Abstract
The mechanism(s) through which fruits, vegetables and whole grains favorably affect health is not well established. Employing an anthocyanin-rich grape as a model, we examined the ability of an agnostic analytical approach employing gene expression microarrays, to generate novel testable hypotheses regarding the mechanisms of action of potentially healthful foods and food components. C57Bl/6 mice were divided into two groups and fed a proatherogenic diet with or without a semi-purified anthocyanin extract (70% anthocyanins) incorporated at a level of 0.1 mg/ml into the drinking water. After six weeks, compared to control mice, mice supplemented with anthocyanins tended to gain more weight and have increased adipose tissue mass, although these effects did not achieve statistical significance. Anthocyanin supplemented mice had significantly reduced relative liver weights and heart weights. Serum lipids and inflammatory cytokines were not different between the groups. Gene expression microarray analysis of the liver and skeletal muscle identified a number molecular pathways significantly affected by anthocyanin treatment. Two distinct clusters emerged. The first cluster included down-regulated pathways in both muscle and liver involving cellular defense while the second included hepatic genes involved in energy metabolism. From these data three hypotheses were developed for future investigation.
INTRODUCTION
Increased consumption of fruits, vegetables and whole grains and lower intakes of foods high in saturated fat are recommended by public agencies1;2 largely because of supporting epidemiological data. For saturated fat, the mechanism underlying the link between high intakes and disease (specifically, cardiovascular disease) is well established. Abundant evidence exists demonstrating an adverse effect of saturated fat on LDL cholesterol levels3, a well established risk factor for cardiovascular disease. However, the mechanism(s) through which fruits, vegetables and whole grains favorably affect health is substantially less well established. We have previously suggested4 that an agnostic approach (e.g., without prior belief as to mechanism of action) employing gene expression microarrays, proteomics and metabolomic analytical methods could be employed to generate novel testable hypotheses regarding the mechanisms of action of potentially healthful foods and food components.
In the present study, we apply gene expression microarray analysis to the investigation of the potential health benefits of an anthocyanin-rich grape extract as a partial test of this hypothesis-generating paradigm. Anthocyanins are polyphenolic compounds that provide color in berries such as grapes, blueberries, strawberries, and blackberries. Consumption in U.S. is estimated at 12.5 mg/day5. Unlike other polyphenols, glycosides of anthocyanins are absorbed intact 6;7 suggestive of a potential unique role among polyphenols in human health. Previous work has focused on antioxidant and anti-inflammatory properties in relation to cardiovascular disease and maintenance of brain function with ageing 8–13. There is also evidence that anthocyanins may have anti-carcinogenic, anti-obesity and anti-diabetic effects as well 14;15;16. The availability of prior data regarding the potential health effects of anthocyanins provides an opportunity to validate our approach through corroboration of hypotheses generated from our analyses with existing published hypotheses.
METHODS
2.1 Anthocyanin-rich extract preparation
An anthocyanin-rich grape extract (ACN-GE) was prepared from the highly pigmented wine grape A-1575. The extracts were prepared by solid phase extraction using Amberlite XAD-7 resin. The final extract contained 67% anthocyanins (Table 1) as assessed by HPLC analysis and was exceptionally rich in malvidin glucosides with moderately high levels of petunidin and delphinidin glucosides. HPLC analysis additionally confirmed the extract to be free of free sugars and organic acids.
Table 1.
Anthocyanin content or A-1575 grape skin extract
| Anthocyanin | Content (mg/g) |
|---|---|
| Cyanidin-(x)gluc | 27 |
| Delphinidin-(x)gluc | 118 |
| Malvidin-(x)gluc | 317 |
| Pelargonidin-(x) gluc | - |
| Peonidin-(x)gluc | 69 |
| Petunidin-(x)gluc | 140 |
| Total | 671 |
Data are mg/g dry weight
-(x)glucosides includes 3-glucoside, 3-acetylglucoside and 3-(p-coumaroyl)glucoside
2.2 Animals and diets
Twenty five-week old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were maintained at constant temperature (22–24°C) under an automated lighting with a 12:12-h light-dark cycle throughout the experiment. The mice were divided into two groups of ten and fed for six weeks a proatherogenic diet (D01022601, Research Diets, New Brunswick, NJ) to increase oxidative stress. The diet provided 39.9% of energy as fat and 1.5 g/kg cholesterol (Table 2). Diets were provided ad libitum for the duration of the study.
