Abstract
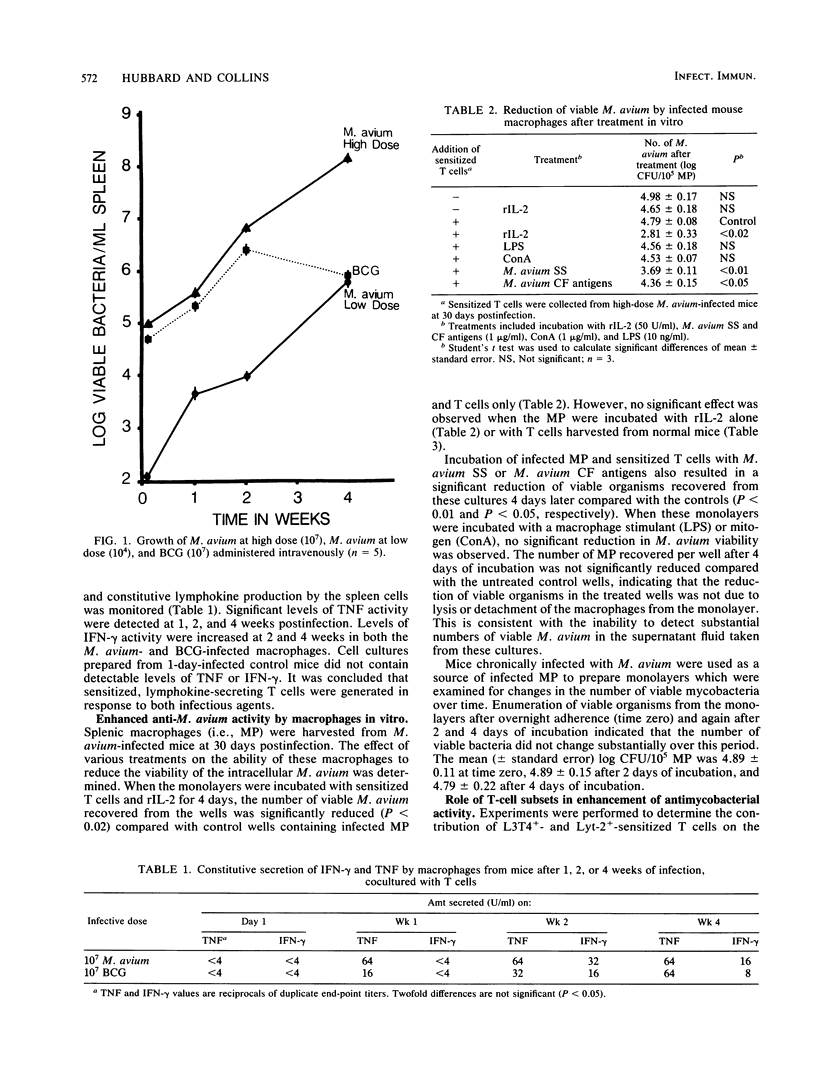
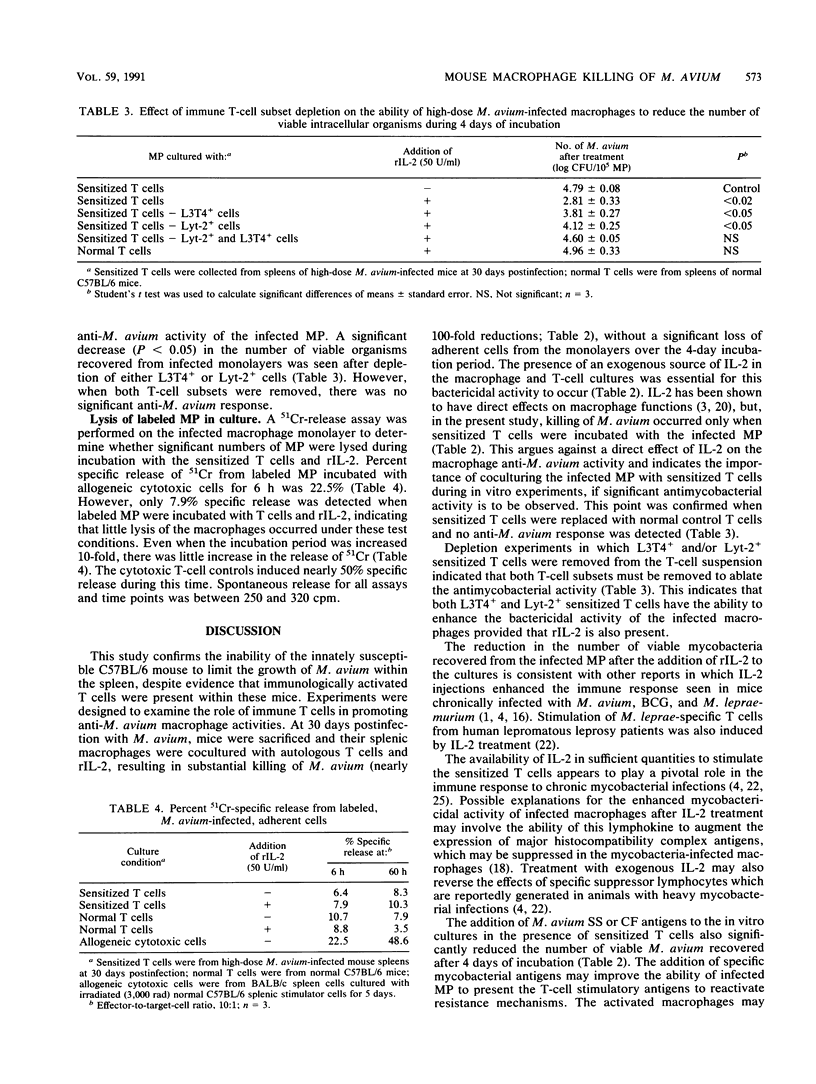
When C57BL/6 mice were infected intravenously with Mycobacterium avium, bacterial growth continued within the spleen until more than 10(8) CFU/g of tissue were attained. This contrasted with Mycobacterium bovis BCG infections where growth declined after 2 weeks. In vivo M. avium-infected splenic macrophages were harvested from chronically infected mice and cultured in vitro for 4 days at 37 degrees C. The number of viable mycobacteria within the resulting macrophage monolayers decreased when cultured in the presence of autologous sensitized T cells and an exogenous source of interleukin-2 (recombinant interleukin-2; 50 U/ml) compared with untreated controls (P less than 0.05). Incubation of the infected macrophages with autologous T cells and soluble M. avium antigens also significantly reduced the number of viable organisms. These results indicate that the mycobactericidal activity of M. avium-infected macrophages can be enhanced in a way that may have important therapeutic implications for patients infected with this opportunistic pathogen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Colizzi V. In vivo and in vitro administration of interleukin 2-containing preparation reverses T-cell unresponsiveness in Mycobacterium bovis BCG-infected mice. Infect Immun. 1984 Jul;45(1):25–28. doi: 10.1128/iai.45.1.25-28.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. In vivo vs. in vitro killing of virulent Mycobacterium tuberculosis. Res Microbiol. 1990 Feb;141(2):212–266. doi: 10.1016/0923-2508(90)90033-m. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterium avium-complex infections and development of the acquired immunodeficiency syndrome: casual opportunist or causal cofactor? Int J Lepr Other Mycobact Dis. 1986 Sep;54(3):458–474. [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- Deo M. G. Immunological approach for control of Mycobacterium avium-intracellulare infections in AIDS-an hypothesis. Int J Lepr Other Mycobact Dis. 1988 Sep;56(3):455–463. [PubMed] [Google Scholar]
