changes elsewhere in the cervical spinal cord, as well as at brainstem levels. Present neuroanatomical data indicate that the crossed axons mediating the CPP derive from decussating contralateral and recrossed ipsilateral axons from the ventral respiratory column (VRC) [77,78]. Otherwise, little is known about changes in descending inputs to PhMNs from other regions of the medulla following cervical spinal cord injury. Within the spinal cord, phrenic neurograms post-C2HMx have demonstrated a delayed, rather than the normally synchronous, onset of recovered ipsilateral PhMN bursting relative to contralateral PhMN output [26]. This raises the possibility that PhMN activity after C2HMx might be affected by a neural substratum that is not exclusively limited to intact monosynaptic projections from the VRC. Whereas anatomical studies have demonstrated the presence of inter-neurons associated with the phrenic circuit of rat [79] and ferret [80], they have been largely dismissed in the rat as having an insignificant role in respiration. However, given their connectivity and potential for modulating PhMN activity, these cells might assume greater functional significance after SCI.
Polysynaptic inputs to PhMNs have been demonstrated by neurophysiological studies in different species [81–83]. Although the existence of propriospinal relays between the medulla and phrenic motoneurons in the rat was previously rejected based on negative neuroanatomical tracing results [77,84], recent observations provide an alternative perspective [85]. Delivery of pseudorabies virus (PRV) to one-half of the diaphragm revealed a substantial population of pre-phrenic interneurons distributed bilaterally throughout the cervical spinal cord in the dorsal horn and around the central canal. When combined with a physiologically targeted anterograde tracing approach, labeled VRC terminals were observed in close proximity to primary dendrites or cell soma of some of these pre-phrenic interneurons [85] (see also Figure 2). Although their role is currently unknown, there is some neurophysiological evidence that activation of polysynaptic spinal pathways can contribute to enhanced phrenic output after C2HMx [76]. Thus, recovery of PhMN activity in the C2HMx model might act in part via spinal interneurons, and this raises consideration for the functional recruitment of preexisting ‘bypass’ pathways following injury, as has been described in other SCI models [86,87].
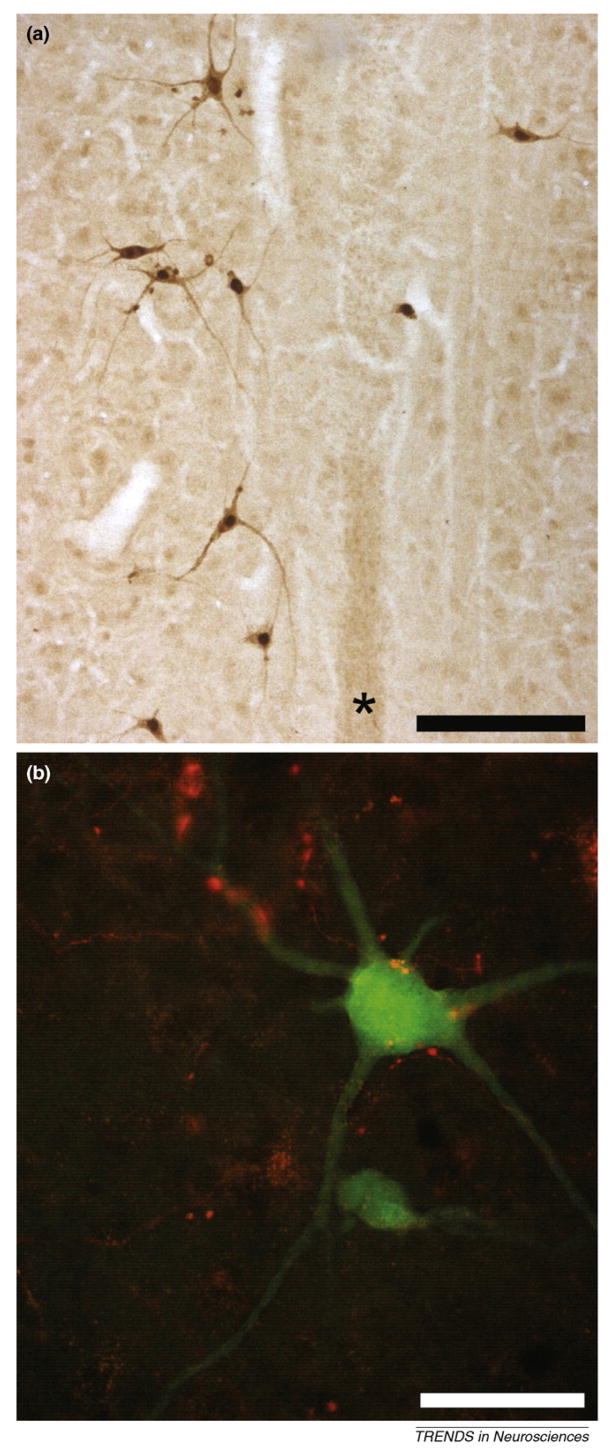
Figure 2.