Table 2.
Composition of base diet
| Ingredient | g/kg diet |
|---|---|
| Casein | 225 |
| L-Cystine | 3.4 |
| Corn starch | 239 |
| Maltodextrin 10 | 80 |
| Sucrose | 127 |
| Cellulose | 56 |
| Soybean Oil | 28 |
| Cocoa Butter | 175 |
| Minerals1 | 51 |
| Vitamins2 | 14 |
| Cholesterol | 1.5 |
Minerals include mineral mix (S10021), dicalcium phosphate, calcium carbonate, and potassium citrate.
Vitamins include vitamin mix (V10001) and choline bitartrate.
A 10 mg/mL stock solution of the ACN-GE was prepared in ethanol. The ACN-GE stock solution was added to the drinking water of one group of mice (ACN group) to provide a final concentration of 0.1 mg/ml ACN-GE and 1% ethanol mice. The control group received drinking water with added ethanol alone. ACN-GE supplemented drinking water was provided in brown water bottles and changed every other day. Preliminary studies demonstrated that under these conditions, the anthocyanin preparations remained stable. Water intake was monitored and did not differ between groups. The experimental design was approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
2.3 Collection of serum and tissues
Two days prior to the end of the experiment, body composition was determined by nuclear magnetic resonance (Brucker model mq10 NMR analyzer, Milton, ON, Canada). At the end of the feeding period the mice were fasted for four hours before blood was obtained by cardiac puncture under anesthesia. The liver, heart, kidney, spleen, adipose tissue depots (subcutaneous, retroperitoneal, epididymal, brown) and sample of thigh skeletal muscle were harvested, weighed and flash frozen in liquid nitrogen. Serum was collected by centrifugation. All tissue and serum samples were stored at −70°C until used for assays.
2.4 Measurement of serum glucose, triglyceride, cholesterol, hormones and cytokines
Serum glucose, triglycerides and cholesterol were measured using commercially available kits. Serum cytokines were measured by multiplexed immunobeads (Luminex, Austin, TX) with reagents purchased from LINCO (LINCOplex, Millipore, St. Charles, MO).
2.5 RNA preparation
RNA was isolated from liver and skeletal muscle using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to manufacturer’s protocol. Potential impurities were removed (RNeasy Mini Kit, Qiagen, Valencia, CA) and the quality of RNA was assessed using a 1.5% agarose gel stained with ethidium bromide.
2.6 Gene expression analysis
Microarrays are prepared by printing oligonucleotides (mouse library, QIAGEN Operon, Inc., Alameda, CA) suspended in 45%(v/v) dimethyl sulfoxide onto poly-lysine-coated, glass microscope slides, using the GeneMachines OmniGrid microarrayer (San Carlos, CA). The mouse oligonucleotide library consists of 70mers that represent over 13,000 well-characterized genes.
Gene-expression microarray analysis was performed using the MICROMAX TSA Labeling and Detection Kit protocol (PerkinElmer Life Sciences, Inc., Boston, MA). Samples from each treatment were pooled to yield a total of 4–6 μg of RNA per biotin (B) or fluorescein (F) label. A total of six slides were used per experiment with three for forward labeling (group 1 is B; group 2 is F) and 3 for reverse labeling (group 1 is F; group 2 is B). Slides were scanned using the ScanArray 5000 (Packard BioChip Technologies, LLC, Billerica, MA) and the data normalized17.
2.7 Statistical Analysis
All phenotypic data are presented as mean ± SEM. Differences between means were assessed by Student’s t-test. P-values <0.05 were considered significant.
Results from microarray array gene expression analysis were analyzed by GenMapp and MAPPFinder (Gladstone Institutes, University of California, San Francisco, CA) to identify molecular pathways or gene groupings significantly affected by the ACN-GE treatment. Pathways significantly affected at an unadjusted P-value of 0.001 were examined. Duplicate or synonymous pathways were removed as were pathways in which the primary difference in gene expression resided in the ‘interaction partners’ rather than the main metabolic pathway.