- Havell E. A. Augmented induction of interferons during Listeria monocytogenes infection. J Infect Dis. 1986 May;153(5):960–969. doi: 10.1093/infdis/153.5.960. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Hill J. O., Awwad M., North R. J. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. J Exp Med. 1989 May 1;169(5):1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989 Jan;139(1):4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- Jeevan A., Asherson G. L. Recombinant interleukin-2 limits the replication of Mycobacterium lepraemurium and Mycobacterium bovis BCG in mice. Infect Immun. 1988 Mar;56(3):660–664. doi: 10.1128/iai.56.3.660-664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. L., Hines D. L. Tumour-induced immunity to H-Y-disparate skin grafts without concomitant priming of CTL. Immunology. 1990 Jan;69(1):85–90. [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Sims M., Feldmann M. Regulation of macrophage accessory cell activity by mycobacteria. II. In vitro inhibition of Ia expression by Mycobacterium microti. Clin Exp Immunol. 1986 Apr;64(1):28–34. [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Andrew P. W. Activation of macrophages for antimycobacterial activity. Immunol Lett. 1985;11(3-4):195–203. doi: 10.1016/0165-2478(85)90168-3. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Growth of Mycobacterium avium in activated macrophages harvested from inbred mice with differing innate susceptibilities to mycobacterial infection. Infect Immun. 1988 Sep;56(9):2250–2254. doi: 10.1128/iai.56.9.2250-2254.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Mononuclear phagocytes as targets for cytolytic T lymphocytes. J Immunol Methods. 1987 Jun 26;100(1-2):99–105. doi: 10.1016/0022-1759(87)90177-3. [DOI] [PubMed] [Google Scholar]
- Zakowski P., Fligiel S., Berlin G. W., Johnson L., Jr Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency. JAMA. 1982 Dec 10;248(22):2980–2982. doi: 10.1001/jama.1982.03330220024029. [DOI] [PubMed] [Google Scholar]