Photomicrographs of longitudinal sections (40 microns) from the cervical spinal cord of an adult rat. Within 48 h following delivery of the transsynaptic tracer pseudorabies virus (PRV) to the left hemidiaphragm, the ipsilateral phrenic motoneuron pool becomes infected with the retrogradely transported virus. Subsequently (64 h; [a,b]), pre-phrenic interneurons become transsynaptically infected. (a) Antibodies against PRV were used to immunocytochemically visualize PRV-infected cells at around laminae VII/X. These interneurons are bilaterally distributed around the central canal and spinal cord midline (*). (b) This pre-phrenic interneuron (green) has been retrogradely infected with the PRV that results in expression of green fluorescent protein. Projections from the ventral respiratory column in this animal were anterogradely labeled using MiniRuby (red). These projections appear in close contact with the soma and dendrites of the interneuron, which could suggest synaptic contact. This finding (described in detail by Lane et al. [85]) indicates that whereas there is substantial monosynaptic bulbospinal-phrenic projection, there is also anatomical evidence for a polysynaptic pathway in the phrenic circuit of the rat. This pathway might serve as an alternative to direct monosynaptic projections from the medulla following spinal cord injury and represent a viable therapeutic target. Scale bar is 200 (a) and 50 (b) micrometers.
There is, therefore, an increasing body of evidence demonstrating that respiratory circuits are modulated by other cells within the spinal cord. Evidence for interneurons contributing to alternative pathways and locomotor neuroplasticity post-SCI has been derived from hemisection lesion models. However, whether these cells play a significant role in various forms of functional recovery after contusion injury has not been determined. In terms of respiratory neuroplasticity, interneurons might provide an alternative substrate to either maintain connectivity with spared PhMNs or form novel functional circuits with accessory respiratory motoneuron pools (e.g. spared intercostal motoneurons). Then again, some interneurons might attenuate the extent of recovery if they are inhibitory. A future challenge is to obtain greater understanding of the functional connectivity of these cells to better define sites of action of various therapeutic approaches which can be complementary to respiratory rehabilitation or other strategies.
Closing commentary: translational relevance of respiratory neuroplasticity models
Recent animal data, based upon the CPP model, continue to highlight the possibility that enhancement of phrenic neuroplasticity represents an achievable therapeutic target in SCI research. However, the pathology and cervical level of human contusions are often inconsistent with preservation of the neural substrate mediating the CPP (Figure 1). Therefore, from a clinical perspective, respiratory neuroplasticity as defined by the CPP might represent a biological phenomenon unique to a particular lesion model. To address this and related issues, this discussion has considered some of the relative translational and theoretical merits of the CPP and other cervical SCI models of respiratory dysfunction.
The preclinical value of experimental models would be considerably enhanced by more comprehensive documentation of the respiratory consequences following human SCI, with greater attention to lesion imaging and neurophysiological changes. In that respect, translation is a bidirectional process, and the significance of animal data is dependent on clinical observations that can influence experimental designs and interpretations. One consideration is that diaphragm dysfunction is a probable consequence of high-to-midcervical SCI in humans, irrespective of injury laterality. Although lesion anatomy in humans is highly variable, the functional outcomes observed both clinically and experimentally are relatively comparable depending on the outcome measures employed. In that regard, despite its relatively unique features, the CPP has provided an appreciation of spinal cord neuroplasticity that can be of significant clinical value.