RESULTS
3.1 Weight gain, body composition and relative organ weights
As compared to control mice, mice supplemented with ACN-GE tended to gain more weight (1.3 g; Figure 1), comprised of 33% fat (0.43g) and 67% fat free mass (0.87g) The ACN-GE mice tended to have increased adiposity, although these effects did not achieve statistical significance (Table 3). ACN-GE supplemented mice had significantly reduced relative liver and heart weights and near significantly reduced kidney weights (P=0.09). Retroperitoneal, epididymal and subcutaneous fat depots, tended to be higher in the ACN group, but were not significantly different from the control group.
Figure 1.
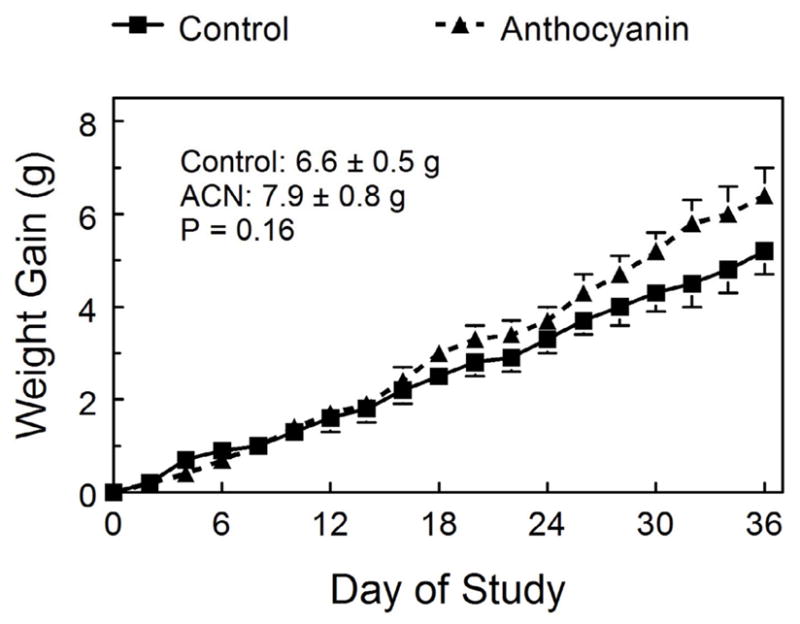
Weight gain of control and ACN-GE treated mice over the course of the study.
Table 3.
Effect of ACN-GE on body composition and relative organ weights
| Tissue | Control Group | ACN Group |
|---|---|---|
| % of body weight | ||
| Total fat | 17.1 ± 0.9 | 19.7 ± 1.9 |
| Liver | 3.81 ± 0.07 | 3.57 ± 0.07* |
| Heart | 0.49 ± 0.02 | 0.44 ± 0.01* |
| Kidney | 1.18 ± 0.03 | 1.10 ± 0.04 |
| Spleen | 0.29 ± 0.01 | 0.27 ± 0.01 |
| Brown fat | 0.23 ± 0.01 | 0.22 ± 0.02 |
| Retroperitoneal fat | 0.54 ± 0.04 | 0.68 ± 0.10 |
| Epididymal fat | 2.47 ± 0.14 | 2.90 ± 0.34 |
| Subcutaneous fat | 1.31 ± 0.07 | 1.48 ± 0.12 |
P<0.05
3.2 Serum glucose, cholesterol, triglycerides, hormones and cytokines
Serum cholesterol, triglycerides and glucose levels were not different between groups (data not shown).
Insulin and leptin levels tended to be higher in the ACN group, but the differences were not significantly different (Table 4). Adiponectin levels were similar between the two groups.
Table 4.
Effect of ACN-GE serum hormones
| Hormone | Control Group | ACN Group |
|---|---|---|
| Insulin (pg/ml) | 138 ± 17 | 178 ± 26 |
| Adiponectin (pg/ml) | 1473 ± 104 | 1446 ± 121 |
| Leptin (pg/ml) | 94 ± 15 | 135 ± 46 |
Control group, N=10; ACN group, N=5
Levels of granulocyte macrophage colony-stimulating factor, interferon-gamma, tumor necrosis factor-alpha, and interleukins 1b, 2, 4, 5, 6, 10 and 12 were all low and not different between the two groups (data not shown).
3.3 Gene expression microarray results
In the liver, only the Wnt signaling pathway met our criteria (unadjusted P≤0.001) for consideration as a pathway up-regulated by ACN-GE supplementation (Table 5). In contrast, nine pathways met our criteria for down-regulation. Of these nine pathways, five are directly related to energy metabolism (metabolism, energy derivation by oxidation of organic compounds, electron transport chain, fatty acid beta-oxidation, and tricarboxylic acid cycle) with three pathways (electron transport chain, fatty acid beta-oxidation, and tricarboxylic acid cycle) specific to the mitochondria. The inclusion of the ‘acute inflammatory response’ genes is in large part due to the down-regulation of a significant number of genes in the ‘complement activation’ subgroup (24% of genes down-regulated; P=0.002). The ‘selenium metabolism-selenoproteins’ includes three significantly down-regulated antioxidant enzymes (glutathione peroxidase 4, ↓47%, selenoprotein K, ↓30%; selenoprotein X1, →40%) and glutathione peroxidase 1 (↓41%, P=0.07).
Table 5.
Molecular pathways significantly (P ≤ 0.001) affected by ACN-GE in the liver
| Pathway Name | Number Measured on Pathway | Percent Changed | Z-Score |
|---|---|---|---|
| Pathways Significantly Up-Regulated | |||
| All pathways (reference) | 10,025 | 6.6 | 0 |
| Wnt Signaling | 269 | 14.9 | 4.8 |
| Pathways Significantly Down-Regulated | |||
| All pathways (reference) | 10,025 | 6.0 | 0 |
| Ribosomal proteins | 69 | 34.8 | 9.4 |
| Electron transport chain | 61 | 24.6 | 5.6 |
| Metabolism | 182 | 16.5 | 5.4 |
| Acute inflammatory response | 67 | 20.9 | 5.1 |
| Cholesterol biosynthesis | 12 | 41. 7 | 4.9 |
| Fatty acid beta-oxidation | 28 | 28.6 | 4.7 |
| Tricarboxylic acid cycle | 18 | 33.3 | 4.5 |
| Selenium metabolism-selenoproteins | 35 | 22.9 | 3.8 |
| Energy derivation by oxidation of organic compounds | 62 | 17.7 | 3.5 |
In muscle, only the ‘translation reactome’ pathway met our criteria for up-regulation. Six pathways were significantly down-regulated. Of these, four pathways dealt directly or indirectly with cellular defenses (response to wounding and its subcategories inflammatory response, and complement activation; immunoglobulin-mediated immune response).
DISCUSSION
The objective of this study was to determine if testable hypotheses could be developed regarding the potential health benefits of foods or food components by using an agnostic analytical and phenotyping approach. Our primary tool in this approach was gene expression profiling by microarray followed by pathway analysis. This allowed us to survey the effects of our dietary supplementation on over 4,500 physiological processes/gene groupings as defined by gene ontology terms. Our selection of an anthocyanin-rich grape skin extract was based in large part by the availability of grape variety (A-1575) which is exceptionally rich in anthocyanins and on published literature suggesting positive health benefits of anthocyanins18. The study was conducted in the C57Bl/6 mouse, a model used extensively in metabolic studies allowing comparison with other studies. Our diet was high in fat and cholesterol which has been used to stress a number of metabolic systems including those involved with lipid and carbohydrate metabolism, oxidative stress, and inflammatory response. The level of the anthocyanin-rich extract was relatively modest and was calculated to provide, on a metabolic body weight basis, an equivalent of approximately 150 mg anthocyanins/day for a 70 kg human. This amount approximates the intake of between 400–750 mls of red wine19
A number of physiological pathways were significantly affected by the addition ACN-GE to the drinking water. Two distinct clusters emerged. The first cluster included down-regulated pathways in both muscle and liver involving cellular defense. The ‘inflammatory response’ gene grouping was down regulated in both liver and muscle. Secondary investigation of additional significantly (P<0.05) down-regulated pathways in the liver identified genes involved in ‘response to oxidative stress’ (16% of genes down-regulated; P=0.008) and ‘response to unfolded proteins’ (17% of genes down-regulated; P=0.008) as additional targets of ACN-GE. From these data we can logically hypothesize that an anthocyanin-rich extract from grape decreases tissue inflammation by reducing oxidative stress.
This first hypothesis, while generated de novo from our data, is not novel. It is well established that anthocyanins are potent antioxidants10–14. Further, both in vitro and in vivo models have demonstrated that anthocyanins from a variety of sources can inhibit the inflammatory process20;20–24. That our agnostic approach independently yielded a hypothesis already under consideration by other investigators partially validates our hypothesis-generating paradigm.