One of several future challenges will be to determine how neuroplasticity relates to respiratory improvements following midcervical contusions. An experimental contusion severe enough to completely replicate a ventilator-dependent human condition is unlikely to evolve. Apart from the logistical and ethical considerations of restricting a conscious animal to mechanical ventilation, studies have shown that diaphragm atrophy occurs within hours of mechanical ventilation [88]. It therefore becomes important to identify how functional parameters of less damaging injuries relate clinically.
As noted, outcome measures exist that can be applied both in humans and animals which can greatly enhance translational power. Studies to date indicate similarities in breathing patterns seen after midcervical contusions in humans and rats. To what extent progressive spontaneous improvements in ventilation entail another form of neuroplasticity after injuries involving gray and/or white matter components remains to be determined by further functional and correlative neuroanatomical investigations.
In principle, each lesion type addressed in this review has translational strengths depending on the issues addressed and evolving concepts. In that regard, the hemisection and contusion models studied can both be instrumental to discovery of pharmacological targets and related molecular mechanisms that can increase the synaptic efficacy of the denervated circuit (i.e. pre- or postsynaptic plasticity) or augment intrinsic neuronal excitability [28,33,62,66,68,71]. Recognition of neuroplasticity as a basis for respiratory recoveries in some patients could influence a paradigm shift in rehabilitation and other treatment approaches to reverse a potentially life-threatening consequence of cervical SCI.
Acknowledgments
Original research of the authors reported in this review was supported by NIH/NINDS RO1 NS054025, the Anne and Oscar Lackner Endowed Chair (P.J.R.) and a Craig H. Neilsen Postdoctoral Fellowship (M.A.L.). The authors also wish to thank Jeffrey Kleim for his helpful comments on the manuscript.
Footnotes
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- 2.Stein RB. The plasticity of the adult spinal cord continues to surprise. J Physiol. 2008;586:2823. doi: 10.1113/jphysiol.2008.155986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbetta M, et al. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci U S A. 2002;99:17066–17071. doi: 10.1073/pnas.262669099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirshblum S, et al. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1811–1817. doi: 10.1016/j.apmr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Edgerton VR, et al. Training locomotor networks. Brain Res Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vavrek R, et al. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- 7.NSCISC. National Spinal Cord Injury Statistical Center; 2008. Spinal cord injury. Facts and figures at a glance. http://www.spinalcord.uab.edu. [Google Scholar]
- 8.Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75:270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 9.DiMarco AF, et al. Inspiratory muscle pacing in spinal cord injury: case report and clinical commentary. J Spinal Cord Med. 2006;29:95–108. doi: 10.1080/10790268.2006.11753863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- 11.Brown R, et al. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. [PMC free article] [PubMed] [Google Scholar]
- 12.Goshgarian H. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer MB, et al. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol. 2008;209:399–406. doi: 10.1016/j.expneurol.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Fuller DD, et al. Respiratory neuroplasticity: respiratory gases, development, and spinal injury. In: Ward DS, et al., editors. Pharmacology and Pathophysiology of the Control of Breathing. Taylor & Francis; 2005. pp. 155–223. [Google Scholar]
- 15.Como JJ, et al. Characterizing the need for mechanical ventilation following cervical spinal cord injury with neurologic deficit. J Trauma. 2005;59:912–916. doi: 10.1097/01.ta.0000187660.03742.a6. [DOI] [PubMed] [Google Scholar]
- 16.Bluechardt MH, et al. Repeated measurements of pulmonary function following spinal cord injury. Paraplegia. 1992;30:768–774. doi: 10.1038/sc.1992.148. [DOI] [PubMed] [Google Scholar]
- 17.Loveridge B, et al. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia. 1992;30:479–488. doi: 10.1038/sc.1992.102. [DOI] [PubMed] [Google Scholar]
- 18.Linn WS, et al. Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord. 2001;39:263–268. doi: 10.1038/sj.sc.3101155. [DOI] [PubMed] [Google Scholar]
- 19.Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124:41–44. doi: 10.1164/arrd.1981.124.1.41. [DOI] [PubMed] [Google Scholar]
- 20.Sinderby C, et al. Electromyographic registration of diaphragmatic fatigue during sustained trunk flexion in cervical cord injured patients. Paraplegia. 1992;30:669–677. doi: 10.1038/sc.1992.131. [DOI] [PubMed] [Google Scholar]