The second cluster centered on energy metabolism in the liver with major metabolic pathways down regulated including the TCA cycle, fatty acid beta oxidation and cholesterol biosynthesis. These pathways are under control of a number of nuclear receptors suggesting the hypothesis that an anthocyanin-rich extract from grape through modulation of the activities of specific nuclear hormone receptors and transcription factors such as liver x-receptor, peroxisome proliferators activated receptors (PPARα, PPARδ, PPARγ), sterol regulatory element binding protein (SREBP)-1c, and/or PPARγ coactivator-1α and –1β, alter substrate metabolism in the liver. The ligands for several of these nuclear receptors are oxidized derivatives of sterols or fatty acids. By virtue of its antioxidant capacity, the ACN-GE may modulate the endogenous levels of specific ligand activators and affect the activities of their nuclear receptors. However, we cannot exclude the possibility of direct interactions of anthocyanins with the nuclear receptors.
Effects of polyphenolic compounds on metabolic pathways involved in energy metabolism are not without precedent. Sesamin, a polyphenolic lignan compound found in sesame oil, was shown to decrease both the enzyme activity and expression levels of enzymes involved in fatty acid synthesis, while increasing the activity of enzymes involved in fatty acid oxidation25. A reduction in the levels of SREBP-1c and conversion of SREBP-1c to its mature form led the authors to conclude that sesamin affected lipid metabolism through modulation of SREBP-1c activity. In a study of purple corn anthocyanin effects, Tsuda et al.26 demonstrated that isolated rat adipose tissue incubated with cyanidin for 24h had elevated expression levels of PPAR-γ. It is noteworthy that PPARγ in adipocytes is thought to induce lipogenesis through modulation of SREBP-1c activity27. Thus both studies suggest involvement of nuclear receptors, consistent with our developed hypothesis.
However, we also note that many of the affected pathways in the liver are localized to the mitochondria. Thus we have also developed a competing hypothesis which states that an anthocyanin-rich extract from grape inhibits mitochondrial biogenesis through modulation of the activity of either PPARγ coactivator-1α or –1β or its downstream targets nuclear respiratory factor-1 and -2. PPARγ coactivator-1α and –1β are induced under conditions of oxidative stress28. Thus, the antioxidant properties of anthocyanins may decrease hepatic oxidative stress and PPARγ coactivator-1α and –1β expression, leading to reduced mitochondrial biogenesis.
Other pathways were clearly influenced by ACN-GE and additional hypotheses may be developed. However for the purpose of this proof-of-concept study, we have focused on the two largest clusters of affected pathways. We have demonstrated that an agnostic approach could be employed to develop testable hypotheses for future investigation into the potential health benefits of foods and food components. This approach could be strengthened by the concomitant application of proteomic and metabolomic methods. In the present study, we also conducted a proteomic analysis of liver proteins. Preliminary analysis of these data confirms the effects of the ACN-GE on the levels of proteins involved in oxidative response and energy metabolism (Lefevre, manuscript in preparation).
Finally, it should be emphasized that the designed outcome of these studies are new hypotheses to be tested in future studies. The microarray gene expression data have not been confirmed by real-time PCR and thus these data should not be taken as definitive evidence of an effect of ACN-GE on the pathways discussed. Additional studies specifically designed to test these developed hypotheses are required.
Table 6.