- 21.Warren VC. Glossopharyngeal and neck accessory muscle breathing in a young adult with C2 complete tetraplegia resulting in ventilator dependency. Phys Ther. 2002;82:590–600. [PubMed] [Google Scholar]
- 22.Axen K, et al. Diaphragmatic function following cervical cord injury: neurally mediated improvement. Arch Phys Med Rehabil. 1985;66:219–222. doi: 10.1016/0003-9993(85)90146-7. [DOI] [PubMed] [Google Scholar]
- 23.McDonald JW, et al. Late recovery following spinal cord injury: case report and review of the literature. J Neurosurg Spine. 2002;97:252–265. doi: 10.3171/spi.2002.97.2.0252. [DOI] [PubMed] [Google Scholar]
- 24.Ditunno JF, Jr, et al. Neurological and functional capacity outcome measures: essential to spinal cord injury clinical trials. J Rehabil Res Dev. 2005;42:35–41. doi: 10.1682/jrrd.2004.08.0098. [DOI] [PubMed] [Google Scholar]
- 25.Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP Panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 26.Fuller DD, et al. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller DD, et al. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- 28.Choi H, et al. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005;25:4550–4559. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merino-Ramirez MA, et al. Diaphragmatic paralysis following minor cervical trauma. Muscle Nerve. 2007;36:267–270. doi: 10.1002/mus.20754. [DOI] [PubMed] [Google Scholar]
- 30.Strakowski JA, et al. Phrenic nerve stimulation in the evaluation of ventilator-dependent individuals with C4- and C5-level spinal cord injury. Am J Phys Med Rehabil. 2007;86:153–157. doi: 10.1097/PHM.0b013e31802edce9. [DOI] [PubMed] [Google Scholar]
- 31.Chiodo AE, et al. Predictors of ventilator weaning in individuals with high cervical spinal cord injury. J Spinal Cord Med. 2008;31:72–77. doi: 10.1080/10790268.2008.11753984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinit S, et al. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- 33.Golder FJ, et al. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinit S, et al. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci. 2007;25:3551–3560. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- 35.Duffin J, Li YM. Transmission of respiratory rhythm: midline-crossing connections at the level of the phrenic motor nucleus? Respir Physiol Neurobiol. 2006;153:139–147. doi: 10.1016/j.resp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Lipski J, et al. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- 37.Fuller DD, et al. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nantwi KD, et al. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- 39.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golder FJ, et al. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minor KH, et al. Spinal cord injury-induced plasticity in the mouse – the crossed phrenic phenomenon. Exp Neurol. 2006;200:486–495. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- 43.Minor KH, Seeds NW. Plasminogen activator induction facilitates recovery of respiratory function following spinal cord injury. Mol Cell Neurosci. 2008;37:143–152. doi: 10.1016/j.mcn.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 44.El-Bohy AA, et al. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- 45.Baussart B, et al. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Fasset DR, et al. Evidence on magnetic resonance imaging of Brown–Se ‘quard spinal cord injury suffered indirectly from a gunshot wound. J Neurosurg Spine. 2008;8:286–287. doi: 10.3171/SPI/2008/8/3/286. [DOI] [PubMed] [Google Scholar]
- 47.Shanmuganathan K, et al. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008;29:655–659. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearse DD, et al. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J Neurotrauma. 2005;22:680–702. doi: 10.1089/neu.2005.22.680. [DOI] [PubMed] [Google Scholar]
- 49.Mihai G, et al. Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J Neurotrauma. 2008;25:1–18. doi: 10.1089/neu.2007.0338. [DOI] [PubMed] [Google Scholar]
- 50.Velardo MJ, et al. Patterns of gene expression reveal a temporally orchestrated wound healing response in the injured spinal cord. J Neurosci. 2004;24:8562–8576. doi: 10.1523/JNEUROSCI.3316-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golder FJ, et al. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 53.Golder FJ, et al. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- 54.Teng YD, et al. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19:7037–7047. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantilla CB, Sieck GC. Invited review: mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- 56.Rowley KL, et al. Respiratory muscle plasticity. Respir Physiol Neurobiol. 2005;147:235–251. doi: 10.1016/j.resp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 58.Guth L. Functional plasticity in the respiratory pathway of the mammalian spinal cord. Exp Neurol. 1976;51:414–420. doi: 10.1016/0014-4886(76)90265-x. [DOI] [PubMed] [Google Scholar]