Molecular pathways significantly (P ≤ 0.001) affected by ACN-GE in skeletal muscle
| Pathway Name | Number Measured on Pathway | Percent Changed | Z-Score |
|---|---|---|---|
| Pathways Significantly Up-Regulated | |||
| All pathways (reference) | 11,424 | 3.3 | 0 |
| Translation reactome | 280 | 8.2 | 4.501 |
| Pathways Significantly Down-Regulated | |||
| All pathways (reference) | 11,424 | 4.3 | 0 |
| Extracellular matrix | 205 | 12.7 | 5.7 |
| Complement activation | 15 | 33.3 | 5.4 |
| Response to wounding | 158 | 12.0 | 4.6 |
| Carbohydrate binding | 140 | 12.1 | 4.4 |
| Inflammatory response | 151 | 11.9 | 4.8 |
| Immunoglobulin mediated immune response | 17 | 23.5 | 4.6 |
Acknowledgments
This work in funded in part by NIH grant: P50AT002776–01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, which funds the Botanical Research Center, and also by USDA to the University of Arkansas Fayetteville, grant number UA AES 2001–102.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MyPyramid. US Department of Agriculture and U.S. Department of Health and Human Services; 2005. http://mypyramid.gov/ (GENERIC) Ref Type: Electronic Citation. [Google Scholar]
- 2.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 3.Third Report of the National Cholesterol Education (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panell III). 2002. National Institutes of Health. (GENERIC) Ref Type: Pamphlet
- 4.Kris-Etherton PM, Lefevre M, Beecher GR, et al. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–538. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Beecher GR, Holden JM, et al. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 6.McGhie TK, Ainge GD, Barnett LE, et al. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J Agric Food Chem. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- 7.Bub A, Watzl B, Heeb D, et al. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur J Nutr. 2001;40:113–120. doi: 10.1007/s003940170011. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Nair MG, Strasburg GM, et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod. 1999;62:802. doi: 10.1021/np990184z. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda T, Horio F, Osawa T. Cyanidin 3-O-beta-D-glucoside suppresses nitric oxide production during a zymosan treatment in rats. J Nutr Sci Vitaminol(Tokyo) 2002;48:305–310. doi: 10.3177/jnsv.48.305. [DOI] [PubMed] [Google Scholar]
- 10.Noda Y, Kaneyuki T, Igarashi K, et al. Antioxidant activity of nasunin, an anthocyanin in eggplant. Res Commun Mol Pathol Pharmacol. 1998;102:175–187. [PubMed] [Google Scholar]
- 11.Noda Y, Kaneyuki T, Mori A, et al. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002;50:166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 12.Cho J, Kang JS, Long PH, et al. Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Arch Pharm Res. 2003;26:821–825. doi: 10.1007/BF02980027. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda T, Horio F, Kato Y, et al. Cyanidin 3-O-beta-D-glucoside attenuates the hepatic ischemia-reperfusion injury through a decrease in the neutrophil chemoattractant production in rats. J Nutr Sci Vitaminol(Tokyo) 2002;48:134–141. doi: 10.3177/jnsv.48.134. [DOI] [PubMed] [Google Scholar]
- 14.Bagchi D, Sen CK, Bagchi M, et al. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Mosc) 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- 15.Briviba K, Abrahamse SL, Pool-Zobel BL, et al. Neurotensin-and EGF-induced metabolic activation of colon carcinoma cells is diminished by dietary flavonoid cyanidin but not by its glycosides. Nutr Cancer. 2001;41:172–179. doi: 10.1080/01635581.2001.9680629. [DOI] [PubMed] [Google Scholar]
- 16.Tsuda T, Horio F, Uchida K, et al. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 17.Yang YH, Dudoit S, Luu P, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 19.Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Shin WH, Seo JW, et al. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food Chem Toxicol. 2007;45:1459–1467. doi: 10.1016/j.fct.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Xia M, Ling W, Zhu H, et al. Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler Thromb Vasc Biol. 2007;27:519–524. doi: 10.1161/01.ATV.0000254672.04573.2d. [DOI] [PubMed] [Google Scholar]
- 22.Pergola C, Rossi A, Dugo P, et al. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide. 2006;15:30–39. doi: 10.1016/j.niox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Tall JM, Seeram NP, Zhao C, et al. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav Brain Res. 2004;153:181–188. doi: 10.1016/j.bbr.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A, Serraino I, Dugo P, et al. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic Res. 2003;37:891–900. doi: 10.1080/1071576031000112690. [DOI] [PubMed] [Google Scholar]
- 25.Ide T, Ashakumary L, Takahashi Y, et al. Sesamin, a sesame lignan, decreases fatty acid synthesis in rat liver accompanying the down-regulation of sterol regulatory element binding protein-1. Biochim Biophys Acta. 2001;1534:1–13. doi: 10.1016/s1388-1981(01)00167-6. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda T, Ueno Y, Aoki H, et al. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem Biophys Res Commun. 2004;316:149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Morrison RF, Farmer SR. Hormonal signaling and transcriptional control of adipocyte differentiation. J Nutr. 2000;130:3116S–3121S. doi: 10.1093/jn/130.12.3116S. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–3. 63–9, 60–63. [PubMed] [Google Scholar]