- 59.Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- 60.Zimmer MB, et al. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30:319–330. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuller DD, et al. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett. 2002;323:25–28. doi: 10.1016/s0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- 62.Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuller DD, et al. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- 64.Teng YD, et al. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci. 2003;23:4182–4189. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinkead R, et al. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30:346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rekling JC, et al. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29:915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- 69.James E, Nantwi KD. Involvement of peripheral adenosine A2 receptors in adenosine A1 receptor-mediated recovery of respiratory motor function after upper cervical spinal cord hemisection. J Spinal Cord Med. 2006;29:57–66. doi: 10.1080/10790268.2006.11753857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker-Herman TL, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 71.Zimmer MB, Goshgarian HG. GABA, not glycine, mediates inhibition of latent respiratory motor pathways after spinal cord injury. Exp Neurol. 2007;203:493–501. doi: 10.1016/j.expneurol.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polentes J, et al. Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol Dis. 2004;16:638–653. doi: 10.1016/j.nbd.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, et al. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–731. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kajana S, Goshgarian HG. Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol. 2008;210:610–680. doi: 10.1016/j.expneurol.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Ling L, et al. Phrenic responses to contralateral spinal stimulation in rats: effects of old age or chronic spinal hemisection. Neurosci Lett. 1995;188:25–28. doi: 10.1016/0304-3940(95)95690-d. [DOI] [PubMed] [Google Scholar]
- 77.Moreno DE, et al. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- 78.Boulenguez P, et al. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res. 2007;1148:96–104. doi: 10.1016/j.brainres.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 79.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 80.Yates BJ, et al. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience. 1999;90:1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- 81.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990;533:141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- 82.Palisses R, et al. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res. 1989;78:624–632. doi: 10.1007/BF00230250. [DOI] [PubMed] [Google Scholar]
- 83.Hayashi F, et al. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol. 2003;94:1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- 84.Goshgarian HG, et al. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- 85.Lane MA, et al. Cervical pre-phrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. doi: 10.1002/cne.21864. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Courtine G, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 88.Levine S, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 89.Patel AS, et al. Diaphragm paralysis definitively diagnosed by ultrasonography and postural dependence of dynamic lung volumes after seven decades of dysfunction. Lung. 2007;185:15–20. doi: 10.1007/s00408-006-0055-7. [DOI] [PubMed] [Google Scholar]
- 90.Coglianese CJ, et al. Rhythmic phrenic nerve activity and respiratory activity in spinal dogs. Respir Physiol. 1977;29:247–254. doi: 10.1016/0034-5687(77)90001-9. [DOI] [PubMed] [Google Scholar]
- 91.Reinoso MA, et al. Respiratory muscle coordination in acute spinal dogs. Respir Physiol. 1996;104:29–37. doi: 10.1016/0034-5687(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 92.Aoki M, et al. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res. 1980;202:51–63. [PubMed] [Google Scholar]
- 93.Goodchild AK, et al. Control of sympathetic, respiratory and somatomotor outflow by an intraspinal pattern generator. Clin Exp Pharmacol Physiol. 2008;35:447–453. doi: 10.1111/j.1440-1681.2008.04913.x. [DOI] [PubMed] [Google Scholar]
- 94.Viala D, Freton E. Evidence for respiratory and locomotor pattern generators in the rabbit cervico-thoracic cord and for their interactions. Exp Brain Res. 1983;49:247–256. doi: 10.1007/BF00238584. [DOI] [PubMed] [Google Scholar]
- 95.Rosenblueth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol. 1936;117:495–513. [Google Scholar]
- 96.O’Hara TE, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- 97.Rosenbaum H, Renshaw B. Descending respiratory pathways in the cervical spinal cord. Am J Physiol. 1949;157:468–476. doi: 10.1152/ajplegacy.1949.157.3.468. [DOI] [PubMed] [Google Scholar]
- 98.Gauthier P, et al. Diaphragm recovery by laryngeal innervation after bilateral phrenicotomy or complete C2 spinal section in rats. Neurobiol Dis. 2006;24:53–66. doi: 10.1016/j.nbd.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 99.Zhou SY, et al. Serotonin2 receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]